Abstract
Cerebral ischemia is the important cause of worldwide disability and mortality, that is one of the obstruction of blood vessels supplying to the brain. In early stage, glutamate excitotoxicity and high level of intracellular calcium (Ca2+) are the major processes which can promote many downstream signaling involving in neuronal death and brain tissue damaging. Moreover, autophagy, the reusing of damaged cell organelles, is affected in early ischemia. Under ischemic conditions, autophagy plays an important role to maintain energy of the brain and its function. In the other hand, over intracellular Ca2+ accumulation triggers excessive autophagic process and lysosomal degradation leading to autophagic process impairment which finally induce neuronal death. This article reviews the association between intracellular Ca2+ and autophagic process in acute stage of ischemic stroke.
Stroke is a type of cerebrovascular disease which commonly occurs in adults and elderly. It remains the worldwide third majority cause of disability and death [1]. The incident of stroke cases infinitely increased to 70% from 1990 to 2019, and the mortality rate escalated to 40% [2]. More than 87% of strokes are presented as ischemia [3]. Cerebral ischemia or ischemic stroke is the insufficient blood supply and nutrient to the brain caused by cerebral artery occlusion leading to brain dysfunction sand damage [4]. The common forms of occlusion resulting in blood flow obstruction to the brain are thrombosis, the ruptured plaque from cerebral stenosis or atherosclerosis, and embolism, the clot formed in vessel and heart [5]. The severity of brain damage depends on occlusion time. Recombinant tissue-type plasminogen activator is recommended for intravenous injection within 4.5 hours after stroke symptoms occurring. The golden hour for ischemic stroke treatment is less than 1 hour [6, 7]. Long-term cerebral artery occlusion without re-perfusion leads to permanent brain injury. There are many homeostatic mechanisms occurring after cerebral vascular obstruction. Excessive glutamate releasing and calcium toxicity is the common stimulator arising in acute stage of ischemia, which can lead to various brain damage pathways [8]. Moreover, it involves the autophagic impairment and neuronal death stimulation during acute phase [9]. This article reviews the underlying mechanism of calcium toxicity related to autophagy up-regulation in acute stage of permanent cerebral ischemia.
Brain is the high energy consumption organ which mainly synthesizes more than 20% of total adenosine triphosphate (ATP) to retain its functions [10]. Neurons drive the neuronal signaling by conveying the electrochemical ions via ATPase ion pumps on cell membrane, called action potential [11, 12]. Neurotransmitters play a vital role in neuronal conducting of neuron to neuron both excitatory and inhibitory signaling [13, 14].
After the cerebral artery occlusion, decreased cerebral blood flow to the brain leads to reduced oxygen and glucose consumption because of depleted ATP produced from mitochondria in neuron and glial cell [15, 16]. These result in ATPase ion pumps failure, known as anoxic depolarization including Na+/K+-ATPase (NKA), Na+/Ca2+-ATPase pumps (NCX) and Ca2+ ATPase channels. Generally, NKA pumps 2 ions of K+ into neurons to evoke action potential and offers energy to NCX pumps which control the concentration of Ca2+, Na+ and K+ between extra- and intracellular space of neuronal cells [17-19]. Impaired ATPase ion pumps affect the flowing of ions into the cell and results in neuronal swelling [20]. Likewise, depleted ATP provokes voltage-gated calcium channel (VGCC) to maintain function resulting in intracellular Ca2+ overload [21] Moreover, these engenders Ca2+ influx into presynaptic area leading to excessive glutamate releasing to synaptic space with glutamate re-uptake failure [22].
Glutamate is an excitatory neurotransmitter related to learning and memory, which is released from presynaptic terminals to stimulate other neuronal cells [23]. Neurons and astrocytes are the two major cells associated with glutamate metabolism [24]. Glutamate releasing activates ionotropic glutamate receptors and ligand-gated ion channels on postsynaptic membranes which rapidly responses to allow ions influx and stimulate various downstream cascades [25]. Under ischemic conditions, excessive glutamate releasing, or glutamate excitotoxicity is the main point which triggers neuronal dysfunction in acute stage [26]. Immoderate glutamate receptor activation promotes Ca2+ transferring into the cell, which can provoke a various cellular signaling resulting to neuronal death [27]. In addition, the dysfunction of ion exchange across neuronal membrane impels exorbitant Na+ influx causing hyperosmotic movement and cell swelling, respectively (Fig. 1) [28, 29].
N-methyl-D-aspartate receptor (NMDAr) is a type of ligand-gated ion channel which plays an important role in early ischemia. Over activation of NMDAr by unnecessary glutamate releasing affects to intracellular Ca2+ accumulation resulting to calcium toxicity, which can promote many signaling cascade associated with neuronal dysfunction and death [30, 31]. Excessive NMDAr stimulation is the major cause of neurotoxicity in acute ischemia. Abnormal Ca2-dependent enzyme activation led to cell death signaling. NMDAr consists of 2 main heterotetrametric forms including GluN1 and GluN2 subunits. These subunits have an important function in neuronal survival and neuronal death. GluN2 subunit is separated into 2 subtypes; GluN2A and GlutN2B. GluN2A is greatly expressed at synapse site of neuron which plays a key role in neuronal survival [32, 33]. The activated GluN2A can activate phosphoinositide 3-kinase (PI3K) by binding to Ca2+ and calmodulin which phosphorylates protein kinase B (Akt), respectively. Phosphorylated Akt generally inhibits pro-apoptotic factors [21, 34]. Moreover, the activated NMDAr stimulates mitogen-activated protein kinases/extracellular signal-regulated kinases (ERK) pathway, the extracellular signaling regulated protein kinase and Ca2+/calmodulin-dependent protein kinase, the calcium signaling, that can provoke cAMP response element-binding protein (CREB) by phosphorylation to motivate anti-apoptotic proteins [35].
Under ischemic condition, excessive glutamate releasing extremely stimulates NMDAr, especially GluN2B subtype, which promote pro-apoptotic cell death signaling cascade [36]. GluN2B is fully located at an extra-synaptic site. Over-synaptic NMDAr can trigger GluN2B subtype to dephosphorylate and inhibit ERK and CREB signaling pathway resulting to pro-apoptotic activation [35, 37]. Furthermore, GluN2B also provokes postsynaptic density protein 95 (PSD95) protein which downstream stimulates neuronal nitric oxide synthase (nNOS) by binding to the N-terminus, called GluN2B/PSD95/nNOS complex, leading to nitric oxide (NO) production [38, 39]. NO interacts with superoxide radical molecules and critically forms reactive nitrogen species (RNS) that are associated with protein oxidation, lipid peroxidation and DNA fragmentation [40].
Death associated protein kinase 1 (DAPK1), the mediator induced programmed cell death, is disinhibited from autophosphorylation suppressing by high level of intracellular Ca2+ activated calmodulin (CaM) and leads to pro-apoptotic activation [41]. Likewise, DAPK1 can directly interact with the C-terminal region of GluN2B tail to escalate the stimulation of GluN2B subtype. GluN2B/DAPK1 binding complex encourages more severity of neuronal damage [42]. Furthermore, activated DAPK1 can interact with programmed cell death 6 (PDCD6), tumor protein 53 and protein kinase D to promote both necrotic and apoptotic neuronal cell death [42-44].
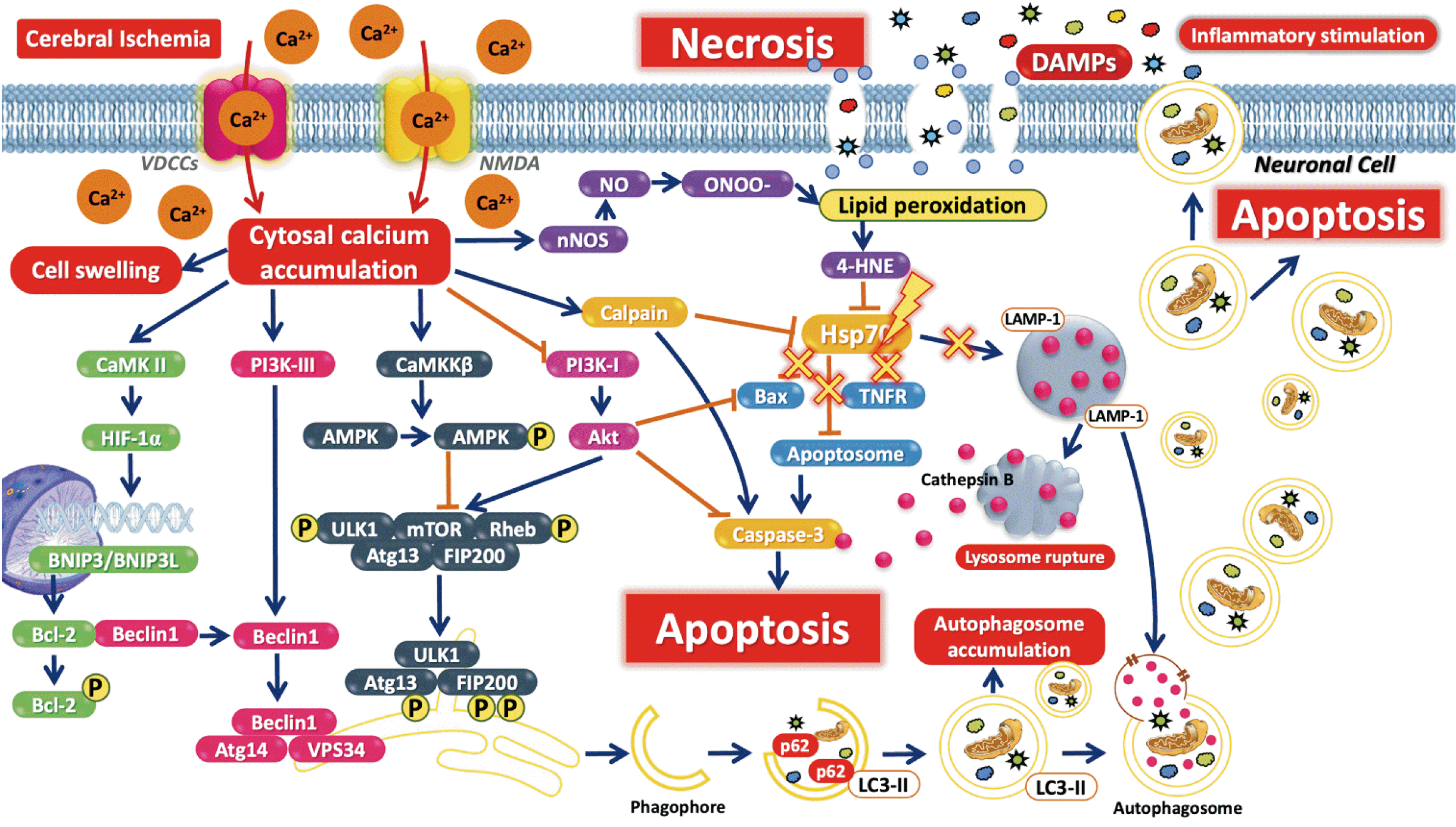
Over NMDAr activation vastly fluxes Ca2+ into the neuron [45]. There is reported Ca2+ quickly influxes into the neuronal cell via NMDAr in the early stage of ischemia [46]. High Ca2+ concentration accumulated in cytoplasm can trigger many downstream cascades of neuronal death signaling [8]. Calpain (CAPN) is a Ca2+-dependent cysteines protease which is greatly found in central nervous system [47]. In the brain, CAPN acts as Ca2+-dependent neuronal function controlling. CAPN is separated into 2 major isoforms which are CAPN1 or μ-calpain and CAPN2 or m-calpain [48]. In ischemic brain, CAPN1 is activated by excessive Ca2+ accumulation. Activated CAPN1 play a key role in neuronal death. CAPN1 is the protease enzyme that hydrolyzes various cytoskeleton proteins causing neuronal dysfunction and apoptotic stimulation [49, 50]. Additionally, overactivated NMDAr downstream stimulates CAPN1 in cytosol which can cleave NCX channels at neuronal membrane resulting in promoting NCX channels dysfunction and poorly control Ca2+ influx [51]. Moreover, CAPN1 also affects to metabotropic glutamate receptor 1 protein at cell membrane that normally motivates brain function and acts as neuroprotective by interacting with nuclear phosphoinositide-3-kinase enhancer to promote PI3K/Akt signaling pathway (Fig. 2) [52].
Autophagy is a self-digestive program for cellular homeostatic maintaining and cell survival by degrading misfold proteins and damaged organelles to produce energy [53]. Macro-autophagy is the general form of autophagic process normally found in eukaryotic cells [54]. The common characteristics are autophagosome forming and fuses with lysosome to degrade the deformed proteins and dysfunctional organelles [55]. The major key of autophagic initiation is mammalian target of rapamycin (mTOR) which is phosphatidylinositol 3-kinase-related kinase family and controls cellular physiology, protein synthesis and autophagy [56]. mTOR regulates autophagy by phosphorylation of Atg1/ULK1 complex to form autophagopore with beclin-1 [57]. Beclin-1 is the autophagy regulator conjugated with PI3K class III to form beclin-1/Vsp34/Atg14/PI3K-III complex and stimulate autophagopore formation [58]. Microtubule-associated proteins 1A/1B light chain 3A (LC3) are the main protein of autophagosome establishing. LC3-II plays an important role in double membrane elongation to form vesicles which engulf the damaged organelles and proteins, known as autophagosome [59]. Autophagosome is fused with lysosome called autophagolysosome to degrade the products by acid hydrolase within lysosome and obtain the final products as free fatty acids and amino acids to generate energy for cell survival [60, 61].
In ischemic brain condition, the research has focused on autophagy in the early stage. It is found that autophagy is the double-edged sword for cerebral ischemia [9, 62]. Excessive autophagy leads to neuronal death which rapidly increases in 3–12 hours after cerebral ischemia [63, 64]. CAPN1, the result of excessive Ca2+ overload, can cleavage beclin-1 lead to defection of autophagosome formation [65]. Moreover, CAPN1 also interrupts the function of Atg protein, the main protein functioning though autophagic process, families resulting in Atg protein dysfunction and autophagic flux impairment [66]. In addition, high level of intracellular Ca2+ and reduced of ATP affects to AMP-activated protein kinase-alpha (AMPKα) phosphorylation, the target of autophagy up-regulation, which inhibits mTOR activation and promote over autophagic flux after ischemic occurring [67]. Incomplete autophagosome formation affects to autophagosome accumulation and trigger neuronal cell death, respectively (Fig. 2).
Heat shock proteins (Hsp70) are associated with protein folding, complex formation, translocation, and protein degradation. It prevents the abnormal protein formation under stress condition by translocating from the cytosol to the nucleus [68-70]. Under cerebral ischemia, a recent study found that Hsp70 translocates to the luminal side of the lysosome to stabilize the lysosomal membrane [71]. The expression of Hsp70 is significantly increased in 6 hours after ischemic occurring [72-74]. Lysosome, the membrane-bound organelle containing hydrolytic enzyme, is an important component of autophagic process [55]. Under ischemic conditions, lysosome is destroyed by CAPN1 which induces lysosomal membrane permeability resulting to lysosomal breakdown and hydrolytic enzyme releasing into cytoplasm. These can trigger cell death signaling in neuronal cell [75, 76].
In cerebral ischemia, ATP depletion in the brain stimulates anoxic depolarization, resulting in NMDAr overexpression and intracellular Ca2+ accumulation. These changes can activate CAPN1 [22, 77], which translocates to the lysosomal membrane, a change that alters lysosomal membrane permeability, causing the membrane to rupture and cathepsin B release into the cytosol [75, 76, 78]. Moreover, NMDA receptor overexpression provokes nNOS activity, leading to NO generation [79]. Over NO generation is the cause of 4-HNE production resulting from lipid peroxidation [79, 80]. CAPN1 and 4-HNE provoke Hsp70 carbonylation, leading to Hsp70 dysfunction and the loss of lysosomal membrane stability. Consequently, the lysosomal membrane ruptures and cathepsin B is released [71, 81-84]. Cathepsin B release can activate caspase-3 via caspase-11, which leads to apoptosis in the brain (Fig. 2) [85-87].
It is reported that blocking NMDAr by NMDAr antagonist can reduce brain damage from excitotoxicity and intracellular Ca2+ accumulation as neuroprotection in acute stage [38, 88]. The researcher found that ifenprodil, the GluN1 and GluN2B subunits inhibitor, reduced neuronal death in transient cerebral ischemic rat together with contribute phosphorylation of CREB protein as neuroprotective effect [89]. Nimodipine, the VGCC, was used to delay brain damage in early cerebral ischemia. It is found that nimodipine injection via intra-arterial administration protects the brain from cerebral ischemic injury after aneurysmal subarachnoid hemorrhage [90]. Treated with icaritin (ICT), the natural compound extracted from traditional Chinese herb (Epimedium Genus), can protect neuronal cell from glutamate-induced neuronal cell damage by inhibiting DAPK1 and GluN2B activation with promoting phosphorylation of ERK and GluN2A expression, which reduce neuronal cell death in ischemic rats [91].
Regulated autophagy is an important process which can maintain cellular energy and promote cell survival under ischemic conditions [92]. The potential therapeutic treatments for autophagic regulation in cerebral ischemia are interesting. Many researchers reported that regulated autophagy by promoting mTOR activation can reduce brain damage in ischemic condition [93, 94]. Furthermore, the alternative treatment with natural compounds also improves autophagy in ischemia [95]. In glutamate-induced excitotoxic neuroblastoma cell, treated with ST2-104, the nona-arginine (R9)-fused CBD3 peptide, reduce Ca2+ accumulation in cytosol with decrease cell death via Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKKβ) regulation. Moreover, ST2-104 control autophagic process via CaMKKβ/AMPK/mTOR pathway. Likewise, ST2-104 protect the rat brain from transient cerebral ischemic injury after transient cerebral ischemia [96]. Neferine is the alkaloid compound extracted from lotus seeds. It demonstrates the attribute of Ca2+ channel blocker [97], which can moderate intracellular Ca2+ level and regulate autophagic flux via Ca2+-dependent AMPK/mTOR pathway leading to reduce of brain infarction in acute permanent ischemic rats [98].
Blocking NMDAr can reduce neuronal cell damage from glutamate and intracellular Ca2+ toxicity in early cerebral ischemia. However, the severity of ischemic brain varies to occlusion time. Treatment with NMDAr antagonist is suitable for acute cerebral ischemia. The effects of treatment are less efficient to prevent brain damage in long-term occlusion [99, 100].
In the early stage of cerebral ischemia, glutamate excitotoxicity and Ca2+ ions overload play an important role in neuronal dysfunction which can disturb the various cellular physiologies and trigger many downstream cascades of cell death. Autophagy is one of the essential processes which is affected after acute cerebral ischemic occurring. Excessive autophagic flux and lysosomal degradation led to neuronal cell death stimulation. Contrarily, regulated autophagy promotes cell survival and protects the neuron from second damage of ischemia.
Notes
References
1. Saini V, Guada L, Yavagal DR. 2021; Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 97(20 Suppl 2):S6–16. DOI: 10.1212/WNL.0000000000012781. PMID: 34785599.

2. Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P. 2022; World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 17:18–29. Erratum in: Int J Stroke 2022;17:478. DOI: 10.1177/17474930211065917. PMID: 34986727.

3. Donnan GA, Fisher M, Macleod M, Davis SM. 2008; Stroke. Lancet. 371:1612–23. DOI: 10.1016/S0140-6736(08)60694-7. PMID: 18468545.

4. Johnstone VP, Shultz SR, Yan EB, O'Brien TJ, Rajan R. 2014; The acute phase of mild traumatic brain injury is characterized by a distance-dependent neuronal hypoactivity. J Neurotrauma. 31:1881–95. DOI: 10.1089/neu.2014.3343. PMID: 24927383. PMCID: PMC4224042.

5. DeSai C, Hays Shapshak A. 2023. Apr. 3. Cerebral ischemia [Internet]. StatPearls;Available from: https://www.ncbi.nlm.nih.gov/books/NBK560510/.
6. Anderson JA. 2014; The golden hour Performing an acute ischemic stroke workup. Nurse Pract. 39:22–9. quiz 29–30. DOI: 10.1097/01.NPR.0000452974.46311.0f. PMID: 25083767.

7. Advani R, Naess H, Kurz MW. 2017; The golden hour of acute ischemic stroke. Scand J Trauma Resusc Emerg Med. 25:54. DOI: 10.1186/s13049-017-0398-5. PMID: 28532498. PMCID: PMC5440901.

8. Singh V, Mishra VN, Chaurasia RN, Joshi D, Pandey V. 2019; Modes of calcium regulation in ischemic neuron. Indian J Clin Biochem. 34:246–53. DOI: 10.1007/s12291-019-00838-9. PMID: 31391713. PMCID: PMC6660593.

9. Chen W, Sun Y, Liu K, Sun X. 2014; Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 9:1210–6. DOI: 10.4103/1673-5374.135329. PMID: 25206784. PMCID: PMC4146291.

10. Davis GW. 2020; Not fade away: mechanisms of neuronal ATP homeostasis. Neuron. 105:591–3. DOI: 10.1016/j.neuron.2020.01.024. PMID: 32078791.

11. Agnati LF, Guidolin D, Cervetto C, Maura G, Marcoli M. 2023; Brain structure and function: insights from chemical neuroanatomy. Life (Basel). 13:940. DOI: 10.3390/life13040940. PMID: 37109469. PMCID: PMC10142941.

12. Bertrand PP. 2003; ATP and sensory transduction in the enteric nervous system. Neuroscientist. 9:243–60. DOI: 10.1177/1073858403253768. PMID: 12934708.

13. Clarke SG, Scarnati MS, Paradiso KG. 2016; Neurotransmitter release can be stabilized by a mechanism that prevents voltage changes near the end of action potentials from affecting calcium currents. J Neurosci. 36:11559–72. DOI: 10.1523/JNEUROSCI.0066-16.2016. PMID: 27911759. PMCID: PMC5125219.

14. Zbili M, Rama S, Debanne D. 2016; Dynamic control of neurotransmitter release by presynaptic potential. Front Cell Neurosci. 10:278. DOI: 10.3389/fncel.2016.00278. PMID: 27994539. PMCID: PMC5136543.

15. Sifat AE, Nozohouri S, Archie SR, Chowdhury EA, Abbruscato TJ. 2022; Brain energy metabolism in ischemic stroke: effects of smoking and diabetes. Int J Mol Sci. 23:8512. DOI: 10.3390/ijms23158512. PMID: 35955647. PMCID: PMC9369264.

16. Liu F, Lu J, Manaenko A, Tang J, Hu Q. 2018; Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 9:924–37. DOI: 10.14336/AD.2017.1126. PMID: 30271667. PMCID: PMC6147588.

17. Suhail M. 2010; Na, K-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res. 2:1–17. DOI: 10.4021/jocmr2010.02.263w. PMID: 22457695. PMCID: PMC3299169.

18. Shen Z, Xiang M, Chen C, Ding F, Wang Y, Shang C, Xin L, Zhang Y, Cui X. 2022; Glutamate excitotoxicity: potential therapeutic target for ischemic stroke. Biomed Pharmacother. 151:113125. DOI: 10.1016/j.biopha.2022.113125. PMID: 35609367.

19. Belov Kirdajova D, Kriska J, Tureckova J, Anderova M. 2020; Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front Cell Neurosci. 14:51. DOI: 10.3389/fncel.2020.00051. PMID: 32265656. PMCID: PMC7098326.

20. de Lores Arnaiz GR, Ordieres MG. 2014; Brain Na(+), K(+)-ATPase activity in aging and disease. Int J Biomed Sci. 10:85–102. DOI: 10.59566/IJBS.2014.10085. PMID: 25018677. PMCID: PMC4092085.

21. Wang F, Xie X, Xing X, Sun X. 2022; Excitatory synaptic transmission in ischemic stroke: a new outlet for classical neuroprotective strategies. Int J Mol Sci. 23:9381. DOI: 10.3390/ijms23169381. PMID: 36012647. PMCID: PMC9409263.

22. Nishizawa Y. 2001; Glutamate release and neuronal damage in ischemia. Life Sci. 69:369–81. DOI: 10.1016/S0024-3205(01)01142-0. PMID: 11459428.

23. Franco R, Rivas-Santisteban R, Lillo J, Camps J, Navarro G, Reyes-Resina I. 2021; 5-hydroxytryptamine, glutamate, and ATP: much more than neurotransmitters. Front Cell Dev Biol. 9:667815. DOI: 10.3389/fcell.2021.667815. PMID: 33937270. PMCID: PMC8083958.

24. Mahmoud S, Gharagozloo M, Simard C, Gris D. 2019; Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 8:184. DOI: 10.3390/cells8020184. PMID: 30791579. PMCID: PMC6406900.

25. Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. 2010; Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62:405–96. Erratum in: Pharmacol Rev 2014;66:1141. DOI: 10.1124/pr.109.002451. PMID: 20716669. PMCID: PMC2964903.

26. Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. 1995; Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 15:961–73. DOI: 10.1016/0896-6273(95)90186-8. PMID: 7576644.

27. Mattson MP. 2003; Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 3:65–94. DOI: 10.1385/NMM:3:2:65. PMID: 12728191.

28. Hellas JA, Andrew RD. 2021; Neuronal swelling: a non-osmotic consequence of spreading depolarization. Neurocrit Care. 35(Suppl 2):112–34. DOI: 10.1007/s12028-021-01326-w. PMID: 34498208. PMCID: PMC8536653.

29. Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. 2009; Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda). 24:257–65. DOI: 10.1152/physiol.00015.2009. PMID: 19675357.

30. Akins PT, Atkinson RP. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin. 2002; 18(Suppl 2):s9–13. DOI: 10.1185/030079902125000660. PMID: 12365832.

31. Besancon E, Guo S, Lok J, Tymianski M, Lo EH. 2008; Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 29:268–75. DOI: 10.1016/j.tips.2008.02.003. PMID: 18384889.

32. von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Köhr G, Seeburg PH, Monyer H. 2007; Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 53:10–7. DOI: 10.1016/j.neuropharm.2007.04.015. PMID: 17570444.

33. Zhou X, Ding Q, Chen Z, Yun H, Wang H. 2013; Involvement of the GluN2A and GluN2B subunits in synaptic and extrasynaptic N-methyl-D-aspartate receptor function and neuronal excitotoxicity. J Biol Chem. 288:24151–9. DOI: 10.1074/jbc.M113.482000. PMID: 23839940. PMCID: PMC3745357.

34. Lai TW, Zhang S, Wang YT. 2014; Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol. 115:157–88. DOI: 10.1016/j.pneurobio.2013.11.006. PMID: 24361499.

35. Wu QJ, Tymianski M. 2018; Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 11:15. DOI: 10.1186/s13041-018-0357-8. PMID: 29534733. PMCID: PMC5851248.

36. Lujan B, Liu X, Wan Q. 2012; Differential roles of GluN2A- and GluN2B-containing NMDA receptors in neuronal survival and death. Int J Physiol Pathophysiol Pharmacol. 4:211–8.
37. Franchini L, Carrano N, Di Luca M, Gardoni F. 2020; Synaptic GluN2A-containing NMDA receptors: from physiology to pathological synaptic plasticity. Int J Mol Sci. 21:1538. DOI: 10.3390/ijms21041538. PMID: 32102377. PMCID: PMC7073220.

38. Li Y, Cheng X, Liu X, Wang L, Ha J, Gao Z, He X, Wu Z, Chen A, Jewell LL, Sun Y. 2022; Treatment of cerebral ischemia through NMDA receptors: metabotropic signaling and future directions. Front Pharmacol. 13:831181. DOI: 10.3389/fphar.2022.831181. PMID: 35264964. PMCID: PMC8900870.

39. Sun Y, Zhang L, Chen Y, Zhan L, Gao Z. 2015; Therapeutic targets for cerebral ischemia based on the signaling pathways of the GluN2B C terminus. Stroke. 46:2347–53. DOI: 10.1161/STROKEAHA.115.009314. PMID: 26173725.

40. Picón-Pagès P, Garcia-Buendia J, Muñoz FJ. 2019; Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta Mol Basis Dis. 1865:1949–67. DOI: 10.1016/j.bbadis.2018.11.007. PMID: 30500433.

41. Sulaiman Alsaadi M. 2019; Role of DAPK1 in neuronal cell death, survival and diseases in the nervous system. Int J Dev Neurosci. 74:11–7. DOI: 10.1016/j.ijdevneu.2019.02.003. PMID: 30763607.

42. Kim N, Chen D, Zhou XZ, Lee TH. 2019; Death-associated protein kinase 1 phosphorylation in neuronal cell death and neurodegenerative disease. Int J Mol Sci. 20:3131. DOI: 10.3390/ijms20133131. PMID: 31248062. PMCID: PMC6651373.

43. Lee JH, Rho SB, Chun T. 2005; Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol Lett. 27:1011–5. DOI: 10.1007/s10529-005-7869-x. PMID: 16132846.

44. Nair S, Hagberg H, Krishnamurthy R, Thornton C, Mallard C. 2013; Death associated protein kinases: molecular structure and brain injury. Int J Mol Sci. 14:13858–72. DOI: 10.3390/ijms140713858. PMID: 23880846. PMCID: PMC3742222.

45. Ludhiadch A, Sharma R, Muriki A, Munshi A. 2022; Role of calcium homeostasis in ischemic stroke: a review. CNS Neurol Disord Drug Targets. 21:52–61. DOI: 10.2174/1871527320666210212141232. PMID: 33583386.

46. Cross JL, Meloni BP, Bakker AJ, Lee S, Knuckey NW. 2010; Modes of neuronal calcium entry and homeostasis following cerebral ischemia. Stroke Res Treat. 2010:316862. DOI: 10.4061/2010/316862. PMID: 21052549. PMCID: PMC2968719.

47. Liu J, Liu MC, Wang KK. 2008; Calpain in the CNS: from synaptic function to neurotoxicity. Sci Signal. 1:re1. DOI: 10.1126/stke.114re1.

48. Bevers MB, Neumar RW. 2008; Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab. 28:655–73. DOI: 10.1038/sj.jcbfm.9600595. PMID: 18073773.

49. Cheng SY, Wang SC, Lei M, Wang Z, Xiong K. 2018; Regulatory role of calpain in neuronal death. Neural Regen Res. 13:556–62. DOI: 10.4103/1673-5374.228762. PMID: 29623944. PMCID: PMC5900522.

50. Yamakawa H, Banno Y, Nakashima S, Yoshimura S, Sawada M, Nishimura Y, Nozawa Y, Sakai N. 2001; Crucial role of calpain in hypoxic PC12 cell death: calpain, but not caspases, mediates degradation of cytoskeletal proteins and protein kinase C-alpha and -delta. Neurol Res. 23:522–30. DOI: 10.1179/016164101101198776. PMID: 11474809.
51. Bano D, Nicotera P. 2007; Ca2+ signals and neuronal death in brain ischemia. Stroke. 38(2 Suppl):674–6. DOI: 10.1161/01.STR.0000256294.46009.29. PMID: 17261713.
52. Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. 2007; Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 53:399–412. DOI: 10.1016/j.neuron.2006.12.020. PMID: 17270736.

53. Reggiori F, Klionsky DJ. 2002; Autophagy in the eukaryotic cell. Eukaryot Cell. 1:11–21. DOI: 10.1128/EC.01.1.11-21.2002. PMID: 12455967. PMCID: PMC118053.

54. Mehrpour M, Esclatine A, Beau I, Codogno P. 2010; Overview of macroautophagy regulation in mammalian cells. Cell Res. 20:748–62. DOI: 10.1038/cr.2010.82. PMID: 20548331.

55. Yim WW, Mizushima N. 2020; Lysosome biology in autophagy. Cell Discov. 6:6. DOI: 10.1038/s41421-020-0141-7. PMID: 32047650. PMCID: PMC7010707.

56. Dunlop EA, Tee AR. 2014; mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol. 36:121–9. DOI: 10.1016/j.semcdb.2014.08.006. PMID: 25158238.

57. Wong PM, Puente C, Ganley IG, Jiang X. 2013; The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 9:124–37. DOI: 10.4161/auto.23323. PMID: 23295650. PMCID: PMC3552878.
58. McKnight NC, Zhenyu Y. 2013; Beclin 1, an essential component and master regulator of PI3K-III in health and disease. Curr Pathobiol Rep. 1:231–8. DOI: 10.1007/s40139-013-0028-5. PMID: 24729948. PMCID: PMC3979578.

59. Tanida I, Ueno T, Kominami E. 2004; LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 36:2503–18. DOI: 10.1016/j.biocel.2004.05.009. PMID: 15325588. PMCID: PMC7129593.

60. Shibutani ST, Yoshimori T. 2014; A current perspective of autophagosome biogenesis. Cell Res. 24:58–68. DOI: 10.1038/cr.2013.159. PMID: 24296784. PMCID: PMC3879706.

61. Settembre C, Fraldi A, Medina DL, Ballabio A. 2013; Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 14:283–96. DOI: 10.1038/nrm3565. PMID: 23609508. PMCID: PMC4387238.

62. Peng L, Hu G, Yao Q, Wu J, He Z, Law BY, Hu G, Zhou X, Du J, Wu A, Yu L. 2022; Microglia autophagy in ischemic stroke: a double-edged sword. Front Immunol. 13:1013311. DOI: 10.3389/fimmu.2022.1013311. PMID: 36466850. PMCID: PMC9708732.

63. Rami A, Langhagen A, Steiger S. 2008; Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 29:132–41. DOI: 10.1016/j.nbd.2007.08.005. PMID: 17936001.

64. Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. 2008; Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 4:762–9. DOI: 10.4161/auto.6412. PMID: 18567942.

65. Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT. 2011; Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2:e144. DOI: 10.1038/cddis.2011.29. PMID: 21490676. PMCID: PMC3122060.
66. Liu Y, Che X, Zhang H, Fu X, Yao Y, Luo J, Yang Y, Cai R, Yu X, Yang J, Zhou MS. 2021; CAPN1 (calpain1)-mediated impairment of autophagic flux contributes to cerebral ischemia-induced neuronal damage. Stroke. 52:1809–21. DOI: 10.1161/STROKEAHA.120.032749. PMID: 33874744.

67. Kim J, Yang G, Kim Y, Kim J, Ha J. 2016; AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 48:e224. DOI: 10.1038/emm.2016.16. PMID: 27034026. PMCID: PMC4855276.

68. Mayer MP, Bukau B. 2005; Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 62:670–84. DOI: 10.1007/s00018-004-4464-6. PMID: 15770419. PMCID: PMC2773841.

69. Sharma D, Masison DC. 2009; Hsp70 structure, function, regulation and influence on yeast prions. Protein Pept Lett. 16:571–81. DOI: 10.2174/092986609788490230. PMID: 19519514. PMCID: PMC2746719.

70. Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. 2019; The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 20:665–80. DOI: 10.1038/s41580-019-0133-3. PMID: 31253954.

71. Balogi Z, Multhoff G, Jensen TK, Lloyd-Evans E, Yamashima T, Jäättelä M, Harwood JL, Vígh L. 2019; Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog Lipid Res. 74:18–30. DOI: 10.1016/j.plipres.2019.01.004. PMID: 30710597.

72. Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. 2001; Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 32:2905–12. DOI: 10.1161/hs1201.099604. PMID: 11739994.

73. Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. 2016; 70-kDa heat shock protein downregulates dynamin in experimental stroke: a new therapeutic target? Stroke. 47:2103–11. DOI: 10.1161/STROKEAHA.116.012763. PMID: 27387989. PMCID: PMC4961549.

74. Zhou XY, Luo Y, Zhu YM, Liu ZH, Kent TA, Rong JG, Li W, Qiao SG, Li M, Ni Y, Ishidoh K, Zhang HL. 2017; Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death Dis. 8:e2618. DOI: 10.1038/cddis.2017.34. PMID: 28206988. PMCID: PMC5386481.

75. Villalpando Rodriguez GE, Torriglia A. 2013; Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta. 1833:2244–53. DOI: 10.1016/j.bbamcr.2013.05.019. PMID: 23747342.

76. Qin AP, Zhang HL, Qin ZH. 2008; Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci Bull. 24:117–23. DOI: 10.1007/s12264-008-0117-3. PMID: 18369392. PMCID: PMC5552511.

77. Terasaki Y, Liu Y, Hayakawa K, Pham LD, Lo EH, Ji X, Arai K. 2014; Mechanisms of neurovascular dysfunction in acute ischemic brain. Curr Med Chem. 21:2035–42. DOI: 10.2174/0929867321666131228223400. PMID: 24372202. PMCID: PMC4066327.

78. Lipton P. 2013; Lysosomal membrane permeabilization as a key player in brain ischemic cell death: a "lysosomocentric" hypothesis for ischemic brain damage. Transl Stroke Res. 4:672–84. DOI: 10.1007/s12975-013-0301-2. PMID: 24323421.

79. Li J, McCullough LD. 2010; Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 30:480–92. DOI: 10.1038/jcbfm.2009.255. PMID: 20010958. PMCID: PMC2852687.

80. Chen H, Kim GS, Okami N, Narasimhan P, Chan PH. 2011; NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol Dis. 42:341–8. DOI: 10.1016/j.nbd.2011.01.027. PMID: 21303700. PMCID: PMC3079796.

81. Yamashima T, Mathivanan A, Dazortsava MY, Sakai S, Kurimoto S, Zhu H, Funaki N, Liang H, Hullin-Matsuda F, Kobayashi T, Akatsu H, Takahashi H, Minabe Y. 2014; Calpain-mediated Hsp70.1 cleavage in monkey CA1 after ischemia induces similar - lysosomal vesiculosis' to Alzheimer neurons. J Alzheimers Dis Parkinsonism. 4:139. DOI: 10.4172/2161-0460.1000139.
82. Yamashima T. 2012; Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem. 120:477–94. DOI: 10.1111/j.1471-4159.2011.07596.x. PMID: 22118687.

83. Koriyama Y, Furukawa A. 2016; HSP70 cleavage-induced photoreceptor cell death caused by N-methyl-N-nitrosourea. Neural Regen Res. 11:1758–9. DOI: 10.4103/1673-5374.194721. PMID: 28123413. PMCID: PMC5204225.

84. Wei R, Wang J, Xu Y, Yin B, He F, Du Y, Peng G, Luo B. 2015; Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience. 301:168–77. DOI: 10.1016/j.neuroscience.2015.05.070. PMID: 26047730.

85. Tontchev AB, Yamashima T. 1999; Ischemic delayed neuronal death: role of the cysteine proteases calpain and cathepsins. Neuropathology. 19:356–65. DOI: 10.1046/j.1440-1789.1999.00259.x.

86. Chaitanya GV, Babu PP. 2008; Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res. 33:2178–86. DOI: 10.1007/s11064-007-9567-7. PMID: 18338260.

87. Lang-Rollin IC, Rideout HJ, Noticewala M, Stefanis L. 2003; Mechanisms of caspase-independent neuronal death: energy depletion and free radical generation. J Neurosci. 23:11015–25. DOI: 10.1523/JNEUROSCI.23-35-11015.2003. PMID: 14657158. PMCID: PMC6741034.

88. Chen J, Hu R, Liao H, Zhang Y, Lei R, Zhang Z, Zhuang Y, Wan Y, Jin P, Feng H, Wan Q. 2017; A non-ionotropic activity of NMDA receptors contributes to glycine-induced neuroprotection in cerebral ischemia-reperfusion injury. Sci Rep. 7:3575. DOI: 10.1038/s41598-017-03909-0. PMID: 28620235. PMCID: PMC5472592.

89. Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, Xu L, Duan WH, Xiong ZQ. 2008; Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 39:3042–8. DOI: 10.1161/STROKEAHA.108.521898. PMID: 18688011.

90. Kotwal A, Ramalingaiah AH, Shukla D, Radhakrishnan M, Konar SK, inivasaiah B Sr, Chakrabarti D, Sundaram M. 2022; Role of nimodipine and milrinone in delayed cerebral ischemia. World Neurosurg. 166:e285–93. DOI: 10.1016/j.wneu.2022.06.150. PMID: 35843579.

91. Liu S, Liu C, Xiong L, Xie J, Huang C, Pi R, Huang Z, Li L. 2021; Icaritin alleviates glutamate-induced neuronal damage by inactivating GluN2B-containing NMDARs through the ERK/DAPK1 pathway. Front Neurosci. 15:525615. DOI: 10.3389/fnins.2021.525615. PMID: 33692666. PMCID: PMC7937872.

92. Wang X, Fang Y, Huang Q, Xu P, Lenahan C, Lu J, Zheng J, Dong X, Shao A, Zhang J. 2021; An updated review of autophagy in ischemic stroke: from mechanisms to therapies. Exp Neurol. 340:113684. DOI: 10.1016/j.expneurol.2021.113684. PMID: 33676918.

93. Yuan J, Zhang Z, Ni J, Wu X, Yan H, Xu J, Zhao Q, Yuan H, Yang L. 2023; Acupuncture for autophagy in animal models of middle cerebral artery occlusion: a systematic review and meta-analysis protocol. PLoS One. 18:e0281956. DOI: 10.1371/journal.pone.0281956. PMID: 36812222. PMCID: PMC9946199.

94. Lu X, Zhang J, Ding Y, Wu J, Chen G. 2022; Novel therapeutic strategies for ischemic stroke: recent insights into autophagy. Oxid Med Cell Longev. 2022:3450207. DOI: 10.1155/2022/3450207. PMID: 35720192. PMCID: PMC9200548.

95. Ahsan A, Liu M, Zheng Y, Yan W, Pan L, Li Y, Ma S, Zhang X, Cao M, Wu Z, Hu W, Chen Z, Zhang X. 2021; Natural compounds modulate the autophagy with potential implication of stroke. Acta Pharm Sin B. 11:1708–20. DOI: 10.1016/j.apsb.2020.10.018. PMID: 34386317. PMCID: PMC8343111.

96. Yao Y, Ji Y, Ren J, Liu H, Khanna R, Sun L. 2021; Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury. Mol Brain. 14:123. DOI: 10.1186/s13041-021-00836-0. PMID: 34362425. PMCID: PMC8344221.

97. Wicha P, Onsa-Ard A, Chaichompoo W, Suksamrarn A, Tocharus C. 2020; Vasorelaxant and antihypertensive effects of neferine in rats: an in vitro and in vivo study. Planta Med. 86:496–504. DOI: 10.1055/a-1123-7852. PMID: 32219782.

98. Sengking J, Oka C, Wicha P, Yawoot N, Tocharus J, Chaichompoo W, Suksamrarn A, Tocharus C. 2021; Neferine protects against brain damage in permanent cerebral ischemic rat associated with autophagy suppression and AMPK/mTOR regulation. Mol Neurobiol. 58:6304–15. DOI: 10.1007/s12035-021-02554-z. PMID: 34498225.

99. Liu CW, Liao KH, Tseng H, Wu CM, Chen HY, Lai TW. 2020; Hypothermia but not NMDA receptor antagonism protects against stroke induced by distal middle cerebral arterial occlusion in mice. PLoS One. 15:e0229499. DOI: 10.1371/journal.pone.0229499. PMID: 32126102. PMCID: PMC7053748.

100. Hu WW, Du Y, Li C, Song YJ, Zhang GY. 2008; Neuroprotection of hypothermia against neuronal death in rat hippocampus through inhibiting the increased assembly of GluR6-PSD95-MLK3 signaling module induced by cerebral ischemia/reperfusion. Hippocampus. 18:386–97. DOI: 10.1002/hipo.20402. PMID: 18172894.

Fig. 1
The underlying mechanism of anoxic depolarization and glutamate excitotoxicity after acute cerebral ischemia. AMPA, glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors; Ca2+, calcium ion; Cl-, chloride ion; Glu, glutamate; Na+, sodium ion; NMDA, N-methyl-D-aspartate receptor; VDCC, voltage-dependent calcium channels.
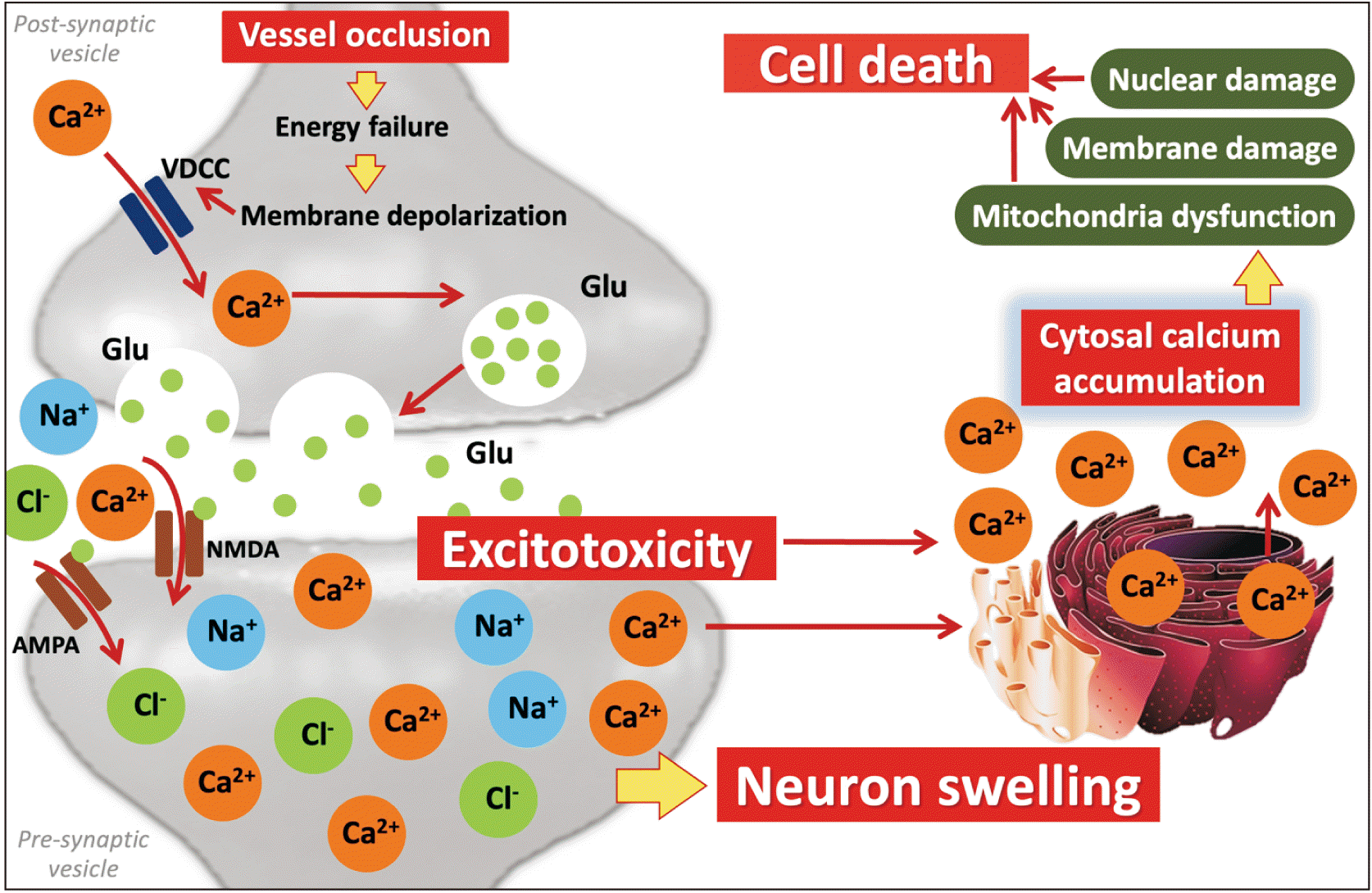
Fig. 2
The underlying mechanism of calcium toxicity induced autophagic cell death and lysosomal degradation related to neuronal cell death. 4-HNE, 4-hydroxynonenal; Akt, protein kinase B; AMPK, 5’ AMP-activated protein kinase; Atg, autophagy related protein; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; BNIP3, Bcl-2 interacting protein 3; Ca2+, calcium ion; CaMKKβ, Ca2+/calmodulin-dependent protein kinase kinase β; CaMKII, Ca2+/calmodulin-dependent protein kinase II; DAMPs, damage-associated molecular patterns; HIF-1, hypoxia-inducible factor 1; Hsp70, heat shock protein 70; LAMP-1, lysosomal associated membrane protein 1; mTOR, mammalian target of rapamycin; nNOS, nitric oxide synthase; NO, nitric oxide; ONOO-, peroxynitrite; PI3K, phosphoinositide 3-kinase; TNFR, tumor necrosis factor receptor; ULK1, UNC-51-like kinase 1; VSP34, phosphatidylinositol 3-kinase VPS34 complex; NMDA, N-methyl-D-aspartate receptor; VDCC, voltage-dependent calcium channels; LC3, light chain 3.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download