Abstract
With recent advances in adeno-associated virus (AAV)-based gene therapy, efficacy and toxicity screening have become essential for developing gene therapeutic drugs for retinal diseases. Retinal organoids from human pluripotent stem cells (hPSCs) offer a more accessible and reproducible human test platform for evaluating AAV-based gene therapy. In this study, hPSCs were differentiated into retinal organoids composed of various types of retinal cells. The transduction efficiencies of AAV2 and AAV8, which are widely used in clinical trials of inherited retinal diseases, were analyzed using retinal organoids. These results suggest that retinal organoids derived from hPSCs serve as suitable screening platforms owing to their diverse retinal cell types and similarity to the human retina. In summary, we propose an optimal stepwise protocol that includes the generation of retinal organoids and analysis of AAV transduction efficacy, providing a comprehensive approach for evaluating AAV-based gene therapy for retinal diseases.
Gene therapy treatments for inherited retinal diseases have developed rapidly, with various approaches ranging from gene supplementation for single-gene disorders, such as Leber’s congenital amaurosis (LCA) (1) and X-linked retinoschisis (XLRS) (2) to more complex strategies, such as gene editing for autosomal dominant retinitis pigmentosa (RP) (3). Currently, dozens of clinical trials are underway for gene therapy in retinal diseases, with the majority utilizing adeno-associated virus (AAV) as a vector to deliver the transgene of interest (4). Therefore, ensuring the safety and efficacy of gene therapy in the retina is crucial for developing effective gene-therapeutic drugs for inherited retinal diseases. To date, preclinical evaluation of AAVs has primarily been conducted using animal models, such as non-human primates and mice. However, these models have limitations in terms of species differences and lack of disease models for retinal diseases. Therefore, novel models that can serve as platforms for testing the efficacy and safety of AAVs and mimic human retinal diseases are necessary.
Retinal organoids can be generated from human pluripotent stem cells (hPSCs) by mimicking the normal process of retinogenesis, which involves the differentiation of stem cells into retinal progenitor cells (5-7). Retinal organoids are three-dimensional structures composed of various cell types found in the human retina, including photoreceptors, bipolar cells, and retinal ganglion cells (8, 9). These retinal organoids can mimic the structure and function of the human retina (10), making them a valuable model for studying retinal development, inherited retinal diseases, and potential therapies. Given their structural similarities and cell-type composition, retinal organoids are considered promising efficacy test platforms for gene therapy. Therefore, human retinal organoids can potentially serve as a bridge between preclinical and clinical applications, with similarity, quantity, and reproduction of healthy and pathological conditions.
To address this issue, we propose a streamlined process for evaluating AAVs using retinal organoids. In this study, we characterized retinal organoids derived from hPSCs using an optimized method. Our qualitative and quantitative assessments of the transduction efficiency of the two AAV serotypes demonstrated that both serotypes effectively transduced various retinal cell types present in the organoids. Overall, we believe that this study provides guidelines for conducting retinal organoid-based AAV efficacy tests, from generating hPSC-derived retinal organoids to testing the efficacy of AAV using different serotypes.
The human embryonic stem cells (hESCs; H9) purchased from WiCell were expanded in mTeSR1 (ST85850; STEMCELL Technologies) on matrigel coated plates at 37℃ and 5% CO2. Cell culture media was replaced daily. hESCs were maintained as described preciously (11). All procedures were conducted in accordance with the approved protocol by Institutional Review Board of the Korea Center for Disease Prevention (P01-201409-ES-01).
Retinal organoids were generated as described with some modification (7). Briefly, hESCs were dissociated into single cell using Gentle Cell Dissociation Reagent (ST07174; STEMCELL Technologies), and 1×104 cells were seeded per well of V-bottom 96-well plate in mTeSR1 supplemented with 10 μM Y-27632 (1293823; Biogems), designated day 0. On day 1, 50 μl of gfCDM media was added, comprised of 41% Iscove’s Modified Dulbecco’s Medium (12440053; Life Technologies), 41% Ham’s F-12 Nutrient Mixture (11765054; Life Technologies), 10% KnockOut Serum Replacement (10828028; Life Technologies), 1% GlutaMAX Supplement (35050038; Life Technologies), 1% Chemically Defined Lipid Concentrate (11905031; Life Technologies), 450 μM 1-Thioglycerol (M6145; Sigma-Aldrich) and 1% Penicillin-Streptomycin (15140122; Life Technologies). 100 μl of gfCDM media was replaced in every other day. The media was supplemented with 1.5 nM BMP4 (120-05ET; PeproTech) on day 7, and this supplemented BMP4 was serially diluted with half media changes every two days. To further neural retina differentiation, the medium was changed to neural retina induction media, comprised of DMEM/F12 (11320-033; Life Technologies), 10% Fetal Bovine Serum (16000-044; Life Technologies), 1% N2 (17502048; Life Technologies), 1% GlutaMAX Supplement, and 1% Penicillin-Streptomycin with 0.5 μM Retinoic Acid (R2625; Sigma Aldrich) and 100 μM Taurine (T8691; Sigma Aldrich) from day 18. The media was changed every other days.
Reporter transgenes of CAG-mCherry were cloned between AAV inverted terminal repeat sequence. The plasmid construction was generated using KOD Multi & Epi DNA polymerase (KME-101; TOYOBO). The mCherry CDS was amplified from pCMV-lox-mCherry-lox (Macrogen) and subcloned into the pscAAV-CAG-RLuc backbone (Cat #83280; Addgene) using HiFi DNA Assembly Master Mix (E2621; New England Biolabs) to create pscAAV-CAG-mCherry. The AAV2 and AAV8 viruses carrying the mCherry CDS were produced by VectorBuilder using a conventional tri-transfection method.
Following 120 days of differentiation, retinal organoid were treated with 1×1010 viral genome of AAV2 and AAV8 in 100 μl medial volume. The half of media was changed at every 2 days. Live image were acquired at regular intervals using Cytation imaging system. Retinal organoids were harvested at 30 days post-transduction.
Total RNA were extracted by TRIzol Reagent (15996018; Life Technologies), and cDNA was synthesized using a PrimeScript RT Master MIX (RR036A; Takara) according to the manufacturer’s instruction. The quantitative real time PCR were performed using a 7500 Fast Real-Time PCR System (Applied Biosystems).
The retinal organoids were fixed with 4% paraforamaldehyde for 1 hour in room temperature. For dehydration, the retinal organoids were incubated in 15% sucrose solution in phosphate buffered saline (PBS) for 1 hour and 30% for 1 day until retinal organoid sink. The dehydrated retinal organoids were embedded in OCT compound and frozen at −80℃. The embedded tissue was sectioned at 10 μm in optimal cutting temperature. For immunostaining, slide were washed three times with deionized water to remove OCT compound, before a blocking solution (3% BSA, 0.1% Tritone-X100 in PBS) was applied for 1 hour at room temperature. After blocking, samples were incubated with the primary antibody at 4℃ and the secondary antibody with Hoechst for 1 hour at room temperature. Finally, slides were rinsed 3 times with PBS-T and air dried for 30 minutes before mount solution and cover slip were applied. Fluorescence images were acquired using LSM800 confocal microscope (Carl Zeiss) and Cytation imaging system.
The statistical significance of any differences among three groups and between two groups was determined using one-way ANOVA with multiple comparisons and Student’s t-tests (two-tailed), respectively. Significance was set at *p<0.05, **p<0.01, and ***p<0.001. The error bars represent the mean±SD.
The efficiency of AAV transduction in retinal organoids was tested using a stepwise process involving the generation of human retinal organoids from pluripotent stem cells, followed by live monitoring and tropism analyses.
As a drug test platform, maintaining consistency in the quality of generated retinal organoids is crucial for obtaining accurate test results. To ensure this consistency, we adopted a one-step approach for generating retinal organoids, covering embryoid body formation to neural retinal differentiation. This method, based on a previously reported protocol (7) with slight modifications, was chosen to minimize retinal organoid variation. Differentiation into retinal organoids was performed using a guided neural retinal differentiation protocol (Fig. 1A, 1B), and characterized by immunostaining for retinal progenitor cells and proliferation markers (Fig. 1C). Four weeks after differentiation, we observed that the visual system homeobox2 (VSX2) was highly expressed, indicating that retinal progenitor cells were differentiated into retinal organoids (12). Furthermore, to validate the cell type composition in early differentiated retinal organoids, the relative mRNA expression of cell type-specific genes was analyzed. Consistent with the immunostaining analysis, retinal progenitor cell markers, such as PAX6 and VSX2, increased in a time-dependent manner until 60 days of differentiation (Fig. 1D). Furthermore, the expression of ganglion cell markers (Fig. 1E) and neural retinal cell markers (Fig. 1F) gradually increased with differentiation, indicating that different neural retinal cell types differentiated in the retinal organoids.
Retinal organoids have emerged as a potential model for retinal degeneration due to successful generation of photoreceptors through in vitro maturation. In this context, the process of generating retinal organoids can be categorized based on maturation stages, with a comparison to human retinal development stages (9, 13). Transcriptome and epigenetic analyses have revealed that retinal organoids typically reach a post-mitotic state with immature photoreceptors within 120∼200 days of differentiation. Therefore, AAV transduction efficiency in retinal organoids has been assessed at various stages of maturation, including D42 (14), D140 (15), and D200 (16) depending on the specific target cell type for transgene delivery by AAV. To optimize our experiments and maximize efficiency of transgene delivery, we tested AAVs in 120-day-old retinal organoids. Before validating the application of AAV-based gene delivery, we performed in-depth characterization of mature retinal organoids on day 120. Retinal organoids are composed of multiple retinal cell types, ATOH7 for retinal ganglion cells (Fig. 2A) and RHO, OPN1SW, and RPE65 genes for photoreceptor cells (Fig. 2B), and thus exhibit a higher expression of marker genes for each retinal cell type. At the time point D120, retinal organoids exhibited distinct layers, including three nuclear layers (outer nuclear layer [ONL], inner nuclear layer [INL], and ganglion cell layer [GCL]), separated by two synaptic layers (outer plexiform layer [OPL] and inner plexiform layer [IPL]) (Fig. 2C). Additionally, the ONL at the apical edge of the retinal organoid was populated with photoreceptor cells labeled with RCVRN. The INL consisted of CRALBP-positive Müller glial cells, which extended radially throughout the retinal organoid (Fig. 2D). Particularly, retinal organoids have been shown to contain different photoreceptor subtypes, including rods and cones, with the expression of rhodopsin in rods and short-wavelength cone opsin (Blue Opsin) in cons (Fig. 2E). In summary, these results demonstrate that the human retinal organoids generated in this study closely resemble the structural morphology and cell-type composition of the human retina. Thus, the retinal organoids generated herein are a suitable in vitro model for testing the efficacy of AAV-based gene therapeutics, providing a streamlined and relevant platform for preclinical studies.
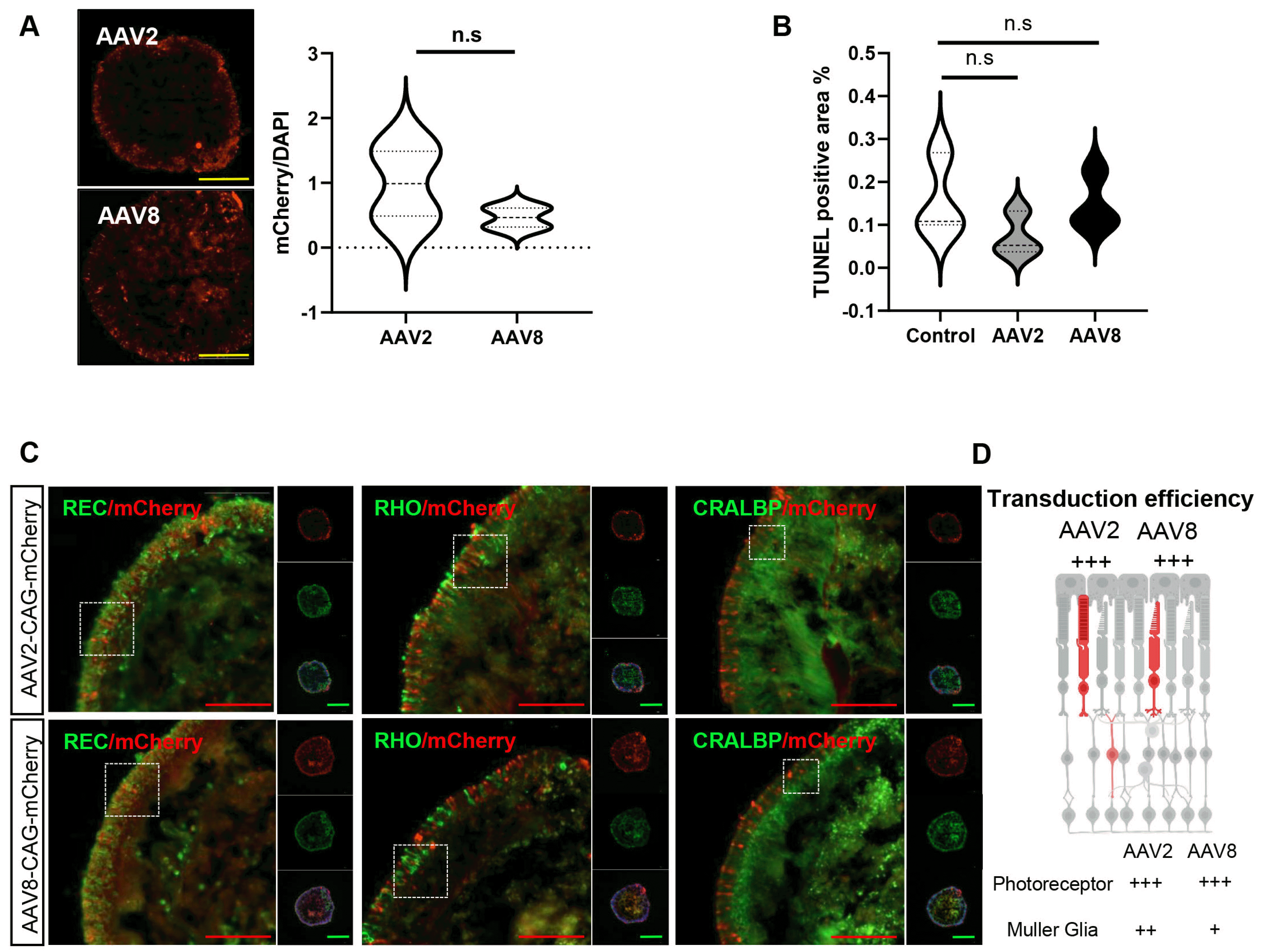
To monitor the AAV transduction efficiency with different AAV serotypes, human retinal organoids derived from the same differentiation batch were exposed to AAV2 and AAV8, which are known to be capable of transduction in the retina (17, 18), at day 120 post-differentiation. In previous reports, AAV efficiently transduced retinal organoids, with a dose dependency ranging from 108 to 1011 vg per organoid (14, 16, 19). Based on preliminary results, wherein 1×1010 genome copies of AAV8 transduced the retinal organoid at a higher success rate compared to 1×109 genome copies, we administered 1×1010 genome copies of each AAV to individual retinal organoids in subsequent experiments (Fig. 3A). Additionally, to exclude any adverse effects resulting from the persistence of AAV infection, the remaining AAV was removed through a series of media dilutions. The transduction efficiency was monitored in live retinal organoids over day 30 post-transduction (Fig. 3B). Reporter gene expression was first observed at day 10 post-transduction and gradually increased until day 30 post-transduction (Fig. 3C). Particularly, at day 30 post-transduction, the retinal epithelia exhibited stable mCherry signal levels without any noticeable morphological changes (Fig. 3D). These results indicated that the AAV2 and AAV8 serotypes successfully transduced organoids without causing significant toxicity or damage.
After live-cell monitoring, the AAV2 and AAV8 tropisms in retinal organoids were analyzed. We found that both AAV2 and AAV8 serotype-transduced retinal organoids were at comparable levels. Transduction efficiency was quantified using the mCherry-positive area normalized to the total cell nuclear area (DAPI), and the resulting mCherry/DAPI pixel ratio was used to assess the transduction efficiency. The quantified mCherry-positive areas were not significantly different between the two groups (Fig. 4A). The TUNEL assay confirmed that neither AAV serotype treatment significantly affected retinal organoid viability (Fig. 4B). Furthermore, reporter mCherry signals were detected across various retinal cell types in retinal organoids day 30 after transduction. Strong mCherry signals were detected in photoreceptor cells (RCVRN-positive), including rod cells (rhodopsin-positive). mCherry-labeled rod cells showed photoreceptor morphology with protruding inner photoreceptor segments in retinal organoids. We also detected mCherry-positive cells within the inner retinal epithelium that co-stained with CRALBP-positive Müller glial cells in the retinal organoids (Fig. 4C). In comparison, we found that AAV2 transduced Müller glial cells more efficiently in human retinal organoids compared to AAV8. Taken together, AAV2 and AAV8 efficiently transduced the major photoreceptor and Müller glial cell types in retinal organoids without affecting cell viability (Fig. 4D).
Recent findings have demonstrated the potential of gene therapy based on AAV for treating congenital retinal disorders. The ongoing development of gene replacement therapy for retinal disease such as LCA and XLRS relies on AAV vectors, specifically AAV2 and AAV8, both renowned for their efficient transduction capabilities in the retina. To determine the optimal dosage threshold and retinal cell type specificity of AAV2 and AAV8, we conducted a comprehensive qualitative and quantitative analysis of transgene expression in retinal organoids using these AAV serotypes. The retinal organoids generated in this study resembled human retinal morphology and contained diverse retinal cell types, including rods, con photoreceptor cells, and Müller glial cells. We utilized these retinal organoids to assess the transduction efficiency of AAV2 and AAV8, employing the mCherry reporter gene and the CAG promoter. Our data indicated that both AAV2 and AAV8 were efficiently transduced in the retinal organoid without causing neurotoxicity. Although, the relative efficiency of rod and con photoreceptor cell transduction by AAV2 was similar to that of AAV8, Müller glial cell transduction was higher for AAV2 than AAV8. In light of these results, we propose that retinal organoids derived from hPSCs serve as a suitable efficacy assay platform with cell type specificity and toxicity test in the context of gene therapy for retinal disorders.
Various studies have been conducted to increase the efficacy of gene therapy, such as AAV capsid modification (20), transgene promoter modification (21), and ITR modification (22), and retinal organoids will play an important role in the initial screening platform. This can complement animal models by bridging the gap between basic research and translational medicine for developing effective gene therapies to treat inherited retinal dystrophies.
As a suitable model for drug testing, comparable to in vivo animal models, the retinal organoid system can provide complex human tissue with high quality, quantity, and even the same genetic background. Previous studies have shown that hPSC-derived retinal organoids mimic the cellular composition with a resembled orientation to the human retina and transcriptome of the fetal retina (8, 9). Moreover, these organoids contain con photoreceptor cells, which possess intrinsic light sensitivity comparable to that of the adult primate fovea (10), indicating that retinal organoids not only replicate cellular composition but also achieve physiological maturation. These findings indicate that hPSC-derived retinal organoids can be applied not only for toxicity and transduction testing but also for evaluating therapeutic efficacy using proper disease models with CRISPR-Cas9 and gene editing technology, thereby minimizing the need for in vivo animal models and improving the success rate by overcoming species differences.
Another crucial aspect that is currently unclear is whether the in vitro AAV treatment approach can replicate the physiological relevance of clinical injection methods. AAV-based gene therapy drugs are typically administered via intravitreal injection in clinical practice, which is the opposite of delivery in retinal organoid models in vitro. Furthermore, it is important to note that the retinal organoid used in this study consisted only of neural retinal cell types, without the presence of the retinal pigment epithelium (RPE). This poses a crucial limitation to the analysis of AAV tropism, which can be addressed using various treatment methods or more intricate models. Efforts to surmount these limitations in retinal organoids have led to the development of retinal organoid-RPE assembloids (23) and human stem cell based retinas on a chip for testing AAV transduction (19). However, challenges persist, particularly concerning tropism and the interaction between RPE and neural retina.
Notes
Authors’ Contribution
Conceptualization: OSK, KSC. Data curation: HJN. Formal analysis: HJN, JEK. Funding acquisition: OSK, KSC. Investigation: HJN, JEK. Methodology: HJN. Project administration: OSK, KSC. Resources: OSK, KSC. Software: SHK. Supervision: SHK, JA. Validation: HJN, JEK. Visualization: OSK. Writing – original draft: OSK. Writing – review and editing: OSK, KSC.
References
1. Cideciyan AV, Hauswirth WW, Aleman TS, et al. 2009; Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 20:999–1004. DOI: 10.1089/hum.2009.086. PMID: 19583479. PMCID: PMC2829287.

2. Cukras C, Wiley HE, Jeffrey BG, et al. 2018; Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 26:2282–2294. DOI: 10.1016/j.ymthe.2018.05.025. PMID: 30196853. PMCID: PMC6127971.

3. Meng D, Ragi SD, Tsang SH. 2022; Therapy in rhodopsin-mediated autosomal dominant retinitis pigmentosa. Mol Ther. 30:2633. Erratum for: Mol Ther 2020;28:2139-2149. DOI: 10.1016/j.ymthe.2020.08.012. PMID: 32882181. PMCID: PMC7545001.

4. Wang D, Tai PWL, Gao G. 2019; Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 18:358–378. DOI: 10.1038/s41573-019-0012-9. PMID: 30710128. PMCID: PMC6927556.

5. Nakano T, Ando S, Takata N, et al. 2012; Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10:771–785. DOI: 10.1016/j.stem.2012.05.009. PMID: 22704518.

6. Zhong X, Gutierrez C, Xue T, et al. 2014; Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 5:4047. DOI: 10.1038/ncomms5047. PMID: 24915161. PMCID: PMC4370190.

7. Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, Sasai Y. 2015; Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun. 6:6286. DOI: 10.1038/ncomms7286. PMID: 25695148.

8. Cowan CS, Renner M, De Gennaro M, et al. 2020; Cell types of the human retina and its organoids at single-cell resolution. Cell. 182:1623–1640.e34. DOI: 10.1016/j.cell.2020.08.013. PMID: 32946783. PMCID: PMC7505495.

9. Finkbeiner C, Ortuño-Lizarán I, Sridhar A, Hooper M, Petter S, Reh TA. 2022; Single-cell ATAC-seq of fetal human retina and stem-cell-derived retinal organoids shows changing chromatin landscapes during cell fate acquisition. Cell Rep. 38:110294. DOI: 10.1016/j.celrep.2021.110294. PMID: 35081356.

10. Saha A, Capowski E, Fernandez Zepeda MA, Nelson EC, Gamm DM, Sinha R. 2022; Cone photoreceptors in human stem cell-derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell. 29:460–471.e3. Erratum in: Cell Stem Cell 2022;29:487-489. DOI: 10.1016/j.stem.2022.02.003. PMID: 35245468. PMCID: PMC9119348.

11. Kwon OS, Na HJ, Ahn J, Chung KS. 2022; Establishment of a human induced pluripotent stem cell line, KRIBBi006-A, from peripheral blood mononuclear cells derived from a healthy male donor. Stem Cell Res. 65:102950. DOI: 10.1016/j.scr.2022.102950. PMID: 36283271.

12. Zou C, Levine EM. 2012; Vsx2 controls eye organogenesis and retinal progenitor identity via homeodomain and non-homeodomain residues required for high affinity DNA binding. PLoS Genet. 8:e1002924. DOI: 10.1371/journal.pgen.1002924. PMID: 23028343. PMCID: PMC3447932.

13. Xie H, Zhang W, Zhang M, et al. 2020; Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Sci Adv. 6:eaay5247. DOI: 10.1126/sciadv.aay5247. PMID: 32083182. PMCID: PMC7007246.

14. Garita-Hernandez M, Routet F, Guibbal L, et al. 2020; AAV-mediated gene delivery to 3D retinal organoids derived from human induced pluripotent stem cells. Int J Mol Sci. 21:994. DOI: 10.3390/ijms21030994. PMID: 32028585. PMCID: PMC7036814.

15. Lane A, Jovanovic K, Shortall C, et al. 2020; Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Reports. 15:67–79. DOI: 10.1016/j.stemcr.2020.05.007. PMID: 32531192. PMCID: PMC7363745.

16. Völkner M, Pavlou M, Büning H, Michalakis S, Karl MO. 2021; Optimized adeno-associated virus vectors for efficient transduction of human retinal organoids. Hum Gene Ther. 32:694–706. DOI: 10.1089/hum.2020.321.

17. Vandenberghe LH, Bell P, Maguire AM, et al. 2011; Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 3:88ra54. Erratum in: Sci Transl Med 2011;3:112er9. DOI: 10.1126/scitranslmed.3002103. PMID: 21697530. PMCID: PMC5027886.

18. Surace EM, Auricchio A. 2008; Versatility of AAV vectors for retinal gene transfer. Vision Res. 48:353–359. DOI: 10.1016/j.visres.2007.07.027. PMID: 17923143.

19. Achberger K, Cipriano M, Düchs MJ, et al. 2021; Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors. Stem Cell Reports. 16:2242–2256. DOI: 10.1016/j.stemcr.2021.08.008. PMID: 34525384. PMCID: PMC8452599.

20. Byrne LC, Day TP, Visel M, et al. 2020; In vivo-directed evolution of adeno-associated virus in the primate retina. JCI Insight. 5:e135112. DOI: 10.1172/jci.insight.135112. PMID: 32271719. PMCID: PMC7259523.

21. Cazier AP, Blazeck J. 2021; Advances in promoter engineering: novel applications and predefined transcriptional control. Biotechnol J. 16:e2100239. DOI: 10.1002/biot.202100239. PMID: 34351706.

22. Pan X, Yue Y, Boftsi M, et al. 2022; Rational engineering of a functional CpG-free ITR for AAV gene therapy. Gene Ther. 29:333–345. DOI: 10.1038/s41434-021-00296-0. PMID: 34611321. PMCID: PMC8983793.

23. Fligor CM, Lavekar SS, Harkin J, et al. 2021; Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Reports. 16:2228–2241. DOI: 10.1016/j.stemcr.2021.05.009. PMID: 34115986. PMCID: PMC8452489.

Fig. 1
Generation of retinal organoid from human embryonic stem cell (hESC). (A) Schematic presentation of the protocol for generating hESC derived retinal organoids (4×). (B) Bright field images of retinal organoid at differentiation day 30. Scale bars=200 μm. (C) Immunofluorescence analysis of retinal cell marker (visual system homeobox2, VSX2) in retinal organoid at differentiate day 30. Yellow scale bars=100 μm. Red scale bars=20 μm. (D-F) Relative expression of retinal cell differentiation marker genes in retinal organoid at each differentiation day.
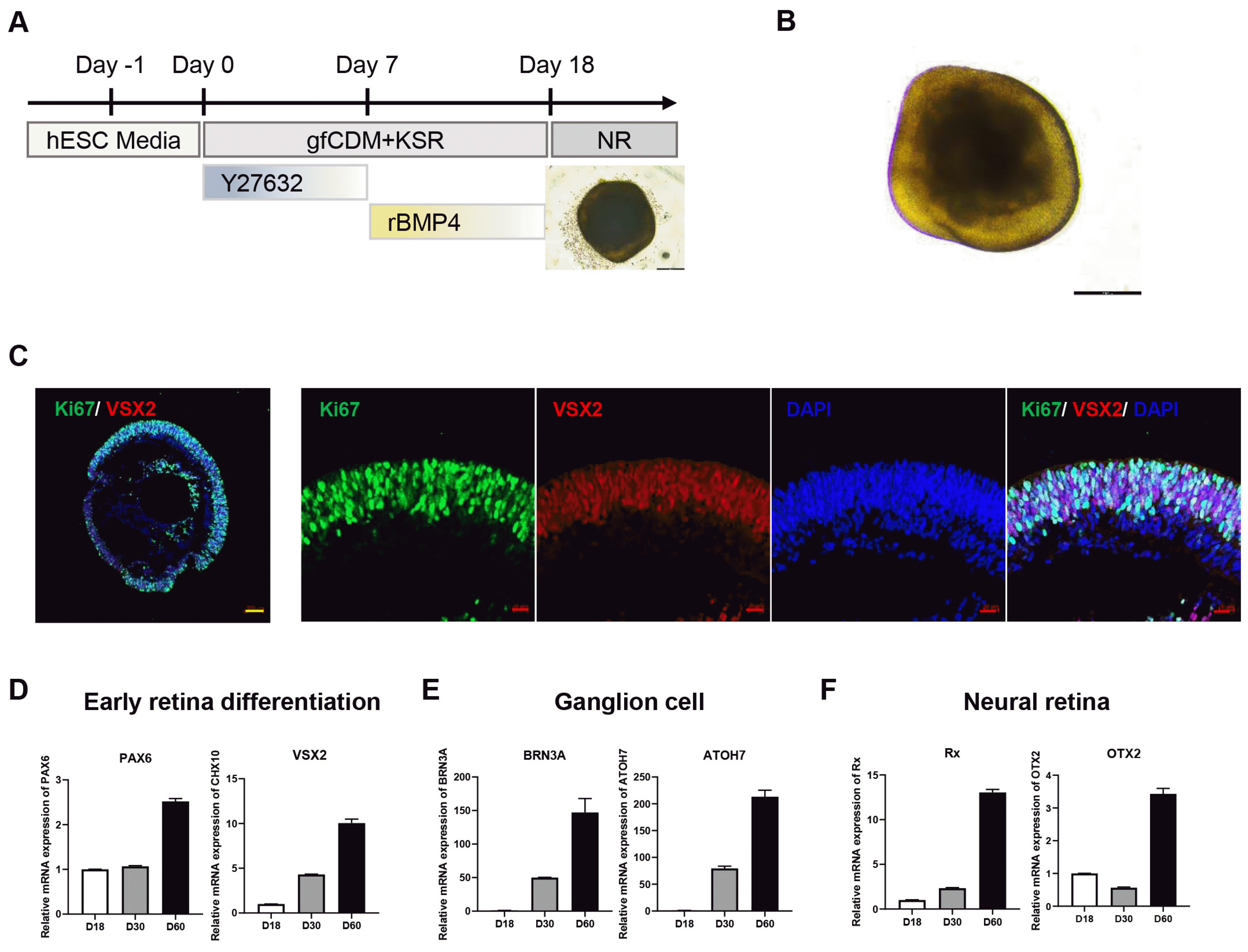
Fig. 2
Characterization of matured retinal organoid. (A) Representative retinal organoid image at differentiate day 120. (B) Relative mRNA expression of retinal cell marker genes in matured retinal organoid. (C) Nuclear staining (Hoe, blue) indicating retinal organoid layering (ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, IPL: inner plexiform layer, GCL: ganglion cell layer with 40× resolution). (D, E) Immunofluorescence analysis of photoreceptor (RCVRN, Blue Opsin, and RHO) and Müller glial cell (CRALBP) markers in matured retinal organoid. Set the upper panel resolution to 10× and the lower panel resolution to 40×. Yellow scale bars=300 μm. Red scale bars=100 μm. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001 using one-way ANOVA.
Fig. 3
Live monitoring of adeno-associated virus (AAV) transduced retinal organoids. (A) Schematic illustration of experimental design (created with BioRender.com). (B) Live cell imaging (4× magnification) of mCherry reporter gene expression in retinal organoid transduced with AAV2 and AAV8. Yellow scale bars=1,000 μm. (C) Quantification of mCherry intensity in indicated time point. (D) Live image (20× magnification) of mCherry intensity after AAV2 and AAV8 transduction in retinal organoid. Red scale bars=200 μm. hPSC: human pluripotent stem cell, hESC: human embryonic stem cell.
Fig. 4
Immunostaining analysis of transduction efficiency of adeno-associated virus (AAV)2 and AAV8 in retinal organoids. (A) Representative image of mCherry expression (left, 10×) and quantification of mCherry expression in retinal organoid (right), mCherrypositive areas were normalized to DAPI areas (cell nuclei). Yellow scale bars=200 μm. (B) Quantification of TUNEL positive cell population after AAV treatment. (C) Immunofluorescence analysis of retinal cell marker with mCherry reporter gene expression in AAV2 and AAV8 treated retinal organoid utilizing a 40× resolution. Red scale bars=100 μm. Green scale bars=300 μm. (D) Graphical summary of mCherry expressing cell type in humanretinal organoid with transduction efficiency. This figure was generated using BiorRender. n.s: not significant.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download