Abstract
To address the limitations of animal testing, scientific research is increasingly focused on developing alternative testing methods. These alternative tests utilize cells or tissues derived from animals or humans for in vitro testing, as well as artificial tissues and organoids. In western countries, animal testing for cosmetics has been banned, leading to the adoption of artificial skin for toxicity evaluation, such as skin corrosion and irritation assessments. Standard guidelines for skin organoid technology becomes necessary to ensure consistent data and evaluation in replacing animal testing with in vitro methods. These guidelines encompass aspects such as cell sourcing, culture techniques, quality requirements and assessment, storage and preservation, and organoid-based assays.
Recently, the need to develop alternative animal testing methods that overcome the limitations of animal testing is increasing. Alternative test methods include organoids which is defined as three-dimensional (3D) cell aggregates that self-organize is uprising technology because they reflect human and disease biology more accurately than animal models and can predict efficacy and toxicity in the human body with high reliability (1-3). In Europe, animal testing for cosmetics has been banned since 2004, and a lot of investment has been made in the development of alternative animal testing methods (4). Based on this background, alternative testing methods using artificial skins for safety evaluation are the first to be developed (5) and put into practice. Currently, a human skin model created by 3D culture of human-derived skin cells has been commercialized and is being used in internationally certified tests to evaluate local toxicity such as skin corrosion/irritation (OECD TG 431, TG 439, TG 498, ISO 10993-23) (6-9).
The human skin model currently being used in certified testing is composed only of skin keratinocytes and has a skin barrier function, so its main function is a skin barrier to prevent the rapid penetration of toxic substances which can evaluate the local skin toxicity (10). Due to the absence of other cell types which compose human skin and accessory organs, there are some limitations in evaluating the efficacy or other safety of products.
In order to overcome the limitations of existing established technologies or to establish alternative animal testing methods for undeveloped areas, it is necessary to continue developing testing methods using the latest skin organoid technology (11, 12), which is gradually increasing in similarity to human skin. Additionally, in order to put these new technologies into practical use, it is needed to establish standard guidelines for skin organoid technology to enable consistent data and evaluation even when skin organoids of various purposes and shapes are used (13).
This guideline sets the scope and standards with reference to the latest scientific technology that is widely known to date, and the contents of this guideline can be modified depending on the level of future scientific and technological development. The purpose of this guideline is to present general standards for skin organoids and test methods using skin organoids that developers or efficacy evaluators of test methods using skin organoids can refer to.
Skin organoids are generated from adult stem cells or pluripotent stem cells and are expected to possess the complexity and function of natural skin. Depending on the purpose, two or more types of stem cells can be combined to produce skin organoids that are more closer to natural human skin (18).
General recommendations: When producing skin organoids by obtaining adult stem cells or pluripotent stem cells from human tissue, a donor compatibility test must be performed when necessary, and documents enabling traceability of the origin cells must be prepared and stored (20). To commercialize skin organoids using induced pluripotent stem cells (iPSCs), the license corresponding to the necessary manufacturing technology through legal procedures must be secured, just as other cell types.
It is important to maintain consistent characteristics and quality of skin organoids used for efficacy or toxicity evaluation, and from this perspective, culture, storage, test items, and standards for origin cells must be established to manage origin cells (21).
Quality control recommendations: In order to maintain consistent characteristics and quality, genetic stability testing (e.g., copy number variation [CNV], single nucleotide polymorphism [SNP] analysis) is required when cells are manufactured through repeated subculture. If there is a possibility that other cells may be mixed in during the cell isolation, culture, and differentiation process, genetic analysis (e.g., short tandem repeat [STR] analysis) that can prove the origin cells are required. Meanwhile, it is necessary to perform basic sterility test, adventitious virus test, and mycoplasma test to ensure stable quality. Additionally, in order to produce skin organoids of consistent quality, each investigator needs to conduct research by establishing standard operate procedures (SOPs) related to culture and storage (22-24).
Cell types and characteristics:
a.Organoids derived from adult stem cells
1)Cell types and characteristics
Skin organoids can be produced using a variety of adult stem cells, such as epidermal stem cells, mesenchymal stem cells, melanocyte stem cells, and hematopoietic stem cells (18, 25). Additionally, depending on the purpose and methods, it can be produced by assembling various stem cells and differentiated cells such as keratinocytes, fibroblasts, immune cells or melanocytes (26), which are induced from a different lineage. When producing a skin organoid by assembling various types of cells, cell-specific markers for each origin cells are needed to be identified before using them to produce skin organoids (27).
2)Quality requirements for cells
When directly collecting human-derived cells, if it is deemed necessary to produce skin organoids with stable quality, a donor compatibility test should be established and conducted (22-24).
Set up a confirmation test using positive markers expressed in the cells used, and set purity test items if there are cells or tissues that may be mixed during the cell separation and culture process and may interfere with the production of consistent skin organoids. If there is a possibility that cells other than the origin cells may be mixed during the culture process, it may affect the quality of skin organoids, so test method to confirm the origin cells (e.g., STR) needs to be set and performed. Additionally, genetic stability testing (e.g., CNV or SNP) is performed to evaluate whether there are any genetic mutations that may affect the quality of skin organoids before use in production. It is important to maintain consistent characteristics and quality of skin organoids used for efficacy or toxicity evaluation, and from this perspective, sterility testing, adventitious virus rejection testing, and testing for basic contaminants such as mycoplasma should be conducted.
If human-derived cells are not directly collected, cells that are confirmed by certificate of analysis (COA) based on quality test results must be used to manufacture skin organoids.
3)Cell suppliers
If a researcher or developer directly secures the origin cells, obtain human cells or tissues according to the legal procedures for research using human derivatives, isolate/cultivate the cells directly through primary culture, and confirm the cell characteristics and quality.
Alternatively, cells can be supplied through an organization that can issue a COA that can confirm cell characteristics and quality.
b.Organoids derived from pluripotent stem cells (ESCs and iPSCs)
1)Cell types and characteristics
Skin organoids using pluripotent stem cells can be produced using embryonic stem cells (ESCs) and iPSCs. The characteristics of pluripotent stem cells can be confirmed by the positive markers of pluripotent stem cell (e.g., octamer-binding transcription factor 4 [OCT4], SRY [sex determining region Y]-box 2 [SOX2], NANOG, etc.), negative markers representing starting materials (e.g., PBMC-CD34, etc.), proliferation ability, embryoid body (EB) formation ability, three germ layer differentiation ability, and teratoma formation ability. To confirm pluripotency, it can be confirmed by in vitro tests through EB formation ability and tripartite differentiation ability, and by in vivo tests through teratoma assay. In the case of the three germ layer differentiation ability test, it can be confirmed through immunofluorescence staining and gene expression after differentiation into ectoderm (OTX2), mesoderm (Brachyury), and endoderm (SOX17). In the case of teratoma assay, a certain amount of pluripotent stem cells is implanted into the back, genitals, and muscles of an immunocompromised mouse and observed for a certain period of time. As for the size of the teratoma, a lump can be felt at the injection site starting from the 30th day, and the subject is sacrificed within 3 months to perform histopathology. The structures of the ectoderm, mesoderm, and endoderm must be confirmed through histopathology.
2)Quality requirements for cells
When directly collecting human-derived cells, if it is deemed necessary to produce skin organoids with stable quality, a donor compatibility test should be established and conducted.
When producing iPSCs using human cells, items and standards must be set to confirm that there are no characteristics and contaminants for the starting cells used for the iPSCs.
After establishing pluripotent stem cells, set a confirmation test using positive markers expressed in the cells used, and set purity test items if there are cells or tissues that may be mixed during the cell separation and culture process and may interfere with the production of consistent skin organoids. Skin organoids derived from pluripotent stem cells also require the test method (e.g., STR) to confirm the origin cells. Additionally, genetic stability testing (e.g., CNV or SNP) should be performed to assess for genetic mutations. Furthermore, sterility testing, adventitious virus rejection testing, and testing for basic contaminants such as mycoplasma should be conducted. These tests can help maintain the characteristics and quality of skin organoids consistently and enhance the reliability of toxicity and efficacy evaluations.
If human-derived cells are not directly collected, cells that are confirmed by COA based on quality test results must be used to manufacture skin organoids.
3)Cell suppliers
If a researcher or developer directly secures the origin cells, obtain human cells or tissues according to the legal procedures for research using human derivatives, isolate/cultivate the cells directly through primary culture, and confirm the cell characteristics and quality.
Alternatively, cells can be supplied through an organization that can issue a COA that can confirm cell characteristics and quality.
Organoids derived from pluripotent stem cells:
a.Essential elements
When producing skin organoids using pluripotent stem cells, various protocols can be applied depending on the components required, but the following conditions can be referred to as examples (28).
1)Media components
As an example, when producing skin organoids after differentiating fibroblasts and keratinocytes from pluripotent stem cells, the following media conditions can be used.
Fibroblast differentiation media (FDM)
FDM1
∙Dulbecco’s modified Eagle’s medium (DMEM)/F12 (3:1) (cat. no. 11965118; Gibco)
∙Fetal bovine serum (cat. no. 10099141; Gibco)
∙Adenine (cat. no. A2786; Sigma-Aldrich)
∙Insulin, human recombinant, zinc solution (cat. no. 12585014; Gibco)
FDM2
∙DMEM/F12 (1:1) (cat. no. 11330032; Gibco)
∙Fetal bovine serum
∙MEM Non-Essential Amino Acid Solution (cat. no. 11140050; Gibco)
Keratinocyte differentiation media (KDM)
KDM1
∙DMEM/F12 (3:1)
∙Fetal bovine serum
∙L-Ascorbic acid (cat. no. A5960; Sigma-Aldrich)
∙Insulin, human recombinant, zinc solution
KDM2
∙Defined Keratinocyte serum free medium (SFM) (cat. no. 10744019; Gibco)
∙L-Ascorbic acid
∙Insulin, human recombinant, zinc solution
∙Adenine
KDM3
∙Defined Keratinocyte SFM+Keratinocyte SFM (1:1) (cat. no. 17005042; Gibco)
As an example, when producing skin organoids containing hair follicles by simulating the skin development process in pluripotent stem cells, the following media conditions can be used.
EB formation
∙Essential 8 Medium (cat. no. A1517001; Gibco)
∙Y-27632 (cat. no. 1293823; BioGems)
Surface ectoderm induction
∙Essential 6 Medium (cat. no. A1516401; Gibco)
∙SB 431542 (cat. no. 1614; Tocris)
Cranial neural crest induction
∙Essential 6 Medium
∙LDN-193189 (BMP inhibitor) (04-0074-10; Stemgent)
Skin maturation
∙Advanced DMEM/F12 50% (cat. no. 12634010; Gibco)
∙Neurobasal Medium 50% (cat. no. 21103049; Gibco)
∙GlutaMAX Supplement 1X (cat. no. 35050061; Gibco)
∙B-27 Supplement, minus vitamin A (cat. no. 12587010; Gibco)
∙N-2 Supplement (cat. no. 17502048; Gibco)
∙2-Mercaptoethanol (cat. no. 21985; Gibco)
2) Growth factors
As an example, when producing skin organoids by differentiating pluripotent stem cells into fibroblasts and keratinocytes, the following essential growth factors are included during the differentiation process
FDM
∙Epidermal growth factor (EGF) (cat. no. AF-100-15; PeproTech)
∙Bone morphogenetic protein 4 (BMP-4) (cat. no. 120-05ET; PeproTech)
KDM
∙Retinoic acid (cat. no. R2625; Sigma-Aldrich)
∙BMP-4
∙EGF
As an example, when producing skin organoids including hair follicles by simulating the skin development process in pluripotent stem cells, the following essential growth factors are included during the differentiation process.
Cranial neural crest induction
∙Basic fibroblast growth factor (bFGF) (cat. no. 100-18B; PeproTech)
Surface ectoderm induction
∙BMP4
∙bFGF
3)Reagents
As an example, when producing skin organoids by differentiating pluripotent stem cells into fibroblasts and keratinocytes, the following reagents are required.
∙Dulbecco’s Phosphate Buffered Saline (DPBS) (cat. no. 14190094; Gibco)
∙TrypLE Express Enzyme (cat. no. 12604013; Gibco)
∙Y-27632 (Rho-associated protein kinase [ROCK] inhibitor)
∙Type I collagen
∙Type IV collagen
∙Transforming growth factor-β (TGF-β) inhibitor
As an example, when producing a skin organoid containing hair follicles by simulating the skin development process in pluripotent stem cells, the following reagents are required.
∙Accutase
∙Y-27632 (ROCK inhibitor)
∙SB 431542 (TGF-β inhibitor)
∙LDN-193189 (BMP inhibitor)
∙2-Mercaptoethanol
b.Culture process and requirements
1)Culture protocols
As an example, the following culture protocol can be used when producing skin organoids by differentiating pluripotent stem cells into fibroblasts and keratinocytes.
[Fibroblast differentiation]
EB generation
Dispense the required amount of iPSCs into a 50 mL conical tube to make 1.0×104 cells/25 μL concentration. Dispense 25 μL into the lid of a 90 mm Petri dish to make 100 EBs. Treat the bottom of the 90 mm Petri dish with DPBS and carefully turn over the lid of the 90 mm Petri dish into which EB was dispensed. Cultivate for 1 day in a 37℃, 5% CO2 incubator.
Fibroblast differentiation
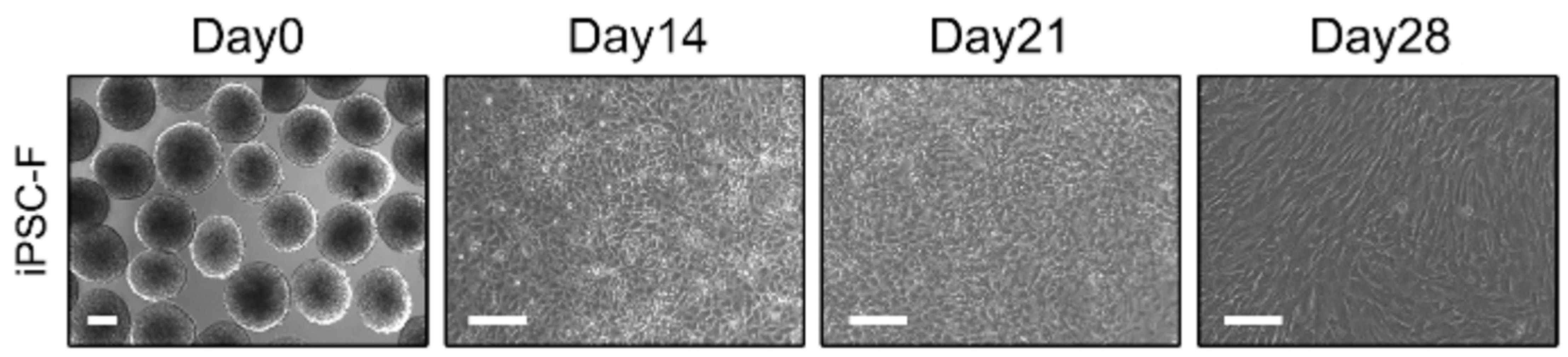
Create a coating dish by treating Matrigel with 20 mL of DMEM/F12 (1:1). One-day-old EBs were placed on a matrigel-coated dish, treated with 100 EBs with FDM1(+EGF) medium, and cultured in a 37℃, 5% CO2 incubator. On the 2nd day, replace the medium with FDM1(+EGF) without ROCK inhibitor. On the 4th day, replace the medium with FDM1(+EGF) containing BMP-4. On the 7th day, change the medium to FDM2 and replace the medium every 2 days. On the 14th day, inoculate the cultured 2×106 cells in a non-coated dish with FDM1(+EGF) medium and replace the medium with FDM1(+EGF) every 2 days. On the 21st day, prepare a coating dish by treating it with type I collagen. Inoculate 2×106 cells with FDM1(+EGF) medium in a type I collagen coated dish and replace the medium with FDM1(+EGF) every 2 days. On day 28, inoculate 2×106 cells in a non-coated dish with FDM1(+EGF) medium and culture in a 5% CO2 incubator at 37℃.
[Keratinocyte differentiation]
EB generation
Proceed in the same way as EB generation of fibroblast differentiation.
Keratinocyte differentiation
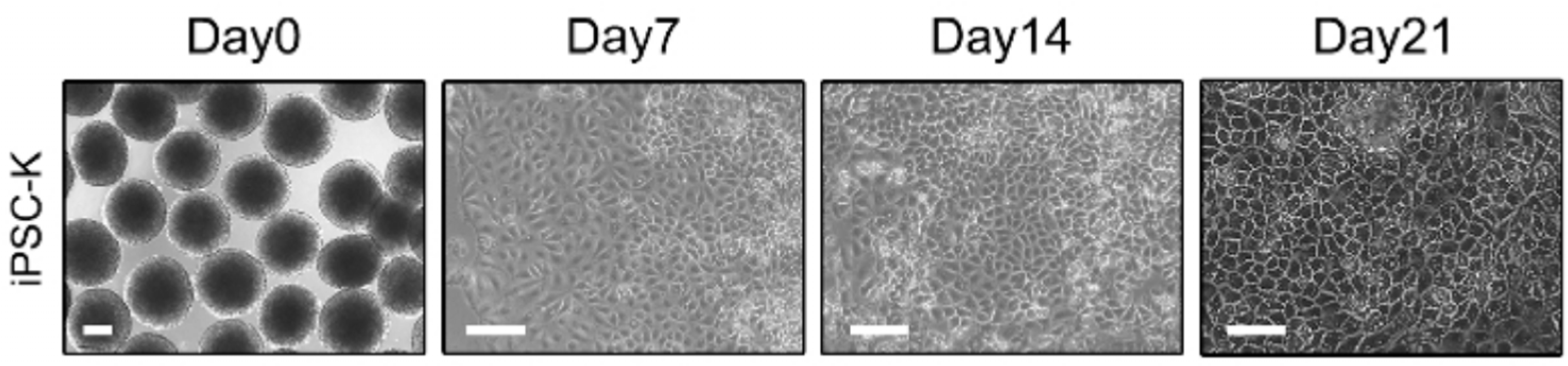
After one day, the EBs are treated with iPSC culture medium containing BMP-4 and seeded in 90 mm Petri dishes. The next day, prepare a coating dish by treating it with type IV collagen. EBs are treated with KDM1(+retinoic acid, BMP-4, EGF) medium containing 10 μM ROCK inhibitor. Treat 100 EBs on a type IV collagen coated dish and incubate in a 37℃, 5% CO2 incubator. On the 2nd day, replace the medium with KDM1(+retinoic acid, BMP-4, EGF) and replace the medium every 2 days. On day 9, change to KDM2(+retinoic acid, BMP-4, EGF) medium and change medium every 2 days. On day 13, change to KDM3(+BMP-4, EGF) medium and change medium every 2 days.
3D skin organoid
Prepare 5 mL of type 1 collagen mixture (PBS, NaOH, dH2O) and store on ice. Add 2×105 iPSC-derived Fibroblasts to 1.5 mL FDM1, mix, and neutralize with type 1 collagen solution (1:1). Place the membrane insert of the transwell plate on a 6-well microplate, transfer the mixture to the insert, and incubate at room temperature for 30 minutes. After gelation is confirmed, add 2 mL of medium to the top of the insert and 3 mL to the bottom of the well. Incubate fibroblasts and collagen matrices at 37℃ in 5% CO2 for 5∼7 days until gelation is complete. After complete gelation, prepare iPSC-derived keratinocyte 1×106 cells. Mix 1×106 cells with 50∼100 μL of low calcium epithelial medium (EP1). All medium from the matrix is aspirated and 1×106 iPSC-derived keratinocytes are seeded on each fibroblast layer. Incubate for 30 minutes at 37℃ and 5% CO2. Add 2 mL of EP1 to the top of the insert and 3 mL of EP1 to the bottom of the well. After 2 days, remove all medium from the membrane insert plate and replace the medium with normal calcium epithelial medium (EP2) for 2 days. After 2 days, remove all medium and add 3 mL of complete cornification medium only to the bottom to create an air-liquid interface. Maintain the 3D skin tissue model at 37℃ in 5% CO2 for up to 14 days, replacing the medium every 2 days. The edges of the insert are cut off to obtain a 3D skin tissue model.
As an example, when producing skin organoids containing hair follicles by simulating the skin development process in pluripotent stem cells, the following culture conditions can be used.
EB formation
Separate hPSC colonies into single cells using accutase, and seed hPSCs at 1 to 3×103/well in a U-bottom low-attachment 96-well plate containing EB formation media.
Surface ectoderm induction
Two days after starting EB formation, one EB per well is transferred to a U-bottom low-attachment 96-well plate containing surface ectoderm induction media containing bFGF and BMP-4, and differentiation begins.
Cranial neural crest induction
Four days after surface ectoderm induction, the media is replaced with cranial neural crest induction media containing bFGF, and culture is performed for approximately 8 days.
Skin organoid maturation
Cranial neural crest induction is performed for approximately 8 days, then replaced with skin maturation media to induce skin organoid formation through self-organi-ation. A clear hair follicle shape is observed between about 75 and 85 days from the start of differentiation, and skin organoids with the structure of the epidermis, dermis, subcutaneous fat, and hair follicles are obtained at about 110 days from the start of differentiation. At the point when hair follicle morphology is observed, skin organoids in a flat form can be obtained through air-liquid interface culture.
As an example, dendritic cells for loading into skin organoids can differentiate under the following conditions.
Differentiation of dendritic cells from PBMC
Using anti-CD14 antibody, monocytes of the myeloid lineage are separated through MACS sorting. Afterwards, monocytes are stimulated with recombinant human interleukin (IL)-4 (250 U/mL) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (1,000 U/mL). After 48 hours, the cells were stimulated again with IL-4 (250 U/mL) and GM-CSF (1,000 U/mL) to obtain immature dendritic cells on day 5 (29, 30).
Differentiation of dendritic cells from iPSCs
When differentiating dendritic cells from iPSCs (ipDCs), differentiation proceeds according to the following steps: (i) progressive differentiation of iPSCs through cells of the hematopoietic lineage and early mesoderm, (ii) regulation of the resulting ipDCs to enhance intrinsic tolerance, and (iii) enrichment of the CD141+ subset.
iPSCs are cultured using mTesR1 medium ssupplemented with GM-CSF, BMP-4, stem cell factor (SCF), and vascular endothelial growth factor (VEGF). Afterwards, the medium is regularly changed every 2 to 3 days using a differentiation medium. BMP-4 is removed on day 5, VEGF is removed on day 14, and SCF is removed on day 19 of culture, leaving only GM-CSF to maintain dendritic cell precursors and immature dendritic cells. IL-4 is added to finally form dendritic cells.
2)Culture environments
The basic culture conditions are 37℃ and 5% CO2 incubator, but in some cases, 3D culture, dynamic culture, air-liquid interface culture and dry condition can be selectively applied.
Quality requirements for organoids:
a.Morphological quality
3D Skin organoid
Skin organoids can have a sheet or sphere shape.
b.Cell composition
The epidermal layer is mainly composed of keratinocytes, and the dermal layer is mainly composed of fibroblasts. Depending on the purpose and use of the skin organoid, it may include adipocytes, melanocytes, hair follicle tissue cells, nerve cells, vascular cells, immune cells, etc. in the subcutaneous fat layer.
c.Organ-specific function
1)Skin barrier function
The most important function of the skin is to protect the human body from external physical, chemical, and biological stimuli by forming a barrier between the external environment and the human body. The skin barrier is made up of tight junctions between keratinocytes located on the outermost side of the epidermis, keratinocyte interstitial lipids, and the granular layer, and has the function of preventing excessive moisture loss and harmful substances from the external environment from entering the body (31). Among Organization for Economic Cooperation and Development test guidelines (OECD TGs), most skin safety evaluation tests (skin irritation, skin corrosion, phototoxicity tests, etc.) are closely related to skin barrier function (32).
2)Skin sensitization reaction
When the skin is exposed to an antigen that causes an immune cell sensitization from the outside, the skin shows a skin sensitization reaction according to four key events (KEs) (33). KE1 is the binding reaction between antigen and skin protein, KE2 is the activation of keratinocytes and secretion of inflammatory cytokines, KE3 is the activation of dermal dendritic cells in the dermal layer and migration to the lymph nodes, and KE4 is the activation of T cells by antigen presented from dendritic cells. This is a reaction in which cells become activated and differentiate into memory T cells. Skin organoids loaded with immune cells, designed to display a skin sensitization response, must be able to display appropriate KE functions depending on the purpose.
3)Skin elasticity
Among the various functions of the skin, it can be considered a representative aesthetic function, and is an item that developers of various products such as cosmetics, health functional foods, and medical devices typically want to evaluate in terms of effectiveness. Skin elasticity is an item that is mainly influenced by both the epidermis and dermis layers, and is mainly related to the matrix protein synthesis ability of the dermis layer (34).
4)Accessory cells and appendage functions
In addition to epidermal cells and dermal cells, melanocytes and immune cells exist within the skin, and sebaceous glands, sweat glands, hair follicles, sensory receptors, and blood vessels exist to perform various roles, and skin organoids must include target cells and appendages for each purpose of use. The cells and appendages of skin organoid must perform the following functions (18).
∙Hair follicle: function to form hair
∙Sebaceous glands: function to synthesize fat components to form the skin fat layer
∙Melanocytes: function to be activated in response to melanocyte formation stimuli such as ultraviolet rays
d.Chromosomal karyotype and identity (STR analysis)
For skin organoids, CNV or SNP analysis is recommended to evaluate for basic chromosomal abnormalities. Additionally, if there is a possibility of mixing of other cells during the process of isolation, culture, and differentiation of the originating cells, STR analysis should be performed on the produced skin organoids (35).
Organoid quality endpoints:
a.Size and number of target cells per size
The size and shape of skin organoids can be manufactured in sheets or spheres of various sizes depending on the purpose. As an example, skin organoids produced by differentiating fibroblasts and keratinocytes can be produced with the following sizes and target cell numbers.
3D skin organoid
6well transwell membrane insert size
Number of target cells required to create 3D skin organoid
∙Fibroblast: 2×105
∙Keratinocyte: 1×106
b.Required target cells and accessory cell composition (cell composition)
Fibroblast
Similar to primary fibroblast, it is flat and elongated, spindle-shaped or star-shaped.
Keratinocyte
It has a polygonal shape similar to primary keratinocyte.
Other accessory cells
Depending on the production method, it may contain adipocytes, melanocytes, hair follicle tissue cells, nerve cells, vascular cells, immune cells, etc.
c.Specific gene expression and functional assay
1)Specific gene expression
After producing skin organoids, the formation of skin cells and tissues can be confirmed through well-known marker proteins or gene expression, and representative cell/tissue-specific markers are as follows.
Epidermal marker
Keratinocyte: OCT4−, Np63+, KRT5+, KRT14+
Skin barrier: Filaggrin
Granular layer: Loricrin
Spinous layer: Cytokeratin 10
Periderm and basal layer, epidermal stem cell: Cytokeratin 15
Basal layer: Cytokeratin 5, Collagen 4
Dermal marker
Fibroblast: OCT4−, Pax6−, Sox1−, CD44+, Col1+, Col3+, Vimentin+
Connective tissue: Collagen 1/3, Vimentin, Fibronectin, Elastin
Hypodermis
Adipocyte, fat tissue: Lipid TOX
Appendages
Hair follicle: Cytokeratin 15
Dermal Papilla/Merkel cells: SOX2
Outer root sheath: Cytokeratin 17
Melanocyte: MelanA
Sebaceous Gl.: Lipid TOX
Neuronal Cells: Tuj-1
2)Functional test
The following test methods can be used for the representative functions of skin organoids, such as skin barrier function, skin sensitization response, skin elasticity, and functions of accessory cells or appendages.
(1)Skin barrier function
The skin barrier function is mainly formed by physical and physiological functions through the stratum corneum and tight junctions between cells and must have sufficient defense ability against the rapid penetration of cytotoxic indicators.
Evaluation of this protective ability is performed by treating skin organoids at specific concentrations and time conditions using toxic standard substances such as Triton X-100. Half maximal effective concentration (EC50, time taken to reduce to 50%) and half maximal inhibitory concentration (IC50, concentration at which cell survival rate decreases to 50% when treated for a specific time) are determined through cell viability evaluation based on MTT assay, and through this, stable skin barrier function can be confirmed. Check whether it appears reproducibly and use it in evaluation tests related to skin barrier function (skin corrosion test, skin irritation test, etc.).
For tests related to skin barrier function, refer to the OECD TG and validation report below:
∙OECD guideline for testing of chemicals 431 (in vitro skin corrosion: human skin model test) (9)
∙OECD guideline for testing of chemicals 439 (in vitro skin irritation: human skin model test) (8)
∙KeraSkinTM SIT validation report (36)
(2)Skin sensitization reaction
Depending on the purpose of evaluating the skin sensitization reaction, the functionality should be evaluated by referring to the OECD TG developed by identifying KEs. If it is difficult to apply the OECD TG test method as is due to the characteristics of skin organoids, a valid test method based on scientific mechanisms can be performed instead of functional evaluation. Depending on the item to check, evaluate functionality by referring to the following OECD TG.
KE1) Binding reaction between skin proteins - OECD TG 442C
KE2) Activation of keratinocytes and secretion of inflammatory cytokines - OECD TG 442D
KE3) Activation of dermal dendritic cells in the dermal layer and migration to lymph nodes - OECD TG 442E
(3)Skin elasticity
A number of efficacy evaluation tests targeting skin tissue have been developed as a form of human study. When evaluating using a human skin model, the evaluation is performed through cell density or the degree of matrix protein formation. Test methods applied to matrix protein evaluation include Picrosirius Red (PSR)/Mason’s trichrome staining, which can evaluate collagen which is a representative connective tissue. In addition, immunostaining is performed for specific cell and matrix protein markers to determine skin elasticity and differences between study groups.
(4)Accessory cells and appendage functions
In addition to epidermal cells and dermal cells, melanocytes and immune cells exist in the skin, and sebaceous glands, sweat glands, hair follicles, sensory receptors, and blood vessels exist to perform various roles. Skin organoids including the necessary cells and appendages can be manufactured according to the purpose, and functionality is needed to be evaluated. Functional testing is performed to ensure that the following elements are confirmed:
∙Hair follicles: Significant increase in hair growth when treated with active substances.
∙Sebaceous glands: Significant changes in fat composition when treated with substances that affect fat formation, such as preventing acne.
∙Melanocytes: Significant increase or decrease in melanocytes due to melanocyte formation stimulants such as ultraviolet rays or treatment with whitening substances.
Methods for each quality evaluation metric:
Characteristics and cell viability: Depending on the purpose of use of skin organoids, standards for size and cell viability evaluation methods should be established and managed. For cell viability, standards should be set to ensure reproducible test results, but the standards should be set in a range of at least 80% or higher.
Confirmation (specific gene expression): Skin organoids may contain various tissue layers and cells depending on the purpose of use and are described in “Specific gene expression and functional assay” section of this document. Referring to the markers for each cell and tissue described in “Specific Gene Expression and Functional Testing,” identify and manage confirmation markers according to the definition of the developed skin organoid. The evaluation method for confirmation markers uses protein or genetic analysis methods such as immunostaining or reverse transcription-polymerase chain reaction (RT-PCR).
Functional test: For toxicity evaluation, skin barrier function must be evaluated, and for efficacy evaluation, appropriate functional tests should be set and performed depending on the purpose. Typically, skin barrier tests related to toxicity evaluation can be performed by referring to the information below:
1) Skin barrier function: Refer to the functional test method mentioned in the “Specific Gene Expression and Functional Testing” section, perform EC50/IC50 evaluation on standard materials, and confirm consistent barrier function. For detailed methods, refer to the OECD TG and validation report below:
∙OECD guideline for testing of chemicals 431 (in vitro skin corrosion: human skin model test) (9)
∙OECD guideline for testing of chemicals 439 (in vitro skin irritation: human skin model test) (8)
∙KeraSkinTM SIT validation report (36)
2) Skin sensitization reaction
Depending on the purpose of evaluating the function for skin sensitization, the functional test for each KE should be evaluated with reference to the following.
KE1) As listed in OECD TG 442C, the haptenation process is evaluated by quantifying the response of the test substance to a synthetic peptide model containing lysine or cysteine. Or, perform an evaluation using a scientifically based test method that can prove the haptenation process.
KE2) As listed in OECD TG 442D, a luciferase assay is performed using the mechanism of activation of antioxidant response element (ARE)-dependent genes by Nrf-2, or a test that can explain the mechanism based on scientific evidence.
KE3) As listed in OECD TG 442E, it can be evaluated that the expression of surface antigen markers such as CD86 or CD54 that can confirm the activity of dendritic cells, or cytokines that can represent the activity of dendritic cells, such as IL-8.
KE4) As listed in OECD TG 429, 442A, 442B, T cell activity is closely related to increased T cell proliferation, so T cell proliferation can be assessed through BrdU assay-based fluorescence activated cell sorting (FACS) or enzyme-linked immunosorbent assay (ELISA) analysis.
3) Skin elasticity: Use PSR/Mason’s trichrome staining or immunostaining for specific matrix proteins to evaluate collagen.
4) Accessory cell/appendage functional test evaluation
∙Hair follicles: Significant changes in hair growth after treatment with active substances are presented through measurements of hair length and hair follicle diameter.
∙Sebaceous glands: Evaluate changes in fat-forming ability of skin organoid tissue through special staining such as Oil Red O staining.
∙Melanocytes: Changes in the number of skin organoid tissues are evaluated through special staining or immunostaining for melanocytes.
Monitoring of quality evaluation results (analysis by batch, cycle, etc.): The stability period of skin organoids may vary depending on culture conditions. For the stability of skin organoids, basic histological evaluation and quality evaluation are conducted by setting two or three test points for the period to be used: the beginning and end point must be included. Stability evaluation is performed using one or more batches, and through this, storage conditions and expiration dates for skin organoids are established to enable stable and consistent test results.
Storage protocol: The freezing and thawing process for skin organoids has not been clearly established to date, but for existing commercialized human skin models, storage and transportation conditions at room temperature have been established. When a storage protocol for skin organoids is developed, the appropriateness of the protocol should be evaluated by considering the following matters and then an appropriate SOP should be established to store and preserve the skin organoids.
Quality assessment factors after storage: Skin organoids can be produced in a variety of ways depending on the purpose, and may be stored during the organoid production process or upon completion of production. When storing skin organoids, quality evaluation should be performed considering the following matters, and storage conditions and periods that can maintain a certain quality should be set and used for tests appropriate for the purpose.
a.Post-thaw viability or post-storage viability
Since the stable survival rate of cells is the most important factor in maintaining the functional properties of skin organoids, a survival rate standard that can represent a certain level of function is set, and the cell survival rate after thawing or storage under specific conditions is determined by that standard.
b.Microorganisms
Considering that trace amounts of microorganisms that were not detected during the manufacturing process of skin organoids may be amplified during the storage process or that external microorganisms may be introduced, sterility tests, adventitious virus and mycoplasma tests should be conducted after storage.
c.Identification of cells
After storage, the identification marker items of the skin organoids are evaluated to confirm whether the basic characteristics of the skin organoids are maintained.
Toxicity test: Skin hazard items designated by OECD or International Organization for Standardization (ISO) include skin corrosion/irritation, skin sensitization, and phototoxicity, and local toxicity evaluations such as skin corrosion/irritation using existing commercialized human skin models are used as internationally certified tests. (OECD TG 431, TG 439, TG 498, ISO 10993-23).
When applying OECD skin hazard evaluation alternation test methods using a new skin organoid, evaluate the characteristics and quality requirements according to this guideline, and then evaluate the existing positve or negative reference material. After evaluating the feasibility of performance, optimizing the test method through preliminary verification studies, and performing main verification of the test method (transferability, proficiency, reproducibility, predictability), accredited certification bodies (OECD, ISO) follow appropriate international review procedures.
Drug efficacy screening and effectiveness evaluation of cosmetic functional ingredients: When using skin organoids for the purpose of efficacy evaluation, it is necessary to use an appropriate skin organoid model that can support the efficacy results or mechanism of action, and sufficient data to support the validity and reliability of the test method depending on the test purpose, and scientific basis must be presented together.
Currently, there are no official guidelines for evaluating the efficacy of pharmaceuticals, and screening is being conducted using self-developed test methods according to the mechanism and indications of each drug. On the other hand, the guidelines for evaluating the effectiveness of cosmetic functional ingredients are published by the regulatory agency on various effectiveness items, focusing on in vitro tests and human application tests, suggesting that skin organoids can be developed and used for these purposes.
Various cell and biochemical instruments, such as CO2 cell culture equipment, tissue slide production equipment, optical microscope, fluorescence microscope, ELISA, PCR, MicroArray, and flow cytometry, can be used in various ways depending on the purpose of use or the toxicity endpoint to be evaluated.
However, it should be noted that calibration, management standards, usage protocols, etc. must be presented in detail so that they can all be applied to good laboratory practice (GLP).
To evaluate a specific toxicity endpoint with the skin organoids produced using a novel method, it is critical to refer to existing test protocols and develop a protocol suitable for skin organoids. The protocol generally consists of stabilization, material handling, cleaning, culture, and final toxicity endpoint evaluation and negative-positive evaluation criteria.
The protocol must be optimized to accurately determine various categories of positive and negative controls, and can be added or deleted to maximize prediction and reproducibility.
The protocol must be clearly described so that it can be passed on to general GLP testing laboratories, and the reasonable range of reference materials and evaluation results for evaluating the proficiency of the protocol must also be presented.
The results of GLP tests should be written and presented according to a standard format.
Test substances and control substances
∙Single composition substance: Information necessary for chemical substance identification, such as IUPAC or CAS name, CAS number, SMILES or InChI code, structural formula, etc.
∙Multi-composition substances, UVCBs (Unknown or Variable composition, Complex reaction products or Biological materials) and mixtures: chemical identity, purity, quantitative occurrence of ingredients, and related physicochemical properties
∙Physical state, pH, volatility, molecular weight, chemical classification and additional relevant physicochemical properties
∙Purity and chemical properties of impurities
∙Pretreatment of test material (e.g., warming, etc., if applicable)
∙Storage conditions and stability
Test information
∙Name and address of test sponsor, test facility, and person in charge of research
∙Description of test method
∙Details of test procedure
∙Description and step-by-step confirmation data on organoid production, including cell line, cell line source, cell passage number, cell density, etc.
∙Reagent supplier, catalog number and lot number
∙Duration of each step in organoid preparation
∙QC inspection data for organoid system
Test conditions and methods
∙Test substance preparation record
∙Test substance exposure conditions and test substance preparation details
∙Lot number of organoid model
∙Time taken to remove the organoid model from the CO2 incubator and expose it to the test substance
∙Concentration of test substance
∙Exposure time of test substance
∙Number of experimental repetitions for each test and number of organoid models used in each experiment
∙Description of modifications or changes to test procedures
∙Proficiency test materials and results
References
1. Jung SY, You HJ, Kim MJ, Ko G, Lee S, Kang KS. 2022; Wnt-activating human skin organoid model of atopic dermatitis induced by Staphylococcus aureus and its protective effects by Cutibacterium acnes. iScience. 25:105150. DOI: 10.1016/j.isci.2022.105150. PMID: 36193049. PMCID: PMC9526179.
2. Corsini NS, Knoblich JA. 2022; Human organoids: new strategies and methods for analyzing human development and disease. Cell. 185:2756–2769. DOI: 10.1016/j.cell.2022.06.051. PMID: 35868278.

3. Wang Y, Wang P, Qin J. 2022; Human organoids and organs-on-chips for addressing COVID-19 challenges. Adv Sci (Weinh). 9:e2105187. DOI: 10.1002/advs.202105187. PMID: 35107217. PMCID: PMC8981475.

4. Silva RJ, Tamburic S. 2022; A state-of-the-art review on the alternatives to animal testing for the safety assessment of cosmetics. Cosmetics. 9:90. DOI: 10.3390/cosmetics9050090.

5. Wang M, Zhang L, Hao H, Yan M, Zhu Z. 2024; Applications of engineered skin tissue for cosmetic component and toxicology detection. Cell Transplant. 33:9636897241235464. DOI: 10.1177/09636897241235464. PMID: 38491929. PMCID: PMC10944590.

6. Deshmukh GR, Kumar KH, Reddy PV, Rao BS. 2012; In vitro skin corrosion: human skin model test - a validation study. Toxicol In Vitro. 26:1072–1074. DOI: 10.1016/j.tiv.2012.04.021. PMID: 22542585.
7. OECD. 2023. Test No. 498: in vitro phototoxicity - reconstructed human epidermis phototoxicity test method. OECD.
8. OECD. 2021. Test No. 439: in vitro skin irritation: reconstructed human epidermis test method. OECD.
9. OECD. 2019. Test No. 431: in vitro skin corrosion: reconstructed human epidermis (RHE) test method. OECD.
10. Moniz T, Costa Lima SA, Reis S. 2020; Human skin models: from healthy to disease-mimetic systems; characteristics and applications. Br J Pharmacol. 177:4314–4329. DOI: 10.1111/bph.15184. PMID: 32608012. PMCID: PMC7484561.

11. Lee J, Koehler KR. 2021; Skin organoids: a new human model for developmental and translational research. Exp Dermatol. 30:613–620. DOI: 10.1111/exd.14292. PMID: 33507537. PMCID: PMC8265774.

12. Lee J, Rabbani CC, Gao H, et al. 2020; Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 582:399–404. DOI: 10.1038/s41586-020-2352-3. PMID: 32494013. PMCID: PMC7593871.

13. Kim J, Koo BK, Knoblich JA. 2020; Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 21:571–584. DOI: 10.1038/s41580-020-0259-3. PMID: 32636524. PMCID: PMC7339799.

14. Clevers H. 2016; Modeling development and disease with organoids. Cell. 165:1586–1597. DOI: 10.1016/j.cell.2016.05.082. PMID: 27315476.

15. Fujii M, Sato T. 2021; Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 20:156–169. DOI: 10.1038/s41563-020-0754-0. PMID: 32807924.

16. Sato T, Vries RG, Snippert HJ, et al. 2009; Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. DOI: 10.1038/nature07935. PMID: 19329995.

17. Wang Y, Lin H, Zhao L, et al. 2023; Standard: human intestinal organoids. Cell Regen. 12:23. DOI: 10.1186/s13619-023-00168-5. PMID: 37314549. PMCID: PMC10267020.
18. Sun H, Zhang YX, Li YM. 2021; Generation of skin organoids: potential opportunities and challenges. Front Cell Dev Biol. 9:709824. DOI: 10.3389/fcell.2021.709824. PMID: 34805138. PMCID: PMC8600117.

19. Ganesh K, Wu C, O'Rourke KP, et al. 2019; A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 25:1607–1614. DOI: 10.1038/s41591-019-0584-2. PMID: 31591597. PMCID: PMC7385919.

20. de Jongh D, Massey EK, Bunnik EM. VANGUARD consortium. 2022; Organoids: a systematic review of ethical issues. Stem Cell Res Ther. 13:337. DOI: 10.1186/s13287-022-02950-9. PMID: 35870991. PMCID: PMC9308907.

21. Geraghty RJ, Capes-Davis A, Davis JM, et al. 2014; Guidelines for the use of cell lines in biomedical research. Br J Cancer. 111:1021–1046. DOI: 10.1038/bjc.2014.166. PMID: 25117809. PMCID: PMC4453835.

22. Sullivan S, Stacey GN, Akazawa C, et al. 2018; Quality control guidelines for clinical-grade human induced pluripotent stem cell lines. Regen Med. 13:859–866. DOI: 10.2217/rme-2018-0095. PMID: 30205750.

23. Steeg R, Mueller SC, Mah N, et al. 2021; EBiSC best practice: how to ensure optimal generation, qualification, and distribution of iPSC lines. Stem Cell Reports. 16:1853–1867. DOI: 10.1016/j.stemcr.2021.07.009. PMID: 34380020. PMCID: PMC8365092.

24. Rohani L, Johnson AA, Naghsh P, Rancourt DE, Ulrich H, Holland H. 2018; Concise review: molecular cytogenetics and quality control: clinical guardians for pluripotent stem cells. Stem Cells Transl Med. 7:867–875. DOI: 10.1002/sctm.18-0087. PMID: 30218497. PMCID: PMC6265634.

25. Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. 2016; Engineering stem cell organoids. Cell Stem Cell. 18:25–38. DOI: 10.1016/j.stem.2015.12.005. PMID: 26748754. PMCID: PMC4728053.

26. Shang Z, Feng H, Xia L. 2023; The suppression effects of fat mass and obesity associated gene on the hair follicle-derived neural crest stem cells differentiating into melanocyte by N6-methyladenosine modifying microphthalmia-associated transcription factor. Int J Stem Cells. 16:135–144. DOI: 10.15283/ijsc22106. PMID: 36823977. PMCID: PMC10226864.

27. Zhao Z, Chen X, Dowbaj AM, et al. 2022; Organoids. Nat Rev Methods Primers. 2:94. DOI: 10.1038/s43586-022-00174-y. PMID: 37325195. PMCID: PMC10270325.

28. Kim Y, Ju JH. 2019; Generation of 3D skin organoid from cord blood-derived induced pluripotent stem cells. J Vis Exp. (146):e59297. DOI: 10.3791/59297.

29. Posch W, Lass-Flörl C, Wilflingseder D. 2016; Generation of human monocyte-derived dendritic cells from whole blood. J Vis Exp. (118):54968. DOI: 10.3791/54968. PMID: 28060313. PMCID: PMC5226452.

30. Sachamitr P, Leishman AJ, Davies TJ, Fairchild PJ. 2018; Directed differentiation of human induced pluripotent stem cells into dendritic cells displaying tolerogenic properties and resembling the CD141+ subset. Front Immunol. 8:1935. DOI: 10.3389/fimmu.2017.01935. PMID: 29358940. PMCID: PMC5766641.
31. Yousef H, Alhajj M, Sharma S. 2024. Anatomy, skin (integument), epidermis. StatPearls Publishing;StatPearls:
32. Caloni F, De Angelis I, Hartung T. 2022; Replacement of animal testing by integrated approaches to testing and assessment (IATA): a call for in vivitrosi. Arch Toxicol. 96:1935–1950. DOI: 10.1007/s00204-022-03299-x. PMID: 35503372. PMCID: PMC9151502.

33. Gądarowska D, Kalka J, Daniel-Wójcik A, Mrzyk I. 2022; Alternative methods for skin-sensitization assessment. Toxics. 10:740. DOI: 10.3390/toxics10120740.

34. Everett JS, Sommers MS. 2013; Skin viscoelasticity: physiologic mechanisms, measurement issues, and application to nursing science. Biol Res Nurs. 15:338–346. DOI: 10.1177/1099800411434151. PMID: 22544517. PMCID: PMC3465619.
35. Furbo S, Urbano PCM, Raskov HH, Troelsen JT, Kanstrup Fiehn AM, Gögenur I. 2022; Use of patient-derived organoids as a treatment selection model for colorectal cancer: a narrative review. Cancers (Basel). 14:1069. DOI: 10.3390/cancers14041069. PMID: 35205817. PMCID: PMC8870458.

36. Han J, Kim S, Lee SH, et al. 2020; Me-too validation study for in vitro skin irritation test with a reconstructed human epidermis model, KeraSkinTM for OECD test guideline 439. Regul Toxicol Pharmacol. 117:104725. DOI: 10.1016/j.yrtph.2020.104725. PMID: 32768665.
37. OECD. 2010. Test No. 429: skin sensitisation: local lymph node assay. OECD.
38. OECD. 2010. Test No. 442A: skin sensitization: local lymph node assay: DA. OECD.
39. OECD. 2018. Test No. 442B: skin sensitization: local lymph node assay: BrdU-ELISA or -FCM. OECD.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download