This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
This study aimed to comprehensively investigate the diverse characteristics of a novel commercial bolus, CLEANBOLUS-WHITE (CBW), to ascertain its suitability for clinical application.
Methods
The evaluation of CBW encompassed both physical and biological assessments. Physical parameters such as mass density and shore hardness were measured alongside analyses of element composition. Biological evaluations included assessments for skin irritation and cytotoxicity. Dosimetric properties were examined by calculating surface dose and beam quality using a treatment planning system (TPS). Additionally, doses were measured at maximum and reference depths, and the results were compared with those obtained using a solid water phantom. The effect of air gap on dose measurement was also investigated by comparing measured doses on the RANDO phantom, under the bolus, with doses calculated from the TPS.
Results
Biological evaluation confirmed that CBW is non-cytotoxic, nonirritant, and non-sensitizing. The bolus exhibited a mass density of 1.02 g/cm3 and 14 shore 00. Dosimetric evaluations revealed that using the 0.5 cm CBW resulted in less than a 1% difference compared to using the solid water phantom. Furthermore, beam quality calculations in the TPS indicated increased surface dose with the bolus. The air gap effect on dose measurement was deemed negligible, with a difference of approximately 1% between calculated and measured doses, aligning with measurement uncertainty.
Conclusion
CBW demonstrates outstanding properties for clinical utilization. The dosimetric evaluation underscores a strong agreement between calculated and measured doses, validating its reliability in both planning and clinical settings.
Keywords: Commercial bolus, Surface dose, Air gap
Introduction
One of the key advantages of external beam radiation therapy lies in its ability to spare the skin, attributed to the skin-sparing effect of megavoltage photon beams that deliver a maximum dose a few centimeters beyond the body’s surface. The region between the surface and the depth of maximum dose (d
max) is known as the build-up region [
1,
2], whose extent varies based on beam quality. However, the photon beam may not deliver sufficient doses for shallow lesions owing to inadequate build-up distance for effective ionization. This challenge can be addressed by applying additional material with water-like physical properties, such as a bolus, directly onto the lesion.
The widespread use of commercial bolus sheets in clinical practice requires thorough confirmation of their physical and biological suitability for application on patients’ skin before use. Despite the close resemblance of bolus material to water in physical properties, the inevitable production of an air gap between the bolus and the skin, especially on curved surfaces, poses a challenge. This air gap can be generated on the beam path, affecting the delivered dose to superficial lesions. Previous studies [
3-
5] have highlighted that smaller irradiated areas and more oblique incident angles result in a reduction of delivered dose, with up to a 10% decrease in surface dose measured for a 1 cm air gap at a 60-degree incident angle in small fields using a 6 MV photon beam [
3]. In order to reduce the air gap on the curved surface, custom bolus was introduced [
6-
9]. The custom bolus production, however, took costs, manufacturing time, and resources including human power. For the economic reason and ease of quick usage, the commercial sheet bolus is popular in use and still very demanding in clinic. Therefore, a commercial bolus with reasonably small air gap even on the curved body surface would be a perfect solution for the clinic.
In this study, we comprehensively investigated the physical, biological, and dosimetric attributes of a newly developed commercial bolus known as CLEANBOLUS-WHITE (CBW; Paprica Lab.). While the manufacturer supplied information regarding the physical and biological properties of the bolus material, our focus was on thoroughly examining its dosimetric characteristics. This involved intensive analysis within the treatment planning system (TPS) and experimentation utilizing a 6 MV photon beam.
Materials and Methods
1. Physical and biological evaluation
The CBW is composed of silicone rubber and exhibits an opaque white coloration. Certification of the bolus’s physical properties was conducted by accredited testing facilities (Huizhou Hongyejie Technology Co., Ltd. and Koptri). The effective atomic number (Zeff) of the bolus material was determined through element analysis conducted at Seoul National University’s national center for inter-university facilities. During the element analysis process, organic elements were assessed through oxygen combustion, while inorganic elements were analyzed using X-ray fluorescence (S8 TIGER; Bruker Co.).
A series of sequential biological stability tests were performed to assess cytotoxicity, skin sensitization, and skin irritation, ensuring the safe application of the product on patients. The cytotoxicity test involved the use of L-929 mouse fibroblast cells. It was conducted using the elution method in accordance with the International Organization for Standardization (ISO) 10993-5:2009 “Tests for In vitro cytotoxicity” and ISO 10993-12:2021 (E) guidelines. Skin sensitization and irritation tests were performed on Guinea pig and New Zealand rabbit skins, respectively, following the protocols outlined in ISO regulations 10993-10:2021 (E) “Test for Skin Sensitization,” ISO 10993-23:2021 (E) “Test for Irritation,” and ISO 10993-12:2021 (E) “Sample Preparation and Reference Materials.” The tests were conducted at Bioneeds India Pvt. Ltd., Karnataka, India, which holds accreditations for good laboratory practice and is also certified by the Association for Assessment and Accreditation of Laboratory Animal Care.
2. Dosimetric evaluation
In accordance with task group report 51 (TG-51) guidelines [
10,
11], percent depth dose at 10 cm (PDD(10)) was assessed using a Farmer Chamber (PTW) for 6, 10, and 15 MV photon beams from the TrueBeam
®system (Varian Medical Systems Inc.). The linac was maintained to deliver 1 cGy/MU at d
max under a field size of 10×10 cm
2 and a source-to-surface distance (SSD) of 100 cm. Chamber parameters derived from a water phantom based on TG-51 standards were employed to correct the measured dose, utilizing an alternative solid water phantom (SWP; Standard Imaging Inc.). PDD(10) measurements were conducted at depths of 1.7, 2.5, 2.9, and 10.2 cm, corresponding to d
max and the reference depth (d
ref) for 6, 10, and 15 MV photon beams, where the depths were relevant to the effective point of measurement of the chamber. CBW with a thickness of 0.5 cm was employed alongside the SWPs to achieve the required depths. Results were compared between including the 0.5 cm CBW in conjunction with the SWPs and using SWPs only for the same depth. Dose measurements were carried out using metal oxide silicon field effect transistor (MOSFET) detectors (Best Medical Canada) with high bias settings.
The computed tomography (CT) number of the CBW was established by comparing the calculated dose in the TPS (EclipseTM v16.1; Varian Medical Systems Inc.) to the measured dose under a 1.5-cm-thick CBW using a 6 MV photon beam. Utilizing the determined CT number, the beam qualities of 6, 10, and 15 MV photon beams were computed with and without the 0.5 cm CBW positioned atop a virtual water surface (30×30×30 cm3). The calculation was performed by the Acuros® XB algorithm (v16.1; Varian Medical Systems Inc.) in the TPS.
The impact of the air gap was assessed in an experiment using a female RANDO phantom (Sun Nuclear) as follows. Treatment plans were devised to deliver a 100 cGy dose of 6 MV static photon beam to specific phantom regions, including the nose, chin, breast, and inguinal area, at an SSD of 100 cm with a field size of 10×10 cm
2. Subsequently, the CBW with a thickness of 0.5 cm was applied to the surface. Two measurement points per treatment area were designated using MOSFET detectors on the RANDO phantom, as depicted in
Fig. 1. The measured doses were then compared with the calculated doses, assuming an ideal CBW sheet bolus without any air gap in the plan (
Fig. 2). This comparison facilitated the inference of the air gap effect based on the difference between the measured and calculated doses.
Results
1. Physical and biological evaluations
The physical properties of CBW were juxtaposed with those of conventional sheet boluses, such as Super-Flex bolus (Radiation Products Design Inc.) and CLEANBOLUS (Paprica Lab.), as outlined in
Table 1.
The results of the element analysis are presented in
Table 2. Based on the weight composition ratios of the elements, the Z
eff of CBW was calculated to be 10.76, indicating a slight increase compared to water (7.42).
Biological testing confirmed the suitability of CBW for clinical application on patients. In experiments conducted with L-929 mouse fibroblast cell lines treated with CBW extract, changes in morphology were observed in no more than 20% of cells, with only slight growth inhibition and minimal reactivity of grade 1 noted (grade 0 indicating negative control cells, and grade 4 indicating destruction of cell layers). Skin irritation tests conducted on New Zealand white rabbits revealed no signs of erythema or edema for up to 72 hours after the removal of test patches. Similarly, in the skin sensitization test, no visible changes were observed within 48 hours following the injection of CBW extract and the removal of test patches. Based on these results, CBW was deemed non-cytotoxic, non-irritating, and non-sensitizing.
2. Dosimetric evaluations
The PDD(10) values for the 6, 10, and 15 MV photon beams are summarized in
Table 3, whether utilizing the SWP with the inclusion of the 0.5 cm CBW thickness or using the SWP alone. Discrepancies in measured doses from the absolute dose measurement in water primarily stemmed from using the SWP.
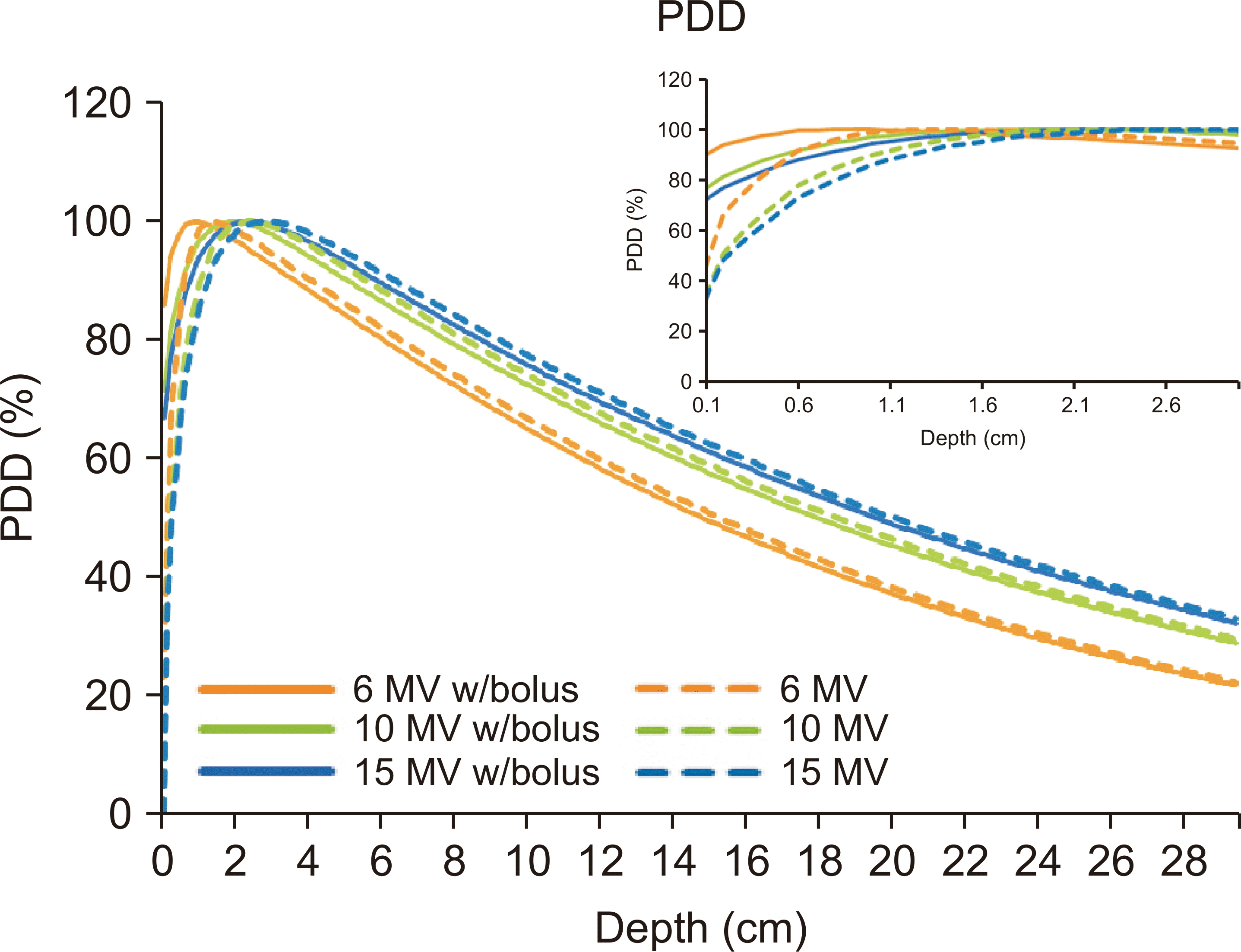
The CT number assigned to the CBW was 0 HU with a 1% uncertainty. For a field size of 10×10 cm
2 and an SSD of 100 cm, the beam quality is illustrated in
Fig. 3, where the dashed lines represent PDDs calculated for 6, 10, and 15 MV photon beams. The solid lines depict PDDs after incorporating a 0.5 cm CBW on the surface of the virtual water phantom. PDDs with the 0.5 cm bolus exhibited approximately twice the surface dose compared to normal PDDs.
A dose of 100 cGy of a static 6 MV photon beam was delivered to the nose, chin, breast, and inguinal area of the RANDO phantom, and the resulting measured doses are listed in
Table 4. The measured doses on the surface of the RANDO phantom (
Fig. 1) under the 0.5 cm CBW closely matched the calculated doses on the CT-scanned RANDO phantom with ideal boluses in the TPS (
Fig. 2), with an agreement within 1%. Each measurement was repeated 3‒5 times per point to ensure accuracy, and the standard deviation was considered to represent the measurement uncertainty.
Discussion
Various aspects of the CBW were evaluated in this study. Biological assessments confirmed its suitability for clinical use with patients. Physically, CBW was identified by its opaque white color and was observed to be less adhesive than other commercial boluses [
12]. The opacity of CBW posed no issues when applied to the patient’s surface after the patient setup was finished, and its reduced stickiness facilitated easier handling for therapists. This characteristic is particularly advantageous for patients with open wounds, as detailed in the subsequent discussion, wherein it is noted that this property does not impact the delivered dose to the patient.
The dosimetric properties of CBW were assessed using a 0.5-cm-thick bolus. Beam quality analysis of the megavoltage photon beam conducted in the TPS demonstrated an increase in surface dose upon the addition of CBW. PDD(10) measurements indicated that the inclusion of CBW did not yield a significant difference compared to using the SWP alone.
The CT number was empirically determined to be 0 HU, while it measured 138.5±8.6 HU in scans utilizing Brilliant CT Big BoreTM (Royal Philips Electronics). Given that the bolus is designed to facilitate adequate dose build-up to superficial lesions, the assessment of the CT number through dosimetric experiments is logical. Additionally, we assigned a CT number of 0 HU to CBW for practical usage in the TPS. This adjustment resulted in a calculated dose in the TPS that was closer to the experimental result by 0.2% compared to using the CT number obtained from the CT scan.
The evaluation of the air gap’s effect involved comparing the measured dose from the experiment with the calculated dose using an ideal CBW in the TPS. This comparison was conducted at two points per virtual lesion located on the nose, chin, breast, and inguinal area of the RANDO phantom. The dosimetrically determined CT number of CBW was aligned with the Hounsfield unit of the ideal bolus in the TPS. The uncertainty of dose measurements using MOSFET detectors was approximately 2 cGy, with the observed dose difference between measured and calculated doses hovering around 1%. This indicates our ability to replicate the planned dose during treatment, even on the curved surface of the RANDO phantom, by employing the CBW.
Conclusions
This study comprehensively assessed the physical, biological, and dosimetric characteristics of the recently developed custom bolus, CBW. Our findings support the suitability of this bolus for clinical application, rendering it a viable alternative to existing commercial options.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download