Abstract
Background
Coronavirus disease (COVID-19) induces inflammation, coagulopathy following platelet and monocyte activation, and fibrinolysis, resulting in elevated D-dimer levels. Activated platelets and monocytes produce microvesicles (MVs). We analyzed the differences in platelet and monocyte MV counts in mild, moderate, and severe COVID-19, as well as their correlation with D-dimer levels.
Methods
In this cross-sectional study, blood specimens were collected from 90 COVID-19 patients and analyzed for D-dimers using SYSMEX CS-2500. Platelet MVs (PMVs; PMVCD42b+ and PMVCD41a+), monocyte MVs (MMVs; MMVCD14+), and phosphatidylserine-binding annexin V (PS, AnnV+) were analyzed using a BD FACSCalibur instrument.
Results
PMV and MMV counts were significantly increased in COVID-19 patients. AnnV+ PMVCD42b+ and AnnV+ PMVCD41a+ cell counts were higher in patients with severe COVID-19 than in those with moderate clinical symptoms. The median (range) of AnnV+ PMVCD42b+ (MV/µL) in mild, moderate, and severe COVID-19 was 1,118.3 (328.1–1,910.5), 937.4 (311.4–2,909.5), and 1,298.8 (458.2–9,703.5), respectively (P=0.009). The median (range) for AnnV+ PMVCD41a+ (MV/µL) in mild, moderate, and severe disease was 885.5 (346.3–1,682.7), 663.5 (233.8–2,081.5), and 1,146.3 (333.3–10,296.6), respectively (P=0.007). D-dimer levels (ng/mL) weak correlated with AnnV+ PMVCD41a+ (P=0.047, r=0.258).
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which may affect the platelet components of patients [1]. When activated, platelets and other cells release microvesicles (MVs), which are known as microparticles or ectosomes and comprise a group of extracellular vesicles (EVs) that originate from the cell membrane after activation under physiological or pathological conditions [2]. All cell types release MVs that are non-nucleated and carry the same surface antigens as their cells of origin; therefore, MVs can be distinguished by their surface antigens [3]. MVs are characterized by surface expression of phosphatidylserine (PS) because upon cell activation, PS is externalized on the outer surface of the cell membrane [4].
The downregulation of angiotensin-converting enzyme (ACE)-2 receptors on the surface of the endothelium of blood vessels accelerates COVID-19 progression from mild to severe [5]. PS is a receptor for coagulation that enhances thrombus formation [6]. As COVID-19 is related to underlying clinical symptoms, disease severity is associated with an increase in circulating MVs [7, 8], which promote pro-adhesive and prothrombotic abilities. Under pathological conditions, MVs express procoagulant phospholipids, which induce inflammation and activate coagulation and thrombosis. Platelet MVs (PMVs) have an eight times higher density and 50–100 times higher procoagulant activity than activated platelets [9]. Monocyte MVs (MMVs) contribute to fibrin-rich thrombus formation [4]. Because of their clinical relevance as disease markers and potential role in mediating COVID-19 severity, MVs have received increasing interest [10].
COVID-19 patients exhibit coagulopathy with fibrin deposition and thrombus formation [11]. COVID-19 affects the hemostatic system via increased levels of inflammatory cytokines and excess thrombin production [12]. Thrombosis is a key factor in COVID-19 that is associated with increased MV counts and can trigger a coagulation cascade [2, 13]. Patients with both cancer and COVID-19 exhibit significant MV formation compared to COVID-19 patients without cancer [14].
D-dimer levels are routinely screened in patients with COVID-19 [15] and reflect the success of fibrinolysis, which is preceded by thrombosis. D-dimer is associated with the activation of blood cells, which subsequently release MVs. CD42b-expressing PMVs (PMVCD42b+) can bind to platelet receptors (glycoprotein [GP] IIb/IIIa), inducing binding to the fibrinogen receptor [9, 7]. Similarly, CD41a-expressing PMVs (PMVCD41a+) can directly bind to fibrinogen receptors to increase platelet aggregation [11]. CD14-expressing MMVs (MMVCD14+) activate the extrinsic coagulation pathway [8].
We investigated PMV (PMVCD42b+ and PMVCD41a+) and MMV (MMVCD14+) counts in COVID-19 patients with varying disease severities, as evinced by clinical symptoms, and assessed whether D-dimer levels correlated with PMV and MMV counts.
This was an analytical, observational study with a cross-sectional design. We recruited patients with COVID-19 presenting at Dr. Soetomo General Academic Teaching Hospital, Surabaya, East Java, Indonesia between September 2020 and December 2021. SARS-CoV-2 infection was confirmed using a nucleic acid amplification test. Ninety hospitalized COVID-19 patients (including 30 with mild, 30 with moderate, and 30 with severe disease) and nine healthy controls (three men and six women), with ages ranging from 19 to 22 yrs, were included in the study.
Clinical degrees were classified based on the Decree of the Minister of Health of the Republic of Indonesia regarding the clinical management of COVID-19 in healthcare facilities, which refers to the WHO recommendations. All patients were diagnosed according to the criteria for classifying mild, moderate, and severe clinical symptoms based on their clinical symptoms and oxygen saturation (SpO2) levels, as follows: mild, patients with symptoms without evidence of viral pneumonia or hypoxia and oxygenation status and SpO2 >95% in room air; moderate, patients with clinical signs of pneumonia but without signs of severe pneumonia, and SpO2 >93% in room air; and severe, patients with clinical symptoms of pneumonia and severe respiratory distress, and SpO2 <93% in room air [16]. The control group consisted of healthy individuals without clinical complaints or symptoms of COVID-19, negative test results for SARS-CoV-2, and no history of comorbidities. The healthy controls were recruited from volunteers at Universitas Nahdlatul Ulama (Surabaya, Indonesia). Blood specimens were collected from the patients and healthy controls after obtaining written informed consent. Ethical approval was obtained from the Ethical Committees in Health Research of Dr. Soetomo General Hospital Surabaya (approval No.: 0030/KEPK/VII/2020) and Universitas Nahdaltul Ulama Surabaya (approval No.: 277/EC//KEPK/UNUSA/2021).
Blood specimens were collected via venipuncture in 3 mL of 0.109 M sodium citrate. D-dimer levels were measured using an INNOVANCE D-dimer assay (OPBPG03C11; Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) in a fully automated coagulation analyzer (CS-2500; Sysmex, Kobe, Japan). According to the institutional protocol, D-dimers were not analyzed in patients with mild clinical symptoms. All specimens were processed by double centrifugation at 2,500×g, 25°C for 15 mins. The platelet-free plasma (PFP) specimens were stored at −80°C until use.
Clinical data, including SpO2, platelet count, monocyte count, prothrombin time (PT), and activated partial thromboplastin time (APTT), of COVID-19 patients with severe and moderate clinical symptoms, but not from routine examination in patients with mild clinical symptoms, were obtained from medical records and the laboratory information system.
The PFP specimens were thawed at 25°C. Approximately 250 µL of PFP supernatant was centrifuged at 18,000×g, 25°C for 30 mins. The supernatant was separated and the pellet was resuspended in 1,000 µL of phosphate-buffered saline (PBS). The supernatant was centrifuged at 18,000×g, 25°C for 30 mins and vortexed for 5 sec. The supernatant was removed and the pellet was resuspended in ±250 µL PBS and vortexed for 5 sec [17, 18].
Up to 50 µL of each specimen was added to 50 µL of Annexin V Binding Buffer (51-66121E; BD Biosciences, Franklin Lakes, NJ, USA; 0.1 M 4-(2-hydroxyethyl)piperazine-1-ethane-sulfonic acid (HEPES)/NaOH [pH 7.4], 1.4 M NaCl, 25 mM CaCl2), 4 µL of phycoerythrin (PE)-conjugated annexin V (51-65875X; BD Biosciences), 15 µL of fluorescein isothiocyanate (FITC)-conjugated CD42b (cat. No. 555472; Becton Dickinson Biosciences, USA), 15 µL of peridinin chlorophyll protein-cyanine5.5 (PerCP-Cy5.5)-conjugated CD41a (HIP8) (340931; BD Biosciences), and 15 µL of allophycocyanin (APC)-conjugated CD14 (555399; BD Biosciences) in a Trucount bead tube (BD Biosciences), vortexed for 5 sec, and incubated at 25°C in the dark for 30 mins to maximize the binding of the fluorochrome antibodies to the MV surface. After incubation, 500 µL of Annexin V Binding Buffer (51-66121E; BD Biosciences; 0.1 M HEPES/NaOH [pH 7.4], 1.4 M NaCl, 25 mM CaCl2) was added. Negative gating was achieved by testing specimens from healthy controls without staining with fluorescence-labeled antibodies (unstained). This strategy was used to determine the quadrant lines, position of the quadrant region, or quadrant boundary of the MVs. The specimens were analyzed using a fluorescence-activated cell sorter (FACS) (FACSCalibur; BD Biosciences) and the data were analyzed using CellQuest Pro software (BD Biosciences).
The absolute MV count was determined using the FACSCalibur flow cytometer. Forward scatter (FSC), side scatter (SSC), and fluorescence data were obtained by setting a logarithmic scale to read smaller sizes. The instrument thresholds for MV gating were optimized using the Megamix-Plus SSC (7803; Biocytex, Marseille, France), which is a mixture of fluorescent beads with varying diameters (0.16, 0.20, 0.24, and 0.5 µm) selected to represent the MV size range (0.1–1 µm), and fluorescence (FL1)/FITC was used as the threshold.
Trucount beads facilitate the calculation of the absolute MV count using the following formula:
Circulating MVs were quantified based on FSC or SSC, MV size characteristics, and annexin V binding. MVs expressing PS on their surface were detected based on annexin V fluorescence. Fluorescence-labeled antibodies were selected to define MVs derived from their cells of origin; platelet GPIbα (CD42b), platelet GPIIb/IIIa (CD41a), and monocytes (CD14). PMVs and MMVs are expressed as PS (AnnV+, PMVCD42b+) and (AnnV+, PMVCD41a+) and PS (AnnV+, MMVCD14+), respectively. Quadrant lines were used to distinguish positive and negative events.
Statistical analyses were conducted using SPSS v.21 (IBM Corp., Armonk, NY, USA). Normality was assessed using the Shapiro–Wilk test. Normally distributed data are presented as mean±SD and non-normally distributed (non-parametric) as median and range. ANOVA test was used for normally distributed data with mean differences in more than two groups. The Kruskal–Wallis test was used to compare numerical variables in more than two groups when the data were non-normally distributed. An independent t-test and the Mann–Whitney U test were used for two groups when the data were normally distributed and for non-normally distributed categorical data, respectively. The chi-square test was used for qualitative data. Spearman’s correlation test was used for non-normally distributed data; correlations between MV counts and certain clinical data of COVID-19 patients were assessed based on Spearman’s correlation coefficients. Differences were considered significant at P<0.05.
As expected, the average age of COVID-19 patients with moderate to severe clinical symptoms was >45 yrs, whereas that patients who had mild clinical symptoms was <45 yrs (P<0.001; Table 1). SpO2 levels decreased with the severity of clinical symptoms (P=0.001; Table 1). Patients with severe clinical symptoms had significantly higher D-dimer levels than those with moderate clinical symptoms (P=0.028; Table 1).
MVs were gated based on FSC and SSC, and the MV location was <1.0 µm (Fig. 1). The selected threshold was FL1/FITC, and a negative gating strategy was used (Fig. 1E). PMVs (PMVCD42b+ and PMVCD41a+) and MMVs (MMVCD14+) were identified using annexin V conjugated with PE (Fig. 1F–1I). Specimens containing MVs stained annexin V-positive (Fig. 1G–1I).
AnnV+ PMVCD42b+, AnnV+ PMVCD41a+, and AnnV+ MMVCD14+ cell counts increased with the severity of clinical symptoms (Fig. 1G–1I; Table 2). AnnV+ PMVCD42b+ cell counts were higher in COVID-19 patients with severe clinical symptoms than in those with mild clinical symptoms (P=0.003) or moderate clinical symptoms (P=0.028). AnnV+ PMVCD41a+ cell counts were higher in COVID-19 patients with severe clinical symptoms than in those with moderate clinical symptoms (P=0.002; Table 3).
Spearman correlation analysis of AnnV+ PMVCD42b+, AnnV+ PMVCD41a+, and AnnV+ MMVCD14+ counts in 60 specimens of COVID-19 patients with clinical symptoms and D-dimer levels revealed that only Ann V+ PMVCD41a+ counts were weakly correlated with D-dimer levels (P<0.05 and P=0.047, r=0.258). AnnV+ PMVCD42b+ (P=0.157, r=0.185) and AnnV+ MMVCD14+ (P= 0.218, r=0.162) counts showed no correlation with D-dimer levels. PMV and MMV counts were not correlated with age (Table 4). The age, sex, condition, and outcome of patients stratified by clinical symptoms (Table 1) were not correlated with the biomarkers. These results confirmed that any differences in the biomarkers were real differences and were not caused by the effects of age, sex, condition, or outcome (Table 4).
In this study, the mean age was lower in the mild COVID-19 group than in the other groups, and the MV count was higher in the mild disease group than in the moderate disease group. Therefore, age may have caused bias in the MV counts because in theory, the number of senescent cells in the body increases with age. However, PMV and MMV counts were not correlated with age. This may explain some of the differences in disease pathophysiology observed among different age groups, although the exact reason remains unclear. Enjeti, et al. [19] reported that median MV counts were significantly negatively correlated with age and were generally similar between women and men. The results of this study are in line with previous findings that MV counts do not correlate with age and sex (Table 4).
Platelet and monocyte counts were within the normal ranges (Table 1), whereas MV counts were increased. Although we did not directly examine parameters of cell activation, this confirmed the increased activation of both cell types and consequent MV release in COVID-19. PMV and MMV counts were increased among COVID-19 patients with mild, moderate, and severe clinical symptoms. We assume that this was due to the invasive effect of SARS-CoV-2 on host cells, which immediately triggers innate immune system modulation. Patients with mild clinical symptoms released large numbers of PMVs and MMVs.
This is consistent with results reported by Rausch, et al. [20], who assessed hyperactive platelets in patients with COVID-19 and found an increase in the amount of circulating PMVs. Abdelmaksoud, et al. [21] reported that the ability of PMVs to promote inflammatory cytokine production can exacerbate the inflammatory response. In COVID-19, PMV production is increased because of secondary inflammatory conditions when the virus is active. Therefore, hematopoietic and endothelial cells are activated to eliminate the incoming viruses.
Non-hyperactive immune cells release MVs in advanced stages, such as in moderate clinical symptoms. With worsening clinical condition in severe COVID-19, MV release increases because of systemic multiorgan failure, further cell activation, and increased MV counts, followed by MV-mediated thrombus formation and coagulation.
PMVCD42b+ express surface-adhesion receptors for von Willebrand factor (vWF) and mediate platelet adhesion to the sub-endothelium damaged by SARS-CoV-2 invasion [22]. Patients with severe, moderate, or mild COVID-19 have different degrees of inflammation, possibly from platelet activation via the P-selectin-mediated GPIbα receptor that binds monocytes to vWF in the endothelium; therefore, PMVCD42b+ contributes to plaque formation by inducing direct interaction between inflammatory monocytes and the endothelium [23, 24].
Bongiovanni, et al. [24] reported increased PMVCD42b+ abundance in annexin V-negative (AnnV–) individuals, in contrast to our findings (PMVCD42b+ abundance in AnnV+ individuals). While not all MVs express PS on their surface, PMVCD42b+ abundantly express PS; therefore, they originate directly from the plasma membrane surface of activated platelets [25]. PMVCD41a+ counts were higher in patients with severe COVID-19 than in those with moderate disease. PMVCD41a+ forms a complex with thrombospondin, fibronectin, vitronectin, fibrinogen, and vWF to aid coagulation [26]. The presence of PMVCD41a+ reflects cell activation because of endothelial dysfunction [27]. PMVCD41a+ mediates cell–cell communication and thereby increases coagulation in COVID-19 patients [28]. PMVCD41a+ binds to the endothelium, blood vessel wall submatrix, and leukocytes, thereby contributing to thrombus formation.
CD14 is highly expressed on the surface of monocytes and MMVCD14+ are released after monocyte activation in response to COVID-19 inflammation. MMV counts increase under pathological conditions, indicating continued monocyte activation, which further promotes MMV release. The interaction between endothelial cells and MMVs indicates chronic inflammation. Therefore, MMVs potentially mediate pathological mechanisms [27]. Using flow cytometry, Tripsciano, et al. [29] found a higher PS density on platelets than on monocytes, which supports our finding of AnnV+ MMVCD14+ MMVs, but without counts significantly differing by COVID-19 severity. We assume that this was because MMVs did not dominate in the circulation when compared with platelets, although they were activated during inflammation (Table 2).
Our results emphasize the role of MVs in COVID-19 patients and their potential as a biomarker. Zahran, et al. [14] reported significant MV formation in cancer patients with COVID-19 when compared with that in COVID-19 patients not affected by cancer. Platelet-derived EVs have shown good diagnostic performance in SARS-CoV-2-positive patients compared with SARS-CoV-2-negative subjects [3].
MVs expressing PS can induce thrombin formation and provide sites for coagulation factors such as FVa, FVIIIa, and FIXa to bind to tenases (FIXa and FVIIIa) and prothrombinase (FXa and FVa), thereby amplifying the coagulation pathway and triggering direct fibrin formation through MV–fibrinogen interactions [30]. PMVs and MMVs reflect cell activation during thrombotic events [29].
Annexin V was used in this study as a marker for the quantification of PMVs (PMVCD42b+, PMVCD41a+) and MMVs (MMVCD14+) expressing PS. MV surface penetration of annexin V is increased when large amounts of PS are expressed on the membrane surface [31].
D-dimer, a product of fibrin degradation by plasmin, is a common activation marker of the coagulation system and an indirect marker of the fibrinolytic process. Increased D-dimer levels in COVID-19 reflect the coagulation activity in viremia and the cytokine storm [15]. In the present study, COVID-19 patients with severe clinical symptoms had increased D-dimer levels compared to those with moderate clinical symptoms (Table 1), which was in line with findings by Yu, et al. [32].
Increased D-dimer levels in COVID-19 patients are associated with cell activation, which is associated with MV coagulation, indicating that MVs are correlated with D-dimer levels [13, 17]. This was supported by our results, which also suggested that annexin V-positive PMV marker (AnnV+ PMVCD41a+) counts correlated with D-dimer levels in patients with COVID-19 [25].
One limitation of this study is that we did not determine the activity of platelets and monocytes. Further studies are required to assess cell activity markers. To validate the results of this study, studies in a larger population comparing COVID-19 patients with a proportional number of healthy donors are required.
In conclusion, PMVs (PMVCD42b+ and PMVCD41a+) in COVID-19 patients are potential biomarkers for assessing disease severity because they indicate enhanced coagulation. This is the first study of this kind in our country, and the results support that the severity of thrombosis in COVID-19 is modulated by PMVs and MMVs.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Mrs. Atika, B.Sc., M.P.H., Department of Public Health and Preventive Medicine, Faculty of Medicine, Universitas Airlangga for helpful discussions on the statistical analysis. The authors are grateful for the support and cooperation of the staff of the Laboratory of Clinical Pathology, Department of Clinical Pathology, Faculty of Medicine, Dr. Soetomo General Academic Teaching Hospital, Surabaya, East Java, Indonesia. The authors would like to thank Editage for English language editing.
Notes
AUTHOR CONTRIBUTIONS
Nunki N and Hernaningsih Y wrote the ethical protocol. Nunki N and Herawati A provided the patient samples. Nunki N and Hernaningsih Y retrieved the laboratory data. Nunki N and Herawati A analyzed the data, Nunki N and Hernaningsih Y conducted the statistical analysis, and Nunki N, Hernaningsih Y, Wardhani P, Moses EJ, and Yusoff NM drafted the manuscript. All the authors have read and approved the final manuscript.
References
1. Fitriah M, Tambunan BA, Kahar H, Nugraha J, Aulia FA, Aryati , et al. 2022; Characteristics of natural killer (NK) cell and T lymphocyte in COVID-19 patients in Surabaya, Indonesia. Res J Pharm Technol. 15:2198–203. DOI: 10.52711/0974-360X.2022.00365.

2. Hashemi Tayer A, Amirizadeh N, Ahmadinejad M, Nikougoftar M, Deyhim MR, Zolfaghari S. 2019; Procoagulant activity of red blood cell-derived microvesicles during red cell storage. Transfus Med Hemother. 46:224–30. DOI: 10.1159/000494367. PMID: 31700504. PMCID: PMC6739704.

3. Cappellano G, Raineri D, Rolla R, Giordano M, Puricelli C, Vilardo B, et al. 2021; Circulating platelet-derived extracellular vesicles are a hallmark of Sars-Cov-2 infection. Cells. 10:85. DOI: 10.3390/cells10010085. PMID: 33430260. PMCID: PMC7825711. PMID: e08565a842624bf78dbc5da03e40b70e.

4. Nomura S, Shimizu M. 2015; Clinical significance of procoagulant microparticles. J Intensive Care. 3:2. DOI: 10.1186/s40560-014-0066-z. PMID: 25705427. PMCID: PMC4336124.

5. Biswas S, Thakur V, Kaur P, Khan A, Kulshrestha S, Kumar P. 2021; Blood clots in COVID-19 patients: simplifying the curious mystery. Med Hypotheses. 146:110371. DOI: 10.1016/j.mehy.2020.110371. PMID: 33223324. PMCID: PMC7644431.

6. Chatterjee V, Yang X, Ma Y, Wu MH, Yuan SY. 2020; Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Heart Circ Physiol. 319:H1181–96. DOI: 10.1152/ajpheart.00579.2020. PMID: 33035434. PMCID: PMC7792704.

7. Canzano P, Brambilla M, Porro B, Cosentino N, Tortorici E, Vicini S, et al. 2021; Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. 6:202–18. DOI: 10.1016/j.jacbts.2020.12.009. PMID: 33649738. PMCID: PMC7904280.

8. Rosińska J, Łukasik M, Kozubski W. 2017; The impact of vascular disease treatment on platelet-derived microvesicles. Cardiovasc Drugs Ther. 31:627–44. DOI: 10.1007/s10557-017-6757-7. PMID: 29164426. PMCID: PMC5730634.

9. Chyrchel B, Drożdż A, Długosz D, Stępień EŁ, Surdacki A. 2019; Platelet reactivity and circulating platelet-derived microvesicles are differently affected by P2Y12 receptor antagonists. Int J Med Sci. 16:264–75. DOI: 10.7150/ijms.28580. PMID: 30745807. PMCID: PMC6367525.
10. Helal O, Defoort C, Robert S, Marin C, Lesavre N, Lopez-Miranda J, et al. 2011; Increased levels of microparticles originating from endothelial cells, platelets and erythrocytes in subjects with metabolic syndrome: relationship with oxidative stress. Nutr Metab Cardiovasc Dis. 21:665–71. DOI: 10.1016/j.numecd.2010.01.004. PMID: 20399083.

11. Balbi C, Burrello J, Bolis S, Lazzarini E, Biemmi V, Pianezzi E, et al. 2021; Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine. 67:103369. DOI: 10.1016/j.ebiom.2021.103369. PMID: 33971404. PMCID: PMC8104913.

12. Loo J, Spittle DA, Newnham M. 2021; COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 76:412–20. DOI: 10.1136/thoraxjnl-2020-216243. PMID: 33408195.

13. Willim HA, Hardigaloeh AT, Supit AI. 2020; Coagulopathy in coronavirus disease-2019 (COVID-19): literature review. Intisari Sains Medis. 11:749–56. DOI: 10.15562/ism.v11i3.766.
14. Zahran AM, El-Badawy O, Ali WA, Mahran ZG, Mahran EEMO, Rayan A. 2021; Circulating microparticles and activated platelets as novel prognostic biomarkers in COVID-19; relation to cancer. PLoS One. 16:e0246806. DOI: 10.1371/journal.pone.0246806. PMID: 33617530. PMCID: PMC7899358. PMID: 170e3c7e8c7c45aba838c32f21352e61.

15. Hernaningsih Y, editor. 2021. Aspek laboratorium COVID-19. Airlangga University Press;Surabaya: 247. https://play.google.com/books/reader?id=IAZWEAAAQBAJ&pg=GBS.PR5&hl=en. Updated on Apr 2022.
16. Ministry of Health of the Republic of Indonesia. 2021; Decree of the Minister of Health of the Republic of Indonesia. KMK Number HK.01.07/MENKES/5671/2021. Regarding clinical management of corona virus disease 2019 (COVID-19) in health service facilities. 2019:1–360.
17. Chandler WL. 2013; Microparticle counts in platelet-rich and platelet-free plasma, effect of centrifugation and sample-processing protocols. Blood Coagul Fibrinolysis. 24:125–32. DOI: 10.1097/MBC.0b013e32835a0824. PMID: 23249614.

18. Menck K, Bleckmann A, Schulz M, Ries L, Binder C. 2017; Isolation and characterization of microvesicles from peripheral blood. J Vis Exp. 55057. DOI: 10.3791/55057-v. PMID: 28117819. PMCID: PMC5408706.

19. Enjeti AK, Ariyarajah A, D'Crus A, Seldon M, Lincz LF. 2017; Aug. Circulating microvesicle number, function and small RNA content vary with age, gender, smoking status, lipid and hormone profiles. Thromb Res. 156:65–72. DOI: 10.1016/j.thromres.2017.04.019. PMID: 28600979.

20. Rausch L, Lutz K, Schifferer M, Winheim E, Gruber R, Oesterhaus EF, et al. 2021; Binding of phosphatidylserine-positive microparticles by PBMCs classifies disease severity in COVID-19 patients. J Extracell Vesicles. 10:e12173. DOI: 10.1002/jev2.12173. PMID: 34854246. PMCID: PMC8636722. PMID: 90e56d7dcd8c4e7da4606b3b096736f2.

21. Abdelmaksoud MF, Abdelmaksoud SS, Abdelsamee HF, Ezzelregal HG, Alfeky MA. 2021; Platelets derived microparticles in COVID-19: correlation to inflammatory and coagulation State. J Appl Hematol. 12:195–202. DOI: 10.4103/joah.joah_102_21. PMID: e5248b4cf38e4b9db9dc2c6fdaed63aa.

22. Kurniawan A, Yanni M. 2020; Endothelial function examination in cardiovascular disease. Hum Care J. 5:638. DOI: 10.32883/hcj.v5i3.817.
23. Rausch L, Lutz K, Schifferer M, Winheim E, Gruber R, Oesterhaus EF, et al. 2021; Binding of phosphatidylserine-positive microparticles by PBMCs classifies disease severity in COVID-19 patients. J Extracell Vesicles. 10:e12173. DOI: 10.1002/jev2.12173. PMID: 34854246. PMCID: PMC8636722. PMID: 90e56d7dcd8c4e7da4606b3b096736f2.

24. Bongiovanni D, Klug M, Lazareva O, Weidlich S, Biasi M, Ursu S, et al. 2021; SARS-CoV-2 infection is associated with a pro-thrombotic platelet phenotype. Cell Death Dis. 12:50. DOI: 10.1038/s41419-020-03333-9. PMID: 33414384. PMCID: PMC7790351. PMID: 888aef1470164dc099f54eb80c115899.

25. Aatonen M, Grönholm M, Siljander PR. 2012; Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost. 38:102–13. DOI: 10.1055/s-0031-1300956. PMID: 22314608.

26. Grobler C, Maphumulo SC, Grobbelaar LM, Bredenkamp JC, Laubscher GJ, Lourens PJ, et al. 2020; Covid-19: the rollercoaster of fibrin(ogen), D-dimer, von Willebrand factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci. 21:5168. DOI: 10.3390/ijms21145168. PMID: 32708334. PMCID: PMC7403995. PMID: 8dd733259f67415c9808ca8e26f6114c.

27. Chiva-Blanch G, Laake K, Myhre P, Bratseth V, Arnesen H, Solheim S, et al. 2017; Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS One. 12:e0172558. DOI: 10.1371/journal.pone.0172558. PMID: 28207887. PMCID: PMC5313202. PMID: 845c93aed6b34b1cab2a4475f67b92a0.

28. Berezin AE, Berezin AA. 2019; Platelet-derived vesicles: diagnostic and predictive value in cardiovascular diseases. J Unexplored Med Data. 4:4. DOI: 10.20517/2572-8180.2019.05.

29. Tripisciano C, Weiss R, Karuthedom George S, Fischer MB, Weber V. 2020; Extracellular vesicles derived from platelets, red blood cells, and monocyte-like cells differ regarding their ability to induce factor XII-dependent thrombin generation. Front Cell Dev Biol. 8:298. DOI: 10.3389/fcell.2020.00298. PMID: 32478066. PMCID: PMC7232549. PMID: cbb2994863cc49abb65a7ff4ea12cd22.

30. Chen F, Liao Z, Peng D, Han L. 2019; Role of platelet microparticles in blood diseases: future clinical perspectives. Ann Clin Lab Sci. 49:161–70.
31. Blair TA, Frelinger AL, Michelson AD. Michelson AD, Cattaneo M, editors. 2019. Flow cytometry. Platelets. 4th ed. Elsevier;Saint Louis: p. 627–51. DOI: 10.1016/B978-0-12-813456-6.00035-7.
32. Yu B, Li X, Chen J, Ouyang M, Zhang H, Zhao X, et al. 2020; Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 50:548–57. DOI: 10.1007/s11239-020-02171-y. PMID: 32524516. PMCID: PMC7286212.

Fig. 1
Flow-cytometric detection of MVs. (A) The MV gate region was separated, defined, and calculated using the SSC-height (log) scale as the threshold to eliminate the 0.16-µm beads and use the 0.2- and 0.5-µm bead clouds to set the MV gate. The gate used for capturing the beads for counting is depicted as region 1 (R1). (B) MVs were calibrated using Megamix-Plus SSC beads in a Trucount tube, using fluorescence (FL1)/FITC as the threshold. (C) FSC and SSC of isolated MVs stained with fluorescence (FL), fluorescein isothiocyanate (FITC)-conjugated CD42b, and Trucount calibrator beads (R1). (D) Analysis of a specimen from a COVID-19 patient using a Trucount tube to obtain the R4 region. R4 comprised Trucount gating beads for counting. (E) MV-negative control, analyzed from healthy controls without staining with fluorescent-labeled antibodies (unstained). (F) The MV population of healthy controls with annexin V-positive staining was analyzed for PMVs (AnnV+ PMVCD42b+, AnnV+ PMVCD41a+) and MMVs (AnnV+ MMVCD14+). (G) The MV population of mild COVID-19 patients with annexin V-positive staining was analyzed for PMVs and MMVs. (H) The MV population of moderate COVID-19 patients with annexin V-positive staining was analyzed for PMVs and MMVs. (I) The MV population of severe COVID-19 patients with annexin V-positive staining was analyzed for PMVs and MMVs. The detected MVs were conjugated with fluorescent FITC for CD42b, PerCP-Cy5.5 for CD41a, APC for CD14, and PE for annexin V.
Abbreviations: MVs, microvesicles; PMVs, platelet microvesicles; MMVs, monocyte microvesicles; Qty, quantity; FSC, forward scatter; SSC, side scatter; FITC, fluorescein isothiocyanate; PerCP-Cy5.5, peridinin chlorophyll protein-cyanine5.5; APC, allophycocyanin; PE, phycoerythrin.
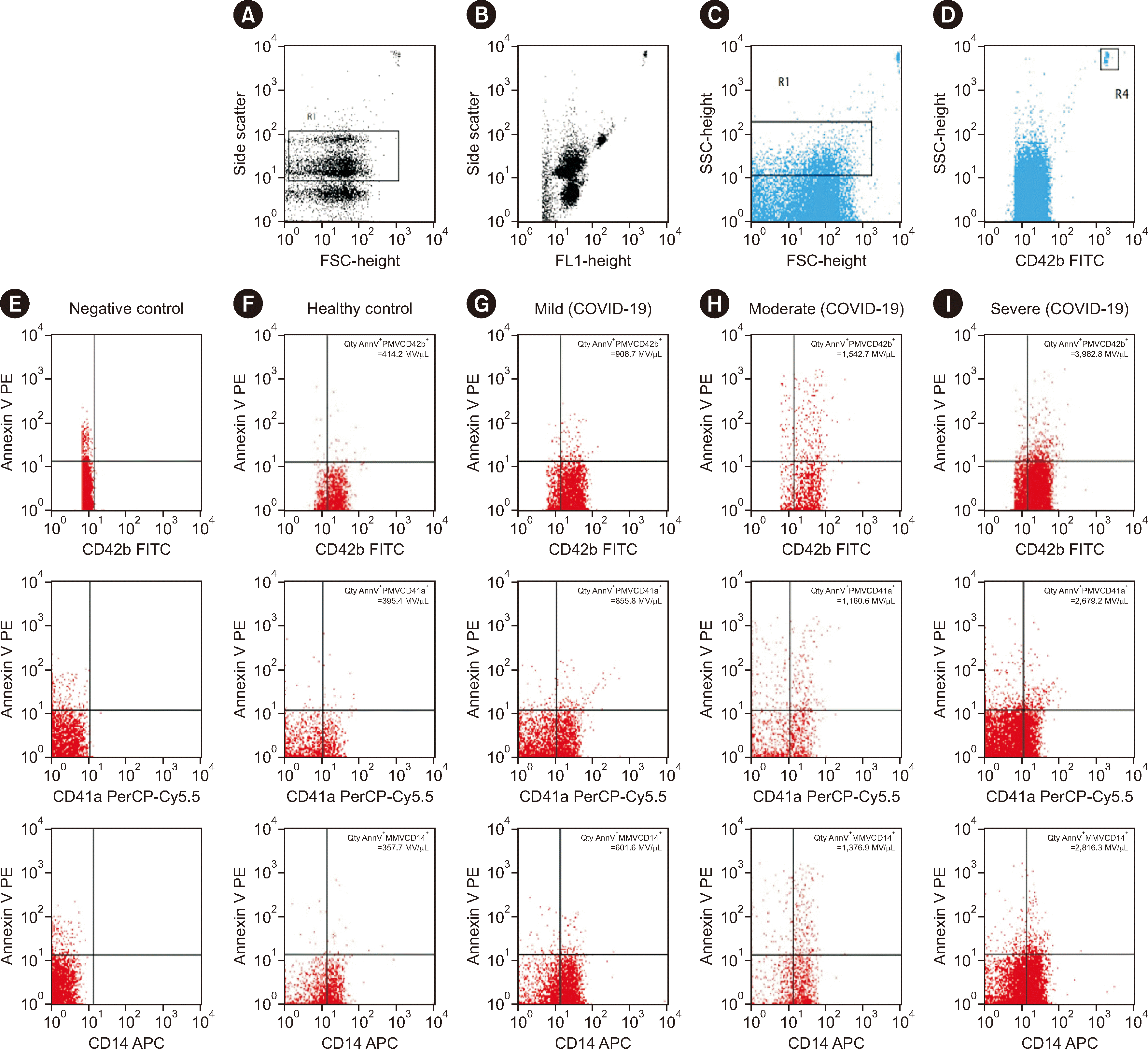
Table 1
Characteristics of COVID-19 patients with mild, moderate, and severe clinical symptoms
| Characteristic | Total (N=90) | COVID-19 clinical symptoms | P | ||
|---|---|---|---|---|---|
| Mild (N=30) | Moderate (N=30) | Severe (N=30) | |||
| Age (yrs), mean±SD | 45.8±13.9 | 35.0±11.2 | 53.2±13.3 | 49.3±10.0 | <0.001* |
| Sex, N (%) | 0.002† | ||||
| Male | 52 (57.8) | 25 (83.3) | 13 (43.3) | 14 (46.7) | |
| Female | 38 (42.2) | 5 (16.7) | 17 (56.7) | 16 (53.3) | |
| Condition, N (%) | <0.001† | ||||
| With comorbidities | 41 (45.6) | 5 (16.7) | 19 (63.3) | 17 (56.7) | |
| Without comorbidities | 49 (54.4) | 25 (83.3) | 11 (36.7) | 13 (43.3) | |
| Outcome, N (%) | <0.001† | ||||
| Survived | 69 (76.7) | 30 (100.0) | 22 (73.3) | 17 (56.7) | |
| Did not survive | 21 (23.3) | N/A | 8 (26.7) | 13 (43.3) | |
| Laboratory findings | |||||
| SpO2 (%), median (range) | 97 (60–100) | 99 (97–100) | 97 (94–99) | 76.5 (60–92) | 0.001‡ |
| PLT count (×103/µL), mean±SD | 308.1±145.4 | N/A | 309.6±153.1 | 306.5±140.0 | 0.935§ |
| Monocyte count (×103/µL), median (range) | 0.8 (0.1–3.3) | N/A | 0.7 (0.1–2.0) | 0.9 (0.2–3.3) | 0.084|| |
| Coagulation parameters | |||||
| PT (sec), median (range) | 11.1 (9.4–21.6) | N/A | 11 (9.7–18.3) | 11.1 (9.4–21.6) | 0.750|| |
| APTT (sec), median (range) | 28.8 (18.2–100) | N/A | 27.9 (18.2–100) | 29.3 (18.5–82.2) | 0.988|| |
| D-dimer (ng/mL), median (range) | 1,980 (380–19,050) | N/A | 1,290 (380–5,360) | 2,825 (540–19,050) | 0.028|| |
Table 2
Differences in MV counts among COVID-19 patients with mild, moderate, and severe clinical symptoms
Table 3
Significant differences among groups with different severity of clinical symptoms
| Biomarker | Clinical symptoms | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| AnnV+ PMVCD42b+ | Mild | 0.003 | ||
| Moderate | 0.554 | |||
| Severe | 0.028 | |||
| AnnV+ PMVCD41a+ | Mild | 0.117 | ||
| Moderate | 0.086 | |||
| Severe | 0.002 | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download