Abstract
Endoluminal ultrasound has become a cornerstone for evaluating anorectal diseases since its introduction as a diagnostic tool nearly three decades ago. This article reviews the applications of 3-dimensional (3D) endoluminal ultrasound, including transrectal (TRUS) and transanal (TAUS), in clinical settings and highlights their efficacies for diagnosing various anorectal conditions. Technological advancements, such as high-resolution imaging and multiplanar display capabilities, enable detailed assessments of anatomic structures, but high-quality images are required to standardize preprocedural and intraprocedural protocols and ensure patient comfort. This article provides insight into examination methodologies, especially those utilizing sophisticated multifrequency transducers that produce in-depth 2D and 3D images. Fundamental anatomical reference points are noted, and the diagnostic importance of understanding rectal wall layers, vasculature, and muscular structures is emphasized. This comprehensive review underscores the importance of standardizing procedures to improve the consistency and reliability of 3D endoluminal ultrasound for diagnosing anorectal diseases.
Introduced nearly 30 years ago, endoluminal ultrasound has been widely used to evaluate the mid to distal rectum, anal canal, and pelvic floor muscles in patients with both malignant and benign anorectal diseases.(1) Advancements in imaging technology, such as 3-dimensional transrectal ultrasound with high-resolution and multiplanar display, have allowed for detailed assessment of anatomic structures, including different layers of the bowel wall, mesorectal lymph nodes, perirectal pelvic organs, and muscular confinement.(2) Research indicates that the accuracy of 3D-transrectal ultrasound (TRUS) is comparable to that of rectal magnetic resonance imaging (MRI) for local staging of rectal cancer.(3) Similarly, 3D-transanal ultrasound (TAUS) has demonstrated high diagnostic accuracy in detecting fistula tracts or abscesses in relation to the sphincter muscle.(4) However, it is important to note that the diagnostic accuracy of both procedures is significantly influenced by the operator's skills and experience.(5,6)
Achieving expertise in learning is key to securing accurate diagnostic observations. Previous research assessing the accuracy and learning trajectory of TRUS in evaluating the stage of rectal cancer suggested that an operator may require the experience of handling 50 to 80 cases to gain proficiency in using TRUS.(7,8) Furthermore, the precision of TRUS can be influenced by different tumor aspects such as its size, developmental stage, and precise anatomical position.(9) Factors unique to individuals, like their sex, age, and prior pelvic surgeries or radiation therapies, can also impact the diagnostic results of TRUS.(3,10) It is essential to control these elements to overcome the learning curve and improve the consistency of diagnosis.
To enhance the precision, consistency, and repeatability of diagnoses, establishing a standardized procedure is critical, particularly in the context of 3D reconstruction following a scan. This article aims to present a systematic approach beginning with preparations before the procedure. It identifies key anatomical landmarks that show the spatial relationships to adjacent pelvic organs and muscle structures during the scanning. Moreover, the article provides guidance on analyzing the results and examining the crucial images once the scan is complete.
For optimal imaging quality, especially when examining the rectum, a clean bowel is critical. TAUS typically does not require bowel preparation. However, if detailed visualization of the distal rectum is needed, bowel preparation can improve the quality of the images. For TRUS, bowel cleansing is a must. Common practice involves administering a laxative, such as 10 mg of bisacodyl, both on the evening before and the morning of the exam to promote bowel movements. It's vital to ensure that the patient drinks plenty of clear fluids to offset any potential dehydration. Often, an oral laxative alone is adequate for clearing the rectum, and no additional cleaning methods are necessary. A digital rectal examination may be conducted to confirm the rectum is empty. If stool is still present, a sodium phosphate enema could be administered immediately before the procedure to further cleanse the rectum. The combination of an oral laxative and an enema usually results in effective bowel preparation.
Sedation is not a necessity for the procedure. TRUS and TAUS are both conducted as outpatient procedures, with TAUS being notably straightforward and comfortable, eliminating the need for sedation. In contrast, TRUS takes a longer duration and is associated with abdominal and pelvic discomfort. While it is common to perform TRUS without sedation, the administration of a sedative may be advantageous for some patients to alleviate anxiety and discomfort, thereby enhancing their experience and satisfaction with the procedure. Sedatives and analgesics, which are not used for inducing general anesthesia and include drugs like benzodiazepines, may be given after a comprehensive assessment and preparation of the patient, similar to the approach for colonoscopy sedation.(11) For integrating sedation into outpatient procedures, establishing practice guidelines address mild to moderate procedural sedation and analgesia that can be consulted.
TRUS or TAUS is usually performed with endoluminal 3D transducer ultrasound systems manufactured by companies, such as BK Medical or Hitachi Medical Systems. We’ve been using the ultrasound system by BK medical shown in Fig. 1, because of the wide range of frequencies to visualize tissue layers in different depths. Therefore, we will describe detailed information regarding how we’ve been using the blind endoprobe to generate detailed images of anorectal anatomy. The endoprobe emits ultrasound waves of high multifrequency between 6 and 16 MHz and has 360° rotational mechanical capability.(12) This transducer features a sophisticated automated system that can capture 300 transaxial 2D images across a 60 mm range, taking a new image every 0.2 mm, completing the process in a minute, all without needing to move the probe inside the cavity.(12) These 2D images are immediately processed into a detailed 3D image that can be manipulated and examined in real time.(12) The 3D data can be saved for later review on the ultrasound system or a personal computer, using specialized 3D Viewer software.(12)
The endoprobe (Fig. 1A) can be covered with a balloon in which a water infusion system is built, as shown in Fig. 2. The water infusion system is essential for dilating the rectum to ensure the rectal wall is fully expanded in all directions. To enhance diagnostic accuracy, it's essential to minimize any potential artifacts. Air bubbles trapped in the water during infusion can cause significant artifacts, which may potentially disrupt the examination and necessitate restarting the preparation process. This can lead to discomfort for patients, especially if exams are prolonged or need to be repeated. Therefore, it is of utmost importance to properly prepare the water infusion system and eliminate all air bubbles before starting the examination. The presence of a skilled assistant is often essential for efficient preparation, tasked with the meticulous yet repetitive process of flushing and withdrawing water into the balloon, a task that is tedious but crucial for removing air bubbles. A skilled assistant not only manages preparation tasks but also anticipates needs and mitigates issues, such as patient complaints which arise from anxiety, providing invaluable support to the operator.
If there are lithotomy chairs, stirrups, or leg supports available, you can position a patient in the lithotomy position. However, not all clinics are fully equipped. To evaluate the anus and rectum, a patient is usually positioned in the left lateral decubitus position. A digital rectal exam must be performed to dilate and relax the anus before inserting the probe. The probe, which is used without filling the rectum with water, can be carefully inserted through the anal canal. It allows for real-time visualization of the anus and the lower part of the rectum in a cross-sectional view. Given that the probe is rigid and the pelvis is enclosed by the bony sacrum and adjacent pelvic organs, it's necessary to adjust the angle of the probe as it moves deeper, shown in Fig. 3. This maneuver requires particular attention to avoid causing any damage to the rectum, especially as the probe approaches the upper rectal area. The highest level where the imaging can start is typically at the level of peritoneal reflection, in between the upper to mid rectum.
For comprehensive observation of the rectal wall, water inflation of the balloon is undertaken. The volume needed to adequately distend the rectum for inspection varies according to individual rectal capacity and compliance of an individual, which is generally around 100 to 120 milliliters (ml) for women and 80 to 100 ml for men. If the individual experiences discomfort or pain during the procedure, it is imperative to stop the infusion immediately to prevent potential harm. Upon achieving adequate distension of the rectum, a 3D scan begins at the level of the peritoneal reflection. As the scanner progresses to the lower segments of the rectum, the introduced water can be gradually reduced by 20 ml after each scan session. A volume of 40 to 60 ml of water is enough to ensure clear imaging of the distal section of the rectum as well as the pelvic floor muscles and the upper part of the anal canal. Ultimately, to ascertain the intricate anatomical details of the muscle structures, the anal canal is best visualized with the application of a high-frequency wave scanning technique.
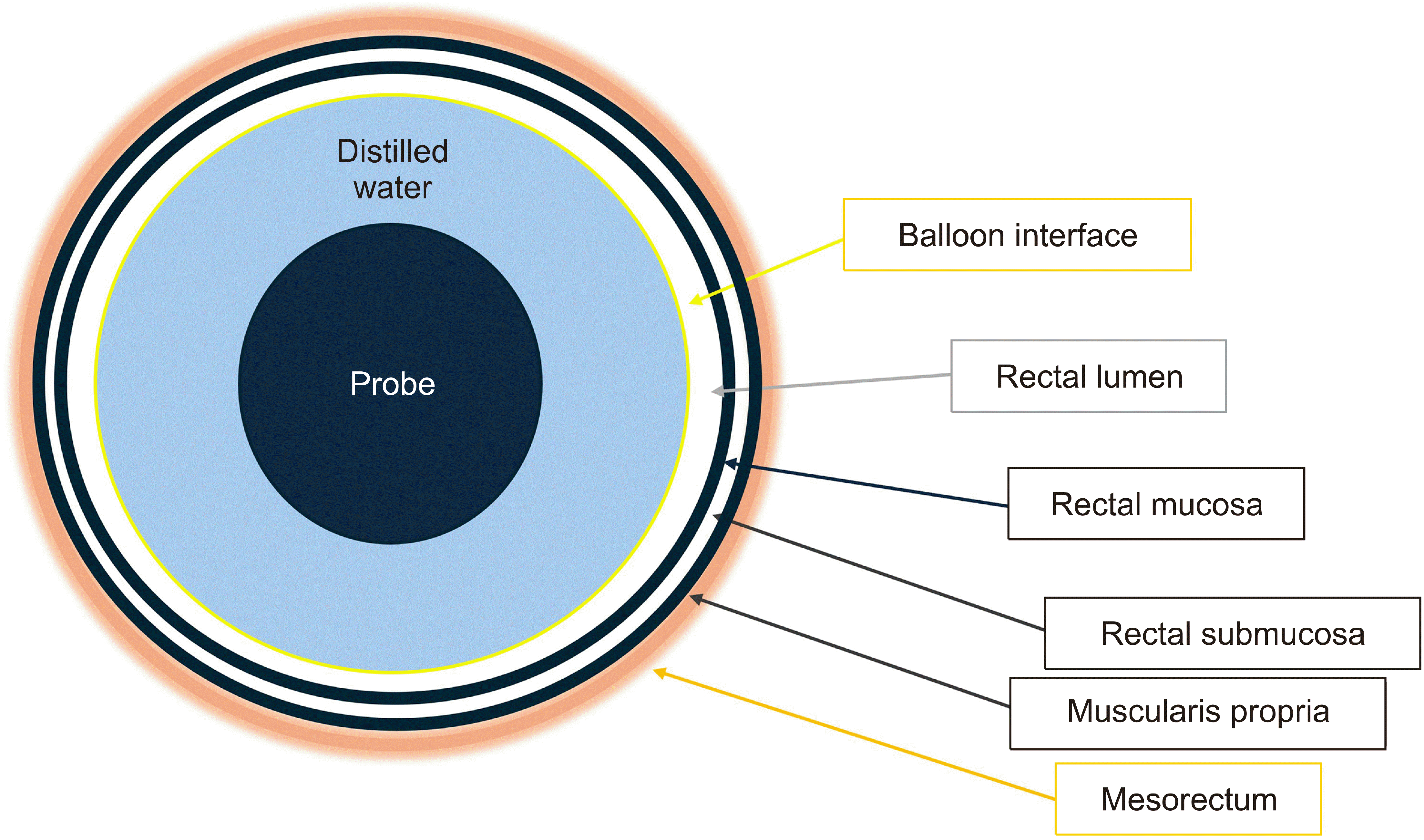
The rectal wall itself can be seen in detail, comprising several layers. In Fig. 4, the structural layers of the rectal wall are depicted diagrammatically. The transducer can be placed at the center of the rectum while the balloon, once infused with water, expands and presses against the rectal lining. If the bowel contains feces, however, artifacts from the feces can become visible in the imaging. The innermost hypoechoic layer is the mucosa, which is the lining that comes into direct contact with the ballon interface. The submucosa lying just outside the mucosa shows hyperechogenicity, reflecting that it contains blood vessels, nerves, and connective tissue. Fig. 5 demonstrates a neuroendocrine tumor embedded in the submucosa. As shown in Fig. 5C, the muscularis propria is the hypoechoic layer following the hyperechoic submucosa, indicating smooth muscle fibers that facilitate the movement and integrity of the rectal wall.
Surrounding the muscularis propria is the mesorectum, which is fatty tissue enveloping the rectum and containing lymph nodes and blood vessels, as shown in Fig. 5C. The superior rectal vessels, located behind and on the sides of the rectum within the mesorectum, are responsible for supplying blood to that area. On an ultrasound scan, they appear as a main tubular structure that branches out into smaller, narrower tubes. As the scan moves caudally, you can see these vessels further divided into even smaller branches.
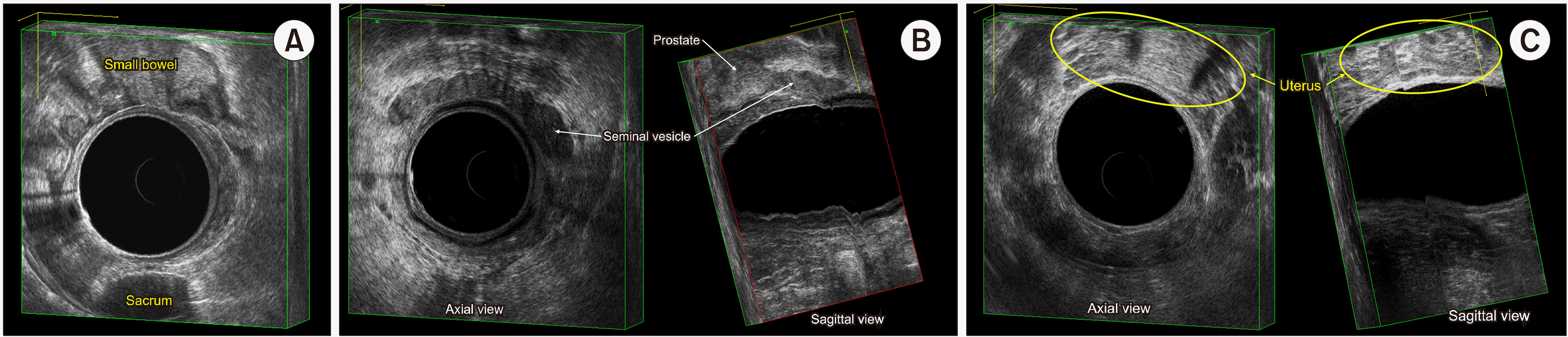
When a rigid endoscope is positioned in the mid to upper portion of the rectum, it allows for the visualization of the respective sections of the rectal wall. Considering the rectum's average length ranges from 12 to 15 cm, the probe may be safely advanced up to 10 to 12 cm, at about the superior Houston valve.(13) Posteriorly, the bony structure of the sacrum, mostly at S3 level, is identified as a landmark behind the rectum (Fig. 6A). The sacrum's curved shape is a distinctive feature on imaging. Anterior to the rectum, in males, the seminal vesicles are seen (Fig. 6B). In females, the uterus is typically visualized in front of the anterior rectal wall unless the patient has undergone a total hysterectomy (Fig. 6C). Above the peritoneum, small bowel loops may be seen floating or moving with peristalsis. The mesorectum of the mid rectum usually contains the superior rectal vessel in the posterior aspect of the rectum. The superior rectal vessels bifurcate into right and left terminal branches.(13)
The distal rectum is an entirely extraperitoneal organ with minimal surrounding mesorectum, which is encased posterolaterally by the pelvic floor muscles, as illustrated in Fig. 7. In males, its anterior wall is adjacent to the posterior aspect of the prostate, while in females it abuts the posterior vaginal wall. A unique anatomical feature within this section is the middle rectal artery, which penetrates the rectal wall most commonly at the 10 and 2 o’clock positions, and less so at the lateral or posterior aspects.
The middle rectal vessels exhibit significant variations in their origin, distribution, and course, causing significant controversy over its anatomy. The middle rectal artery typically arises from branches of the internal iliac artery, most commonly from the internal pudendal artery, followed by the gluteal artery, a common gluteal-pudendal trunk, or trifurcation with these arteries.(14) The entry site into the mesorectum can be visualized depending on the location of originating arteries, e.g. if it’s the internal pudendal artery, it will be ventrolateral, as shown in Fig. 8. This interindividual variation in the anatomy of the middle rectal vessels may bring different patterns of lymphatic drainage in the distal rectum.(15)
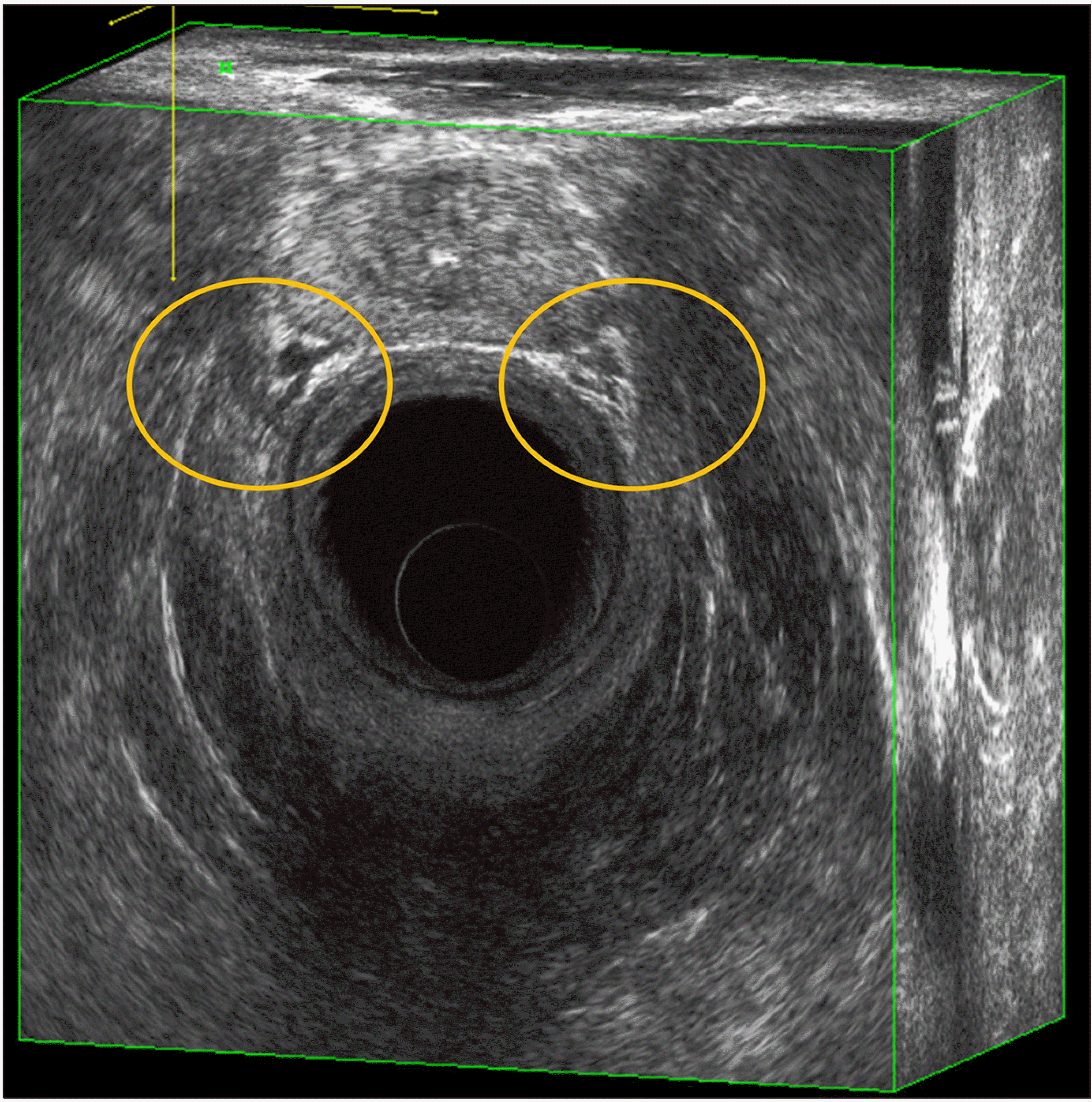
Using low-frequency waves, the pelvic outlet can be visualized. The pubococcygeus muscle and iliococcygeus muscle can be delineated vaguely. At the same time, the obturator internus muscle can be visualized laterally on both sides. As the rectum gets surrounded by pelvic floor muscles, its gross appearance changes into funnel shape, forming the anorectal junction. At this point, the inner circular muscle of the muscularis propria gets thickened and continues as the internal anal sphincter muscle. The outer longitudinal muscle of the muscularis propria appears as distinct fibers inserting into the external sphincter muscles. These muscles coordinate the function of the anal sphincter complex, facilitating the anorectal inhibitory reflex and controlling the continence.(13) By allowing precise control of rectal distension with a water-infusion system, endoluminal ultrasound provides exceptional visualization of the anal canal and rectum, making it highly effective in pinpointing the exact transition zone between them.
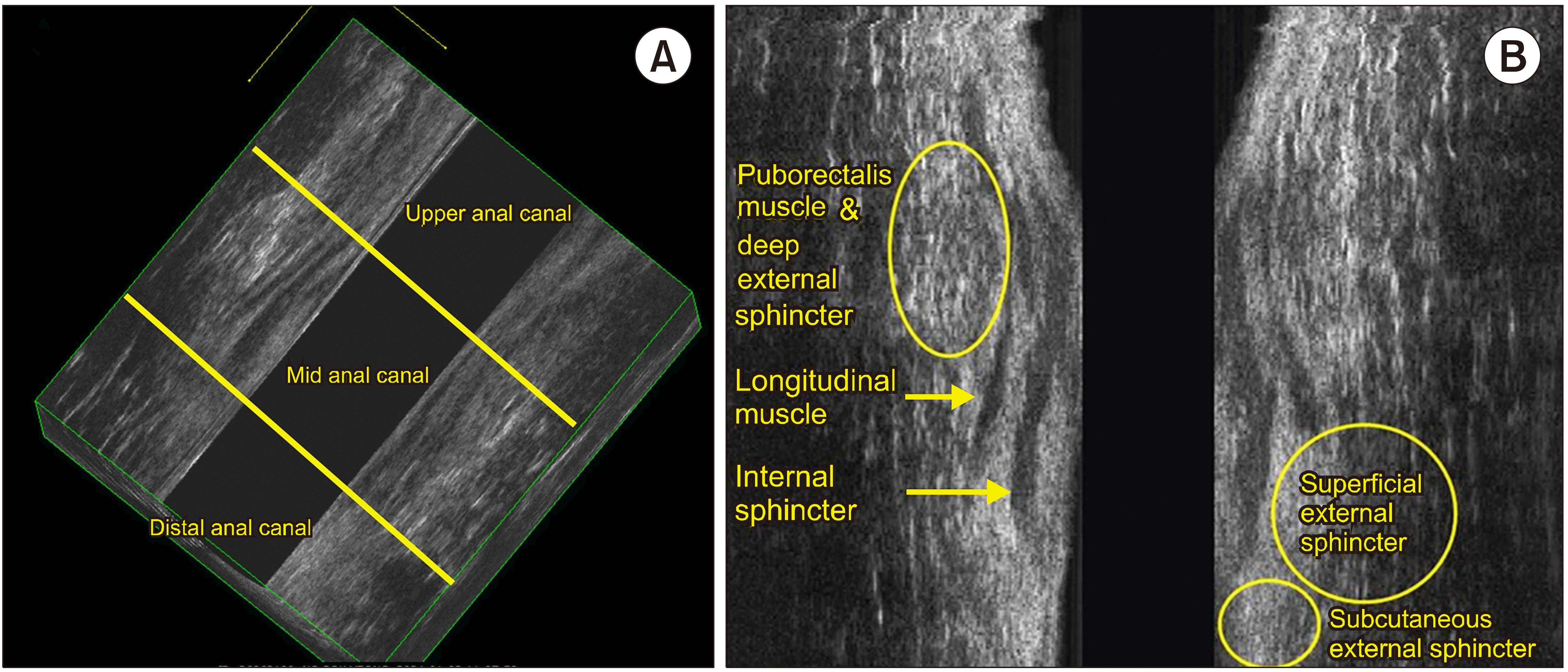
The anal canal, the terminal part of the gastrointestinal tract, is best visualized with high-frequency waves. Sonographic definition of anal canal refers to the “surgical” or “functional” anal canal, which is from the anal verge to the anorectal ring.(13) The squamous-columnar junction, the mucocutaneous transition from the anoderm to the mucous membrane of the rectum, lies at the inner most layer. Then, a complex arrangement of multiple muscle layers compiled as a cylindrical structure, with the subsequent layers of internal sphincter, the intersphincteric space with the longitudinal muscle fibers running down, and the outer striated skeletal muscle layers of external sphincter and puborectalis muscle.
The anal canal is typically divided into three segments – the upper, mid, and distal anal canal, shown in Fig. 9. These divisions are based on the anatomy of the muscles that comprise and surround the canal, as well as their appearance on the ultrasound.
(1) Upper anal canal: At this level, the most notable feature is the puborectalis muscle, which forms a U-shaped sling. This muscle is part of the levator ani group and is important for maintaining fecal continence. On the ultrasound, it appears as a hyperechogenic, brightly reflecting, structure due to its dense muscular composition.
The internal anal sphincter muscle, which is involuntary and made of smooth muscle, is seen within the embrace of the puborectalis muscle. It typically appears hypoechoic, less bright or dark, in comparison to the puborectalis muscle because it is less dense.
(2) Mid anal canal: This segment is characterized by the presence of two distinct muscular structures:
The internal sphincter ring, which is a continuation of the smooth muscle of the rectum and maintains involuntary control over defecation.
The external sphincter muscles, which are voluntary striated muscles that surround the internal sphincter. The external sphincter has mixed echogenicity (varying levels of brightness) or may appear hyper-echogenic (very bright) due to its composition of muscle and fibrous tissue.
(3) Distal anal canal: In this lowest part of the anal canal, only the subcutaneous part of the external anal sphincter is present. The internal anal sphincter does not extend to this level.
The subcutaneous external anal sphincter is responsible for fine control of continence and is visible on ultrasound as it is close to the skin surface.
The distinction between these layers is important for diagnostic purposes and for guiding surgical procedures. For example, in conditions like anal fistula, understanding this anatomy helps in planning treatments that preserve sphincter function. Additionally, in cancer staging, delineating these structures accurately can influence surgical decisions and prognosis.
There is an ongoing debate about the merits of using 3D endoluminal ultrasound as the first-line diagnostic tool for anorectal disease. A systematic review and meta-analysis by Luglio et al. compared TRUS to pelvic MRI for local staging of mid-to-distal rectal cancer, which suggests that TRUS had comparable or even better sensitivity and specificity for both tumor depth (T) and lymph node involvement (N) staging compared to pelvic MRI.(3) Currently, TRUS is the preferred method for early rectal cancer recommended by the European Society for Medical Oncology clinical practice guideline.(16) Several studies have demonstrated the high accuracy of TRUS in distinguishing benign and malignant rectal tumors, while pelvic MRI tended to overdiagnose adenomas as cancer.(10,17) Addi-tionally, Liu et al. assessed the pre-surgical accuracy of TRUS in differentiating three sub-stages of T3 rectal cancer (T3a <5 mm, T3b 5-10 mm, T3c >10 mm) based on extramural depth of tumor invasion. They found an overall accuracy of 88.0%, 86.8%, and 76.2% for each sub-stage, respectively.(18) Notably, this accuracy correlated with survival outcomes, implying that TRUS can potentially refine risk stratification within the T3 stage.
However, the diagnostic accuracy for predicting lymph node metastasis is limited, as reflected by a wide range from 64% to 100%.(19) Although mesorectal lymph nodes can be visualized, TRUS can only show the lymph nodes located in mid to distal mesorectum, restricting accurate assessment of nodal involvement in the proximal mesorectum and lateral pelvic lymph nodes.(20) Moreover, the diagnostic accuracy in re-assessment after neoadjuvant therapy with radiation is contested. TRUS has shown varying levels of accuracy, from 38% to 75%, highlighting inconsistencies in tumor staging after treatment.(21) Additionally, TRUS is limited in their ability to detect total cancer remission and have a low sensitivity of around 25% in predicting a complete pathological response.(22) Overstaging was common, mainly due to chemoradiation-induced changes such as fibrosis, edema, inflammation, and necrosis.(23) Nevertheless, strides are being made toward improving diagnostic techniques. Zhang et al. have identified sonographic signatures, such as alterations in muscle layer thickness, tumor angulation, and shape uniformity that might enhance the prediction of therapeutic outcomes.(24) In an attempt to improve the diagnostic accuracy, the authors suggested a pathological tumor response prediction model using such parameters to re-assess rectal cancer patients after neoadjuvant chemoradiotherapy.
For benign anal disease, such as anal fistula, TAUS is also a preferred diagnostic method due to its excellent accuracy. Previous studies demonstrated that with an overall accuracy of up to 93%, it can identify a primary track and internal opening.(25-27) Injecting 3% hydrogen peroxide solution into the external opening can further enhance the diagnostic accuracy by highlighting a pathologic changes in the anal canal.(28,29) Moreover, studies comparing preoperative 3D-TAUS assessments with intraoperative findings have revealed a strong correlation in both the height and type of anal fistulas, supported by a high kappa coefficient, thereby solidifying the technique's reliability.(30) In the study assessing the impact of preoperative 3D-TAUS on the outcomes of fistula surgery, it was found that patients who underwent the 3D-TAUS assessment prior to their procedure experienced more favorable results compared to those who did not.(31) Specifically, the group that received the 3D-TAUS assessment had a reduced rate of fistula recurrence and a lower occurrence of postoperative incontinence. Additionally, they demonstrated improved anal sphincter function, as evidenced by higher resting and squeezing pressures.
With the advent of technology, 3D ultrasound in the rectum and anus provides high-quality images with better resolution compared to 2D ultrasound. The anatomical relationship between the rectum and adjacent tissues and organs is clearly visualized. The five layers of the rectal wall can be displayed and viewed from various angles and sections with real-time recording and excellent spatial perception, ensuring reproducibility and reducing inter-observer variabi-lity.(20) Nonetheless, the precision of diagnoses achieved through the use of 3D endoluminal ultrasonography highly depends on the skill level of the clinician conducting the examination. Indeed, a profound understanding of anatomical structure and significant professional experience are critical components for accurate interpretation of the images obtained. The operator-dependent nature of the test may explain the lack of reproducibility in a clinical setting in large multicenter studies.(32,33) To have a good understanding of anatomical relationships depending on the location within the rectum, a certain training period to overcome the learning curve for both performance and interpretation is required.(20) Additionally, examiners with limited experience may encounter challenges due to a lack of full preparation. The process involves distending the rectum with a water-infusion method, which may result in discomfort or even pain for the patient. Moreover, if the bowel is not properly cleaned or there are errors in configuring the water-infusion system, it could lead to artifacts in the images. Such complications could prolong the exam time, leading to greater discomfort and inconvenience for the patient.
While 3D endoluminal ultrasound presents certain challenges, its use provides valuable insights for specialists examining the rectum, anus, and the neighboring pelvic anatomy and musculature. This method also offers a unique opportunity for physicians to interact closely with patients, which reinforces strong relational ties and promotes a caring, empathetic atmosphere. A doctor can assess bowel function in extensive detail via face-to-face engagements, yielding a rounded portrait of the patient’s medical condition. The diagnostic precision can be significantly enhanced when integrated with advanced imaging techniques such as elastography, enhanced chromoendoscopy, or multispectral optoacoustic imaging. Addi-tionally, employing machine learning tools to identify anatomical irregularities can further sharpen diagnostic acumen.
REFERENCES
1. Felt-Bersma RJ, Cazemier M. 2006; Endosonography in anorectal disease: an overview. Scand J Gastroenterol Suppl. (243):165–74. DOI: 10.1080/00365520600664292. PMID: 16782637.

2. Hünerbein M, Pegios W, Rau B, Vogl TJ, Felix R, Schlag PM. 2000; Prospective comparison of endorectal ultrasound, three-dimensional endorectal ultrasound, and endorectal MRI in the preoperative evaluation of rectal tumors. Preliminary results. Surg Endosc. 14:1005–9. DOI: 10.1007/s004640000345. PMID: 11116406.

3. Luglio G, Pagano G, Tropeano FP, Spina E, Maione R, Chini A, et al. 2021; Endorectal ultrasonography and pelvic magnetic resonance imaging show similar diagnostic accuracy in local staging of rectal cancer: an update systematic review and meta-analysis. Diagnostics (Basel). 12:5. DOI: 10.3390/diagnostics12010005. PMID: 35054171. PMCID: PMC8775222.

4. Varsamis N, Kosmidis C, Chatzimavroudis G, Apostolidou Kiouti F, Efthymiadis C, Lalas V, et al. 2023; Preoperative assessment of perianal fistulas with combined magnetic resonance and tridimensional endoanal ultrasound: a prospective study. Diagnostics (Basel). 13:2851. DOI: 10.3390/diagnostics13172851. PMID: 37685389. PMCID: PMC10486944.

5. Li J, Chen SN, Lin YY, Zhu ZM, Ye DL, Chen F, et al. 2021; Diagnostic accuracy of three-dimensional endoanal ultrasound for anal fistula: a systematic review and meta-analysis. Turk J Gastroenterol. 32:913–22. DOI: 10.5152/tjg.2021.20750. PMID: 34872892. PMCID: PMC8975359.

6. Schaffzin DM, Wong WD. 2004; Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer. 4:124–32. DOI: 10.3816/CCC.2004.n.015. PMID: 15285819.

7. Liu ZL, Zhou T, Liang XB, Ma JJ, Zhang GJ. 2014; Learning curve of endorectal ultrasonography in preoperative staging of rectal carcinoma. Mol Clin Oncol. 2:1085–90. DOI: 10.3892/mco.2014.352. PMID: 25279202. PMCID: PMC4179823.

8. Kolev NY, Tonev AY, Ignatov VL, Zlatarov AK, Bojkov VM, Kirilova TD, et al. 2014; The role of 3-D endorectal ultrasound in rectal cancer: our experience. Int Surg. 99:106–11. DOI: 10.9738/INTSURG-D-13-00227.1. PMID: 24670018. PMCID: PMC3968834.

9. Marusch F, Koch A, Schmidt U, Zippel R, Kuhn R, Wolff S, et al. 2002; Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 34:385–90. DOI: 10.1055/s-2002-25292. PMID: 11972270.

10. Oien K, Forsmo HM, Rösler C, Nylund K, Waage JE, Pfeffer F. 2019; Endorectal ultrasound and magnetic resonance imaging for staging of early rectal cancers: how well does it work in practice? Acta Oncol. 58(Suppl 1):S49–54. DOI: 10.1080/0284186X.2019.1569259. PMID: 30736712.

11. 2018; Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: a report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 128:437–79. DOI: 10.1097/ALN.0000000000002043. PMID: 29334501.
12. Santoro GA, Wieczorek AP, Stankiewicz A, Woźniak MM, Bogusiewicz M, Rechberger T. 2009; High-resolution three-dimensional endovaginal ultrasonography in the assessment of pelvic floor anatomy: a preliminary study. Int Urogynecol J Pelvic Floor Dysfunct. 20:1213–22. DOI: 10.1007/s00192-009-0928-4. PMID: 19533007.

13. Oliveira LCC. 2020. Anorectal physiology: a clinical and surgical perspective. Springer;Cham: DOI: 10.1007/978-3-030-43811-1.
14. Heinze T, Fletcher J, Miskovic D, Stelzner S, Bayer A, Wedel T. 2023; The middle rectal artery: revisited anatomy and surgical implications of a neglected blood vessel. Dis Colon Rectum. 66:477–85. DOI: 10.1097/DCR.0000000000002531. PMID: 36630321.

15. Yoo RN, Cho HM, Kye BH, Lee YS, Kim YS. 2023; Reappraisal of the lymphatic drainage system of the distal rectum: functional lymphatic flow into the presacral space and its clinical implication in rectal cancer treatment. Biomedicines. 11:274. DOI: 10.3390/biomedicines11020274. PMID: 36830812. PMCID: PMC9952975.

16. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. 2017; Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28(Suppl 4):iv22–40. DOI: 10.1093/annonc/mdx224. PMID: 28881920.

17. Burdan F, Sudol-Szopinska I, Staroslawska E, Kolodziejczak M, Klepacz R, Mocarska A, et al. 2015; Magnetic resonance imaging and endorectal ultrasound for diagnosis of rectal lesions. Eur J Med Res. 20:4. DOI: 10.1186/s40001-014-0078-0. PMID: 25586770. PMCID: PMC4304171.

18. Liu Q, Zang Y, Zhou D, Chen Z, Xin C, Zang W, et al. 2024; The importance of preoperative T3 stage substaging by 3D endorectal ultrasonography for the prognosis of middle and low rectal cancer. J Clin Ultrasound. 52:249–54. DOI: 10.1002/jcu.23623. PMID: 38041543.
19. Restivo A, Zorcolo L, Marongiu L, Scintu F, Casula G. 2015; Limits of endorectal ultrasound in tailoring treatment of patients with rectal cancer. Dig Surg. 32:129–34. DOI: 10.1159/000375537. PMID: 25791387.

20. Pinto RA, Nahas SC, Rizkalah Nahas CS, Sparapan Marques CF, Ribeiro Junior U, et al. Corrêa Neto IJ. 2017; Efficacy of 3-dimensional endorectal ultrasound for staging early extraperitoneal rectal neoplasms. Dis Colon Rectum. 60:488–96. DOI: 10.1097/DCR.0000000000000781. PMID: 28383448.

21. Cuicchi D, Castagna G, Cardelli S, Larotonda C, Petrello B, Poggioli G. 2023; Restaging rectal cancer following neoadjuvant che-mo-radiotherapy. World J Gastrointest Oncol. 15:700–12. DOI: 10.4251/wjgo.v15.i5.700. PMID: 37275455. PMCID: PMC10237020.

22. Liu S, Zhong GX, Zhou WX, Xue HD, Pan WD, Xu L, et al. 2018; Can Endorectal Ultrasound, MRI, and mucosa integrity accurately predict the complete response for mid-low rectal cancer after preoperative chemoradiation? A prospective observational study from a single medical center. Dis Colon Rectum. 61:903–10. DOI: 10.1097/DCR.0000000000001135. PMID: 29944579.

23. Evans J, Patel U, Brown G. 2011; Rectal cancer: primary staging and assessment after chemoradiotherapy. Semin Radiat Oncol. 21:169–77. DOI: 10.1016/j.semradonc.2011.02.002. PMID: 21645861.

24. Zhang X, Fan J, Zhang L, Wang J, Wang M, Zhu J. 2021; Association between three-dimensional transrectal ultrasound findings and tumor response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: an observational study. Front Oncol. 11:648839. DOI: 10.3389/fonc.2021.648839. PMID: 34178635. PMCID: PMC8223675.

25. Santoro GA, Fortling B. 2007; The advantages of volume rendering in three-dimensional endosonography of the anorectum. Dis Colon Rectum. 50:359–68. DOI: 10.1007/s10350-006-0767-z. PMID: 17237912.

26. Sun MR, Smith MP, Kane RA. 2008; Current techniques in imaging of fistula in ano: three-dimensional endoanal ultrasound and magnetic resonance imaging. Semin Ultrasound CT MR. 29:454–71. DOI: 10.1053/j.sult.2008.10.006. PMID: 19166042.

27. Sun Y, Cui LG, Liu JB, Wang JR, Ping H, Chen ZW. 2018; Utility of 360° real-time endoanal sonography for evaluation of perianal fistulas. J Ultrasound Med. 37:93–8. DOI: 10.1002/jum.14307. PMID: 28708274.

28. Kruskal JB, Kane RA, Morrin MM. 2001; Peroxide-enhanced anal endosonography: technique, image interpretation, and clinical applications. Radiographics. 21 Spec No:S173–89. DOI: 10.1148/radiographics.21.suppl_1.g01oc13s173. PMID: 11598256.

29. Mantoo S, Mandovra P, Goh S. 2020; Using preoperative three-dimensional endoanal ultrasound to determine operative procedure in patients with perianal fistulas. Colorectal Dis. 22:931–8. DOI: 10.1111/codi.14993. PMID: 31991037.

30. Kołodziejczak M, Santoro GA, Obcowska A, Lorenc Z, Mańczak M, Sudoł-Szopińska I. 2017; Three-dimensional endoanal ultrasound is accurate and reproducible in determining type and height of anal fistulas. Colorectal Dis. 19:378–84. DOI: 10.1111/codi.13580. PMID: 27943527.

31. Ding JH, Bi LX, Zhao K, Feng YY, Zhu J, Zhang B, et al. 2015; Impact of three-dimensional endoanal ultrasound on the outcome of anal fistula surgery: a prospective cohort study. Colorectal Dis. 17:1104–12. DOI: 10.1111/codi.13108. PMID: 26331275.

32. Marusch F, Ptok H, Sahm M, Schmidt U, Ridwelski K, Gastinger I, et al. 2011; Endorectal ultrasound in rectal carcinoma--do the literature results really correspond to the realities of routine clinical care? Endoscopy. 43:425–31. DOI: 10.1055/s-0030-1256111. PMID: 21234855.

33. Ashraf S, Hompes R, Slater A, Lindsey I, Bach S, Mortensen NJ, et al. 2012; A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 14:821–6. DOI: 10.1111/j.1463-1318.2011.02830.x. PMID: 21920011.

Fig. 1
The anorectal ultrasound system. (A) The main body of the ultrasound system with endoluminal probe (model flexFocus 500 BK medical, Peabody, Massachusetts). (B) The endoluminal probe (type 2052).
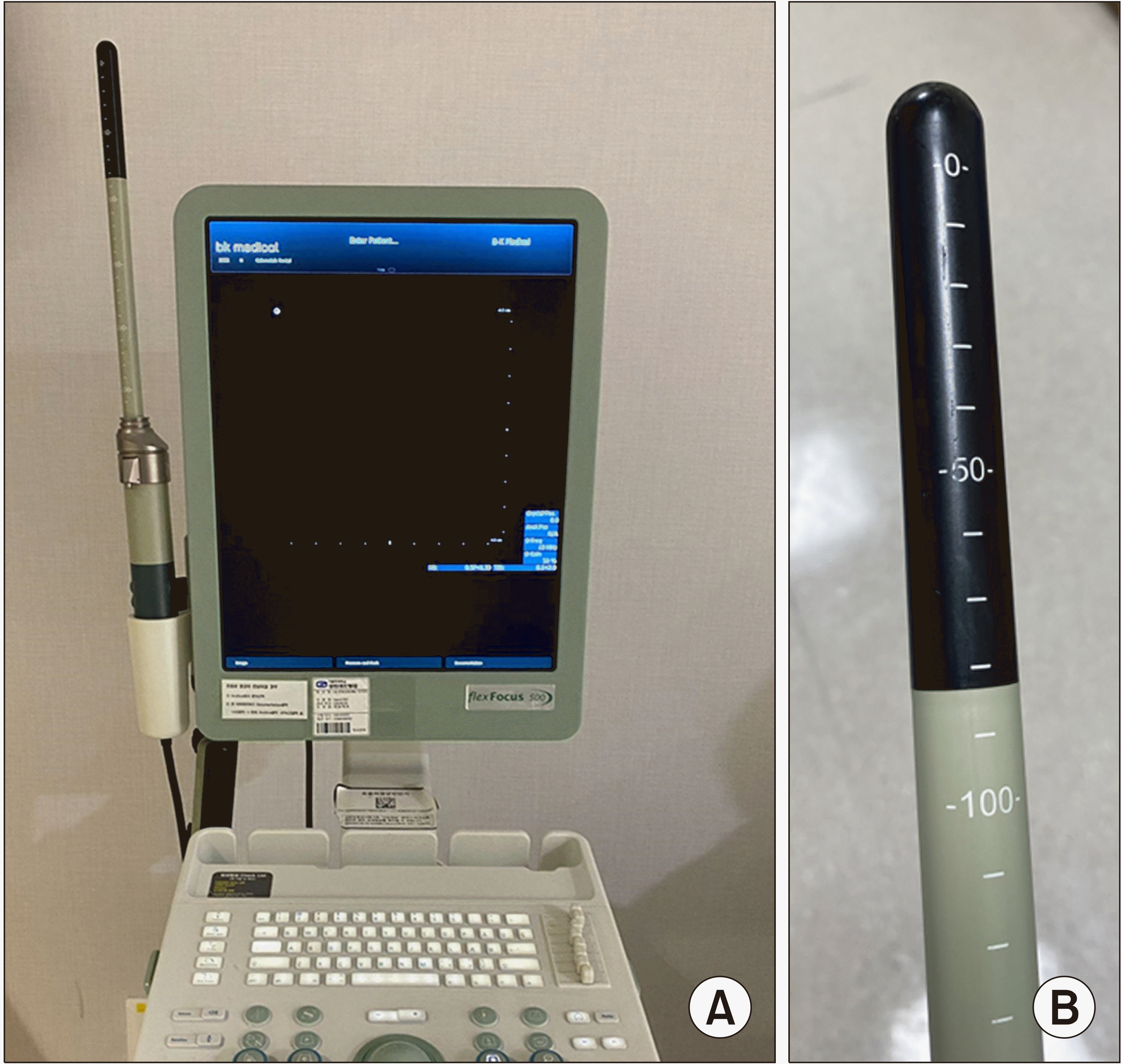
Fig. 2
A water infusion system is built with a balloon encasing the probe, and saline is delivered into the balloon to distend the rectum.

Fig. 3
The direction of probe inser-tion. (A) The initial direction of inserting a probe. (B) The direction of inserting a probe in the distal to mid rectum.
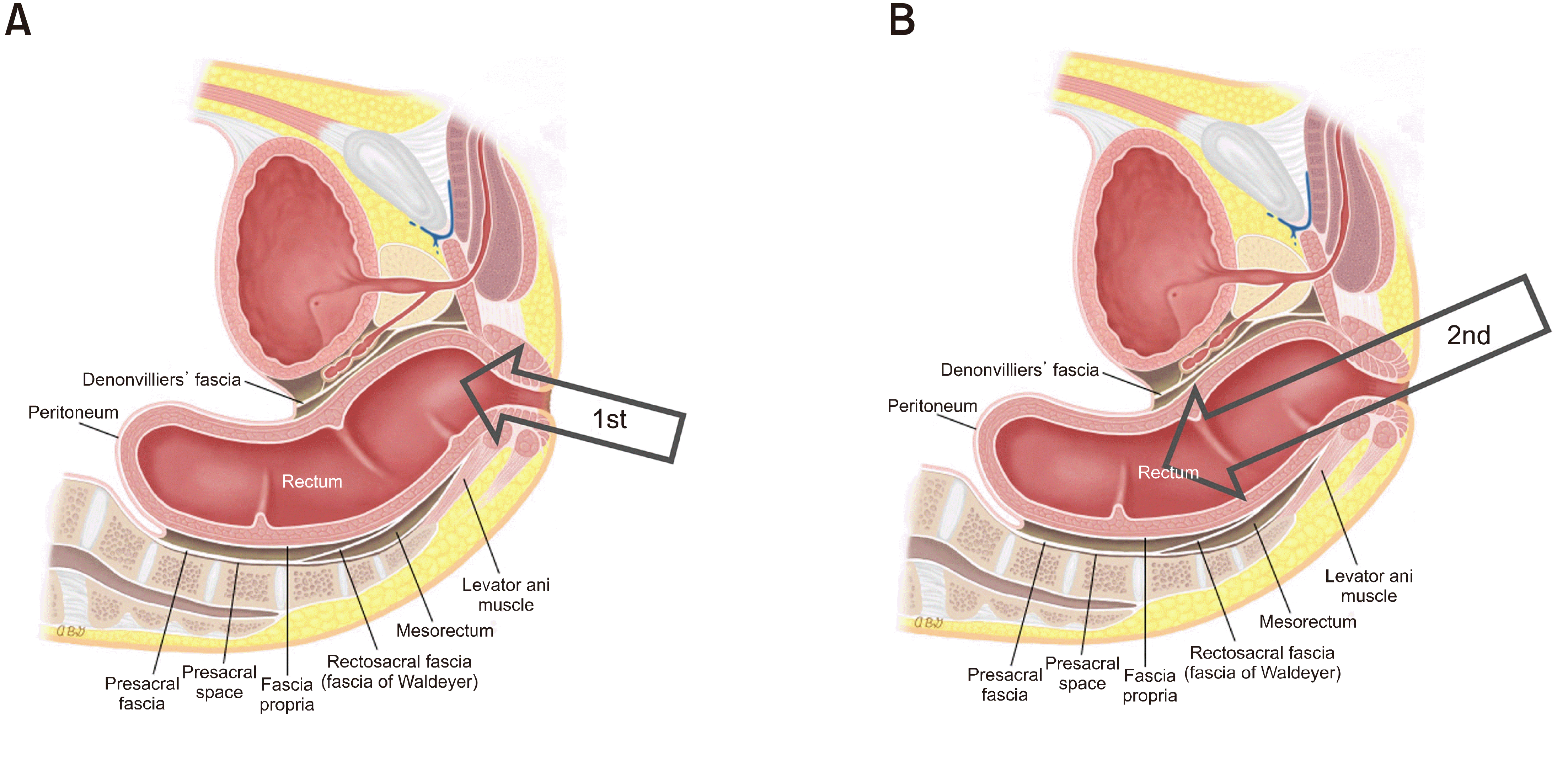
Fig. 5
A submucosal tumor within the submucosal layer. (A) Sonographic appearance. (B) Endoscopic view of submucosal tumor. (C) An example of detailed rectal wall layers.

Fig. 6
Various anatomical views of the distal rectum. (A) In the axial view, the prostate gland is positioned at 12 o’clock in male. (B) The coronal view reveals the distinct expansion of the distal rectum at the anorectal junction, indicating the transition from the rectum to the anal canal. (C) The sagittal view illustrates the distal rectum's location in relation to the pelvic floor muscles, showcasing how it rests against these muscles posteriorly. (D) In the axial view, the vagina is positioned at 12 o’clock in female. (E) The coronal view reveals the distinct expansion of the distal rectum at the anorectal junction, indicating the transition from the rectum to the anal canal. (F) The sagittal view illustrates the distal rectum's location in relation to the pelvic floor muscles, showcasing how it rests against these muscles posteriorly.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download