Abstract
To compare the outcomes of low central venous pressure (CVP) to standard CVP during laparoscopic liver resection. The study design was a systematic review following the PRISMA statement standards. The available literature was searched to identify all studies comparing low CVP with standard CVP in patients undergoing laparoscopic liver resection. The outcomes included intraoperative blood loss (primary outcome), need for blood transfusion, mean arterial pressure, operative time, Pringle time, and total complications. Random-effects modelling was applied for analyses. Type I and type II errors were assessed by trial sequential analysis (TSA). A total of 8 studies including 682 patients were included (low CVP group, 342; standard CVP group, 340). Low CVP reduced intraoperative blood loss during laparoscopic liver resection (mean difference [MD], –193.49 mL; 95% confidence interval [CI], –339.86 to –47.12; p = 0.01). However, low CVP did not have any effect on blood transfusion requirement (odds ratio [OR], 0.54; 95% CI, 0.28–1.03; p = 0.06), mean arterial pressure (MD, –1.55 mm Hg; 95% CI, –3.85–0.75; p = 0.19), Pringle time (MD, –0.99 minutes; 95% CI, –5.82–3.84; p = 0.69), operative time (MD, –16.38 minutes; 95% CI, –36.68–3.39; p = 0.11), or total complications (OR, 1.92; 95% CI, 0.97–3.80; p = 0.06). TSA suggested that the meta-analysis for the primary outcome was not subject to type I or II errors. Low CVP may reduce intraoperative blood loss during laparoscopic liver resection (moderate certainty); however, this may not translate into shorter operative time, shorter Pringle time, or less need for blood transfusion. Randomized controlled trials with larger sample sizes will provide more robust evidence.
Despite advances in surgical techniques, intraoperative bleeding during liver surgery remains a concern. Intraoperative blood loss is considered a cause of perioperative complications in patients undergoing liver surgery [1]; hence, several operative and non-operative strategies are applied to reduce the risk of bleeding. The strategies include intermittent clamping the portal vein and hepatic artery (Pringle manoeuvre) [2], sophisticated liver dissection techniques, use of stapling devices, ultrasonic dissectors, bipolar cautery, and hydrodissectors for parenchymal transection [3], and maintaining a low central venous pressure (CVP) during parenchymal transection [4,5].
Although the learning curve is steep and there are technical challenges, laparoscopic liver resection has become more popular in the past decade even for lesions in anatomically challenging segments [6,7]. However, the application of haemostasis techniques may be more challenging during laparoscopic liver resections compared with the open approach [8]. It has been shown that low CVP can reduce intraoperative bleeding during liver resection [5]; however, the impact of low CVP on intraoperative bleeding, specifically in laparoscopic liver resection is not known. Therefore, this systematic review and meta-analysis aimed to compare the outcomes of low CVP to standard CVP during laparoscopic liver resection.
The design of the study was according to the Preferred Reporting Items for systematic reviews and Meta-Analyses statement standards [9], and followed a predefined protocol.
Study design: Comparative cohort studies and randomized controlled trials (RCTs) that compared low CVP with standard CVP in patients undergoing laparoscopic liver resection were of interest to this review.
Population: Adult patients undergoing laparoscopic liver resection for any indications were eligible. The liver resection procedures of interest comprised both anatomical and non-anatomical resections.
Intervention and comparison: Low CVP, defined as pressure ≤ 5 cmH2O, was the eligible intervention and standard CVP, defined as pressure > 5 cmH2O, was the eligible comparison.
Outcomes: Intraoperative blood loss was the primary outcome. The secondary outcomes comprised the need for blood transfusion, mean arterial pressure, operative time, Pringle time, and total complications.
Using proper keywords, thesaurus headings, and search limits, a literature search strategy was constructed by two separate authors who had adequate experience in evidence synthesis (Appendix 1). There were no language restrictions. The date for the last search was 15 July 2023. The electronic sources searched comprised MEDLINE, CINAHL, Scopus, International Standard Randomised Controlled Trial Number Registry, World Health Organization International Clinical Trials Registry, CENTRAL, ClinicalTrials.gov, the European Association for Grey Literature Exploitation, and System for Information on Grey Literature. The reference lists of the relevant original studies and reviews were also evaluated.
Two separate authors reviewed the titles and abstracts of the articles, obtained the full texts of potentially eligible articles, and included the eligible studies. The following relevant data from the eligible studies were recorded on an electronic data collection sheet:
• First author’s name
• Publication year
• Journal name
• Study design
• Included population
• Study sample size
• Definitions of low and standard CVP
• Outcomes
Any disagreements about study selection or data collection were resolved by a third author when required.
The risk of bias (RoB) of RCTs was judged using the Cochrane RoB tool [10], and the RoB of observational studies was evaluated using the Risk Of Bias In Non-Randomized Studies of Interventions (ROBINS-I) tool [11]. Two independent authors conducted the RoB assessment and a third author resolved the disagreements when required.
Review Manager 5.4 software and trial sequential analysis (TSA) 0.9.5.5 Beta software were used. Random effects modelling was used to compute mean difference (MD) with 95% confidence level for continuous variables and odds ratio (OR) with 95% confidence for dichotomous variables. An individual patient was the unit of analysis and we based the analyses on intention to treat data. The I2 using the Cochran Q test (χ2) was calculated to measure statistical heterogeneity and was interpreted as below:
• I2 0%–25% suggests low heterogeneity
• I2 25%–75% suggests moderate heterogeneity
• I2 75%–100% suggests high heterogeneity
When an outcome was reported by ten studies, we aimed to construct funnel plots for publication bias risk assessment. TSA was modelled to explore the possibility of type I and type II errors in the primary outcome analysis as long as it was adequately reported (by at least five RCTs). The possibility of type I and II errors was determined using the O’Brien-Fleming α-spending function and futility boundaries, respectively.
Leave-one-out analysis and independent meta-analysis of studies with low RoB were planned as sensitivity analyses of the primary outcome.
The GRADE system was followed to determine the certainty of evidence for each outcome [12].
The literature search yielded 277 articles; 266 studies were excluded due to irrelevance. After full-text review, three more articles were excluded as they were systematic reviews (Fig. 1). The remaining eight articles [13-20] met the eligibility criteria of this study. The eligible studies included 7 RCTs and 1 retrospective cohort study, enrolling a total of 682 patients; 342 in the low CVP group and 342 in the standard CVP group (Table 1).
The outcomes of RoB assessment based on the aforementioned tools are shown in Fig. 2.
Intraoperative blood loss: Examination results of 682 individuals (8 studies) demonstrated that low CVP reduced intraoperative blood loss (MD, –193.49 mL; 95% confidence interval [CI], –339.86 to –47.12; p = 0.01). The heterogeneity (statistical) was high (I2 = 99%, p < 0.001) and the GRADE system suggested moderate certainty (Fig. 3). The information size calculated via TSA (950 patients) was not achieved; however, Z-curve intersected the conventional and alpha-spending boundaries in favour of low CVP, hence the possibility of type I and II errors was minimal and the conclusion was robust (Fig. 4).
Need for blood transfusion: Examination results of 416 individuals (4 studies) demonstrated comparable need for blood transfusion between low CVP and standard CVP (OR, 0.54; 95% CI, 0.28–1.03; p = 0.06) (Fig. 3). The heterogeneity (statistical) was low (I2 = 0%, p = 0.85) and the GRADE system suggested moderate certainty.
Mean arterial pressure: Examination results of 364 individuals (3 studies) demonstrated comparable mean arterial pressure between low CVP and standard CVP (MD, –1.55 mm Hg; 95% CI, –3.85–0.75; p = 0.19) (Fig. 3). The heterogeneity (statistical) was low (I2 = 0%, p = 0.75) and the GRADE system suggested moderate certainty.
Pringle time: Examination results of 404 individuals (3 studies) demonstrated comparable Pringle time between low CVP and standard CVP (MD, –0.99 minutes; 95% CI, –5.82–3.84; p = 0.69) (Fig. 3). The heterogeneity (statistical) was high (I2 = 96%, p < 0.001) and the GRADE system suggested moderate certainty.
Operative time: Examination results of 454 individuals (4 studies) demonstrated comparable operative time between low CVP and standard CVP (MD, –16.38 minutes; 95% CI, –36.68–3.39; p = 0.11) (Fig. 3). The heterogeneity (statistical) was high (I2 = 95%, p < 0.001) and the GRADE system suggested low certainty.
Total complications: Examination results of 274 individuals (4 studies) demonstrated comparable risks of total complications between low CVP and standard CVP (OR, 1.92; 95% CI, 0.97–3.80; p = 0.06) (Fig. 3). The heterogeneity (statistical) was low (I2 =0%, p = 0.66) and the GRADE system suggested moderate certainty.
We conducted a systematic review to compare the outcomes of low CVP and standard CVP during laparoscopic liver resection. Analysis of 8 studies including 682 patients showed that low CVP reduced intraoperative blood loss during laparoscopic liver resection; however, it did not have any effects on the need for blood transfusion, mean arterial pressure, Pringle time, operative time, or total complications. Sensitivity analysis suggested consistency of the results for intraoperative blood loss and TSA suggested that meta-analysis for intraoperative blood loss was not subject to type I or II errors. The GRADE system suggested moderate certainty.
For the first time in the literature, the current study assessed the impact of low CVP on intraoperative bleeding, specifically during laparoscopic liver resection. However, the results of the current study can be compared with the results of previous reviews, which did not limit the included population to laparoscopic liver resection. Liu et al. [5] conducted a systematic review including patients who had open or laparoscopic liver resection and concluded that low CVP is effective in reducing blood loss during liver resection. In another study, Hughes et al. [21] concluded that low CVP reduced intraoperative bleeding during open liver resection. Our findings are consistent with those of the aforementioned reviews.
The reduced intraoperative blood loss associated with low CVP during laparoscopic liver resection can be simply explained. It is well-recognized that venous bleeding is the main source of bleeding during liver resection, especially when hepatic inflow is reduced via Pringle manoeuvre or hepatic hilum occlusion [22,23]. Considering that CVP is directly related to the hepatic sinusoidal pressure, reducing the pressure in the inferior vena cava will reduce the former resulting in a drop of the pressure in hepatic sinusoids hence reducing intraoperative blood loss [22,23]. The findings of the current study support the above mechanism.
The current study is subject to the following limitations. Although most of the included studies had a randomized design, the included population characteristics, detail of liver resections, and detail of randomizations were poorly reported. Moreover, the available evidence is limited to studies from the same country, which may affect the generalisability of the findings. Consequently, the available data was not adequate for performing meta-regression analysis or subgroup analyses based on the baseline characteristics of the included population and type of liver resection; hence, confounding bias and selection bias cannot be excluded. Laparoscopic insufflation pressure may help to reduce intraoperative blood loss when it exceeds CVP pressure during laparoscopic liver resection. Therefore, variation in insufflation pressure among the included studies and between the treatment arms may be an important confounding factor. The insufflation pressure used during laparoscopic pressure was poorly reported by the included studies; consequently, it was not possible to perform meta-regression analysis or subgroup analyses based on insufflation pressure; this can be considered as another potential source of confounding bias. Although the intraoperative blood loss as the primary outcome was well-reported by the included studies, the secondary outcomes were not reported by all of the included studies; hence, our secondary outcomes results are less robust. The statistical heterogeneity was high for the primary outcome; nevertheless, the findings remained consistent through sensitivity analyses and TSA. Moreover, we downgraded evidence certainty due to high statistical heterogeneity. Because we included less than 10 studies, we were not able to assess publication bias risk.
While the available evidence may be subject to confounding bias and selection bias, the best available evidence suggests that low CVP may reduce intraoperative bleeding during laparoscopic liver resection (moderate certainty); however, this may not translate into shorter operative time, shorter Pringle time, or less need for blood transfusion. RCTs with larger sample sizes will provide more robust evidence. The results of the current study provide a robust basis for power analysis and hypothesis synthesis for future randomised trials.
References
1. Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. 2002; Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 236:397–406. discussion 406–407. DOI: 10.1097/00000658-200210000-00001. PMID: 12368667. PMCID: PMC1422593.
2. Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2009; (1):CD007632. DOI: 10.1002/14651858.CD006409.pub3. PMID: 19160283. PMCID: PMC10654807.

3. Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. 2005; How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 242:814–822. DOI: 10.1097/01.sla.0000189121.35617.d7. PMID: 16327491. PMCID: PMC1409877.
4. McNally SJ, Revie EJ, Massie LJ, McKeown DW, Parks RW, Garden OJ, et al. 2012; Factors in perioperative care that determine blood loss in liver surgery. HPB (Oxford). 14:236–241. DOI: 10.1111/j.1477-2574.2011.00433.x. PMID: 22404261. PMCID: PMC3371209.

5. Liu TS, Shen QH, Zhou XY, Shen X, Lai L, Hou XM, et al. 2021; Application of controlled low central venous pressure during hepatectomy: a systematic review and meta-analysis. J Clin Anesth. 75:110467. DOI: 10.1016/j.jclinane.2021.110467. PMID: 34343737.

6. Hajibandeh S, Kotb A, Evans L, Sams E, Naguib A, Hajibandeh S, et al. 2023; Procedural outcomes of laparoscopic caudate lobe resection: a systematic review and meta-analysis. Ann Hepatobiliary Pancreat Surg. 27:6–19. DOI: 10.14701/ahbps.22-045. PMID: 36245071. PMCID: PMC9947369.

7. Hajibandeh S, Hajibandeh S, Dave M, Tarazi M, Satyadas T. 2020; Laparoscopic versus open liver resection for tumors in the posterosuperior segments: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 30:93–105. DOI: 10.1097/SLE.0000000000000746. PMID: 31929396.

8. Rhu J, Kim SJ, Choi GS, Kim JM, Joh JW, Kwon CHD. 2018; Laparoscopic versus open right posterior sectionectomy for hepatocellular carcinoma in a high-volume center: a propensity score matched analysis. World J Surg. 42:2930–2937. DOI: 10.1007/s00268-018-4531-z. PMID: 29426971.

9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. 2009; The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339:b2700. DOI: 10.1136/bmj.b2700. PMID: 19622552. PMCID: PMC2714672.

10. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Cochrane handbook for systematic reviews of interventions. Chapter 8: assessing risk of bias in a randomized trial [Internet]. Available from: https://training.cochrane.org/handbook/current/chapter-08. Cochrane;2011. cited 2018 Oct 10.
11. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.

12. Schünemann H, Brożek J, Guyatt G, Oxman A. 2013. GRADE handbook for grading quality of evidence and strength of recommendations [Internet]. GRADE;Available from: https://gdt.gradepro.org/app/handbook/handbook.html. cited 2023 Aug 20.
13. Zhang Y, Li L, Xu J, Cheng W. 2023; Effects of controlled low central venous pressure combined with dexmedetomidine on the blood loss, renal function and cognitive function in patients undergoing laparoscopic hepatectomy. Signa Vitae. 19:182–187.
14. Wu G, Chen T, Chen Z. 2021; Effect of controlled low central venous pressure technique on postoperative hepatic insufficiency in patients undergoing a major hepatic resection. Am J Transl Res. 13:8286–8293.
15. Pan YX, Wang JC, Lu XY, Chen JB, He W, Chen JC, et al. 2020; Intention to control low central venous pressure reduced blood loss during laparoscopic hepatectomy: a double-blind randomized clinical trial. Surgery. 167:933–941. DOI: 10.1016/j.surg.2020.02.004. PMID: 32216964.

16. Gan Q. 2020; Application effect of controlled low central venous pressure technique in laparoscopic hepatectomy. Chin Mod Med. 27:62–64.
17. Chen X, Hu CH, Peng YH, Luo H, Yang P. 2018; Prospective randomized controlled study on controlled low central venous pressure in laparoscopic hepatectomy. Chin J Min Inv Surg. 208:15–19.
18. Chen J, Xiao X, Wang JX, Xin CH, Wu JJ, Cai WH. 2017; Clinical application of low central venous pressure in laparoscopic hepatectomy. J Hepatobiliary Surg. 25:423–426.
19. Deng DJ, Zhang YN, Zeng ZW, Lin YH, Luo R, Zhang WQ. 2017; Transesophageal echocardiography monitoring for laparoscopic hepatectomy combined with low central venous pressure. Lingnan Mod Clin Surg. 17:528–534.
20. Zhang YN, Luo R, Deng JD, Zhang WQ, Lin WX, Zeng ZW, et al. 2017; Clinical application of controlled low central venous pressure in laparoscopic hepatectomy. Lingnan Mod Clin Surg. 17:423–431.
21. Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ. 2015; Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford). 17:863–871. DOI: 10.1111/hpb.12462. PMID: 26292655. PMCID: PMC4571753.

22. Wang WD, Liang LJ, Huang XQ, Yin XY. 2006; Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 12:935–939. DOI: 10.3748/wjg.v12.i6.935. PMID: 16521223. PMCID: PMC4066160.

23. Lai PB, Chui PT, Leow CK, Lau WY. 1998; Correlation between blood loss and inferior vena caval pressure during liver resection. Br J Surg. 85:1158. DOI: 10.1046/j.1365-2168.1998.00570.x. PMID: 9501812.
Fig. 2
Risk of bias summary and a graph showing the authors’ judgements about each risk of bias item for randomized controlled trials (A) and observational studies (B).
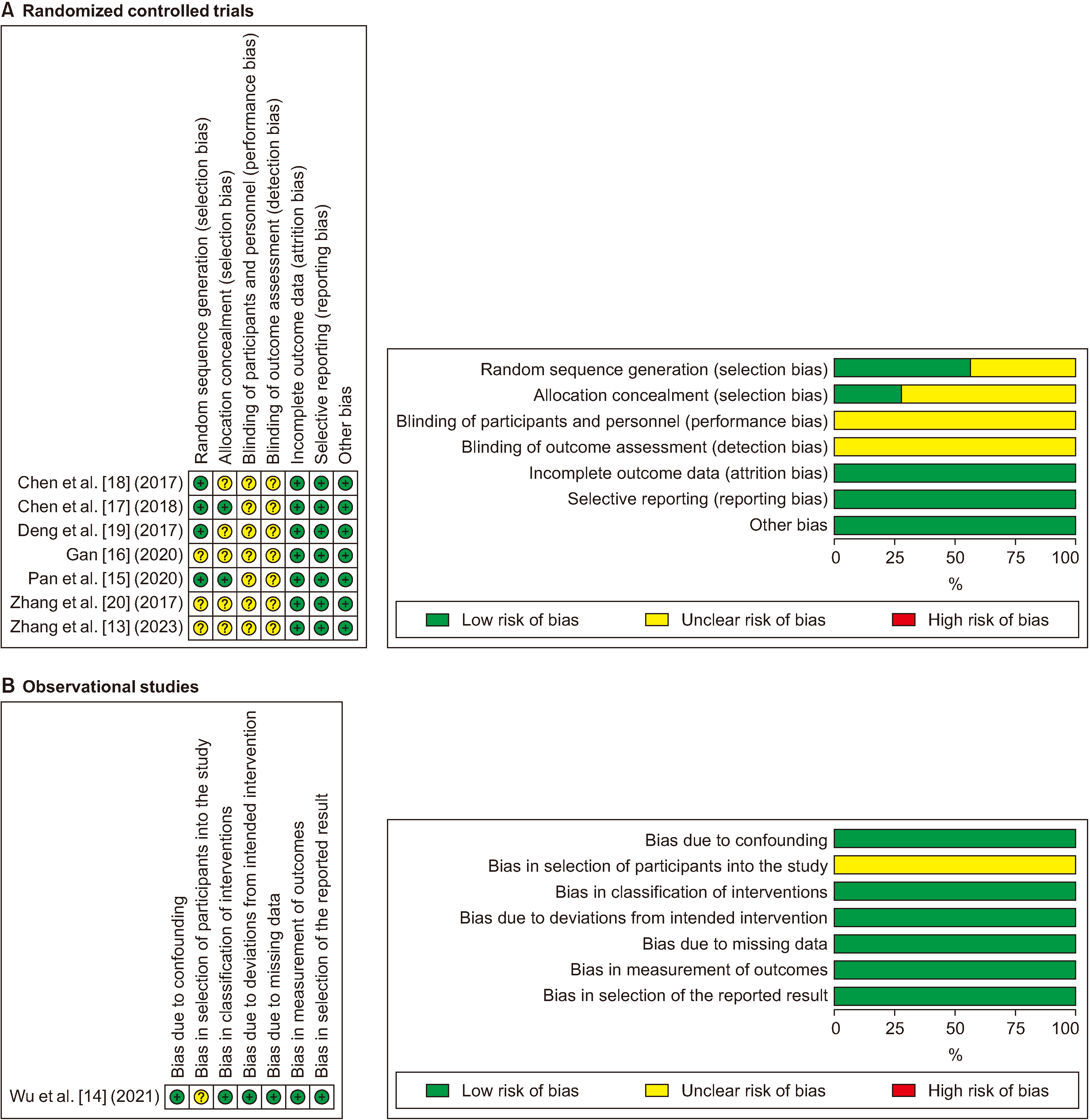
Fig. 3
Forest plots for the comparison between low CVP and standard CVP. (A) Intraoperative blood loss. (B) Need for blood transfusion. (C) Mean arterial blood pressure. (D) Pringle time. (E) Operative time. (F) Total complications. CVP, central venous pressure; SD, standard deviation; CI, confidence interval; IV, inverse variance; M-H, Mantel-Haenszel.
Fig. 4
Results of trial sequential diagnosis for intraoperative blood loss. CVP, central venous pressure.
Table 1
Baseline characteristics of the included studies
| Study | Country | Journal | Design | Included population | Sample size | Low CVP definition | Standard CVP definition | Interventions for maintaining low CVP in the low CVP group | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Total | Low CVP | Standard CVP | ||||||||
| Zhang et al. [13], 2023 | China | Signa Vitae | RCT | Patients undergoing laparoscopic liver resection | 90 | 45 | 45 | CVP between 2–4 cmH2O | CVP between 6–12 cmH2O |
Intravenous nitroglycerin Dorsal elevated position |
| Wu et al. [14], 2021 | China | American Journal of Translational Research | Retrospective cohort | Patients undergoing laparoscopic major liver resection | 168 | 84 | 84 | CVP between 1–5 cmH2O | CVP between 6–10 cmH2O |
15° head over feet position Limiting infusion volume Vasoactive drugs |
| Pan et al. [15], 2020 | China | Surgery | RCT | Patients undergoing laparoscopic liver resection | 146 | 73 | 73 | CVP between 0–5 cmH2O | CVP higher than 5 cmH2O |
Nitro compounds Diuretics Opioids |
| Gan [16], 2020 | China | Modern Chinese Medicine | RCT | Patients undergoing laparoscopic liver resection | 100 | 50 | 50 | CVP between 3–5 cmH2O | CVP between 6–12 cmH2O |
Limiting infusion volume Intravenous nitroglycerin Diuretics |
| Chen et al. [17], 2018 | China | China Journal of Minimally Invasive Surgery | RCT | Patients undergoing laparoscopic liver resection | 52 | 27 | 25 | CVP between 2–4 cmH2O | CVP between 5–10 cmH2O |
Limiting infusion volume Intravenous nitroglycerin |
| Chen et al. [18], 2017 | China | Journal of Hepatobiliary Surgery | RCT | Patients undergoing laparoscopic liver resection | 36 | 18 | 18 | CVP between 0–5 cmH2O | CVP between 6–12 cmH2O |
Reversed Trendelenburg’s position Intravenous nitroglycerin Diuretics |
| Deng et al. [19], 2017 | China | Lingnan Modern Clinics in Surgery | RCT | Patients undergoing laparoscopic liver resection | 40 | 20 | 20 | CVP between 0–5 cmH2O | CVP between 6–12 cmH2O | Limiting infusion volume |
| Zhang et al. [20], 2017 | China | Lingnan Modern Clinics in Surgery | RCT | Patients undergoing laparoscopic liver resection | 50 | 25 | 25 | CVP between 3–5 cmH2O | CVP between 6–12 cmH2O |
Limiting infusion volume Intravenous nitroglycerin |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download