Abstract
This study offers a comprehensive overview of brain organoids for researchers. It combines expert opinions with technical summaries on organoid definitions, characteristics, culture methods, and quality control. This approach aims to enhance the utilization of brain organoids in research. Brain organoids, as three-dimensional human cell models mimicking the nervous system, hold immense promise for studying the human brain. They offer advantages over traditional methods, replicating anatomical structures, physiological features, and complex neuronal networks. Additionally, brain organoids can model nervous system development and interactions between cell types and the microenvironment. By providing a foundation for utilizing the most human-relevant tissue models, this work empowers researchers to overcome limitations of two-dimensional cultures and conduct advanced disease modeling research.
Necessity of research using organoids that mimic the human nervous system: The emergence of neural analogs composed of human cells that mimic the nervous system (brain/neural organoids) has been highlighted as a new approach to understanding the human brain (1, 2). These organoids are considered next-generation ex vivo models that can reproduce the anatomical structure and physiological characteristics of nervous tissue, and, from the perspective of regulatory science, are considered to have high usability in toxicology studies.
As a technique for modeling the complex characteristics of the central nervous system (CNS) ex vivo, brain/neural organoids are characterized by their ability to reproduce nervous system development, and the complex structure and electrophysiology of neuronal networks, while simultaneously reflecting interactions between different cell types and various microenvironmental factors. As such, by using brain/neural organoid techniques to create a model with the highest similarity to real human tissue, it is possible to overcome the limitations of conventional two-dimensional cell culture models and to perform high-caliber disease model research (3-6).
Future developmental directions include ‘advanced modeling’ via simultaneous differentiation, coculture, and connection between multiple brain regions or central and peripheral nervous systems (PNSs), as well as the use of these advanced models for ‘exploring precise functions and understanding disease mechanisms based on interactions and connectivity between neurons’ (7, 8). From the perspective of regulatory science, this is expected to form the basis for toxicity and efficacy assessments.
Increasing commercial usability through the construction of human brain models: Globally, there has been active research using brain/neural organoids and human pluripotent stem cell (hPSC)-derived brain cells to ensure the efficiency and accuracy of studies on human brain diseases. Recent cases demonstrating the production of stem cell-derived brain/neural cells and brain/neural organoids have enabled the development of human disease models that closely mimic the genotypes and phenotypes of patients with brain disease (Fig. 1) (9-11). As a result, interest in brain/neural organoids is growing in the commercial sector, and technologies are being developed that can act as a bridge for the translation of basic research outcomes to industry (12-14).
To date, research has largely relied on animal models due to significant limitations in acquiring tissue samples from patients with brain disease. The reliance on such animal models has led to the failure of new drug development due to genetic and physiological differences between species (15). In contrast, brain organoids have emerged as a promising alternative, as they closely mimic the structural, genetic, and physiological features of human brain tissue, and have been shown to recapitulate the pathological hallmarks of various neurological disorders in prior studies (16). Driven by these advantages, there is a growing effort to rapidly commercialize brain organoid-based modeling of intractable neurological disease and drug discovery technologies (17, 18). Despite these advantages, the fabrication of brain organoids remains a complex and challenging process due to the lack of standardization in production methods, posing potential risks for their application in drug discovery pipelines. Moreover, organoids inherently lack reproducibility and quantifiability for use in disease modeling and drug screening, indicating that there is an urgent need for the development of related technologies (19-21).
In the standard guidelines, we gathered and reflected on the opinions of brain organoid experts from industry and academia, presented a technical summary of organoid-related terms and definitions, the characteristics of organoids, as well as the cells, culture methods, and quality requirements of brain organoids for facilitating the utilization of brain organoids in research.
Limitations of two-dimensional cell culture models and animal experiments: Disease model and new drug development studies using patient-induced pluripotent stem cells (iPSC)-derived cell models are important in pre-clinical research. By using cells that reflect the genetic information of patients, it is possible to reproduce disease phenotypes and to rapidly test, ex vivo, the toxicity and efficacy of novel drug candidates (22, 23). However, current disease model drug research platforms for new drug development using patient cell models rely on two-dimensional culture systems, in which cells are distributed in a single layer. This method has limitations, as it is difficult to recreate the cellular heterogeneity and intercellular interactions observed in vivo, and it is impossible to reflect the microenvironment of the tissue as opposed to cells (24). This may cause unpredictable errors when the model is used to recreate biological phenomena in tissue. Ultimately, the critical limitations of these two-dimensional cell culture models suggest that research results obtained using these models lack reliability and accuracy. There have been studies using cell models differentiated from patient iPSCs to investigate the mechanisms of pathogenesis for various intractable diseases accompanied by genetic variants, intending to recreate patient phenotypes ex vivo, and elucidating the basic causes of disease (22, 23). However, most of these studies used two-dimensional cell culture models, implying that only a very limited level of trait expression was observed.
Furthermore, brain disease models are often sporadic/idiopathic, rather than being caused by genetic factors, meaning that two-dimensional cell research based on genome sequencing is limited. Recently, there have been reports of brain disease caused by the spread of infectious diseases and increased exposure to hazardous environmental substances, such as fine dust and microplastics (25); thus, there is a growing need for models that can mimic the human brain.
Rat models exposed to microplastics reportedly show symptoms of autism spectrum disorder (ASD), and people exposed to fine dust are known to show cognitive impairment, delayed brain development, encephalitis, injury to the blood–brain barrier, and accumulation of toxic brain proteins, similar to dementia. Nevertheless, only the final outcomes have been verified, and the mechanisms of brain disease development have not been elucidated.
Children whose mothers were exposed to infection during pregnancy show a much higher risk of ASD, and some studies have reported that this is affected by inflammatory cytokines (26, 27). However, only a small fraction of the factors involved in the development of ASD have been identified, making it crucial to develop a model to investigate these phenomena more broadly.
As described above, human tissue is highly complex, as it is made of organic combinations of many different types of cells with different developmental origins that allow it to perform specific functions. The brain not only contains the most varied cell types of all organs in the human body but also has unique structural properties. To maximize the accuracy and reliability of research platforms, it is essential to develop advanced, ex vivo culture models that can recreate the anatomical and microenvironmental characteristics of actual organs.
Usability of brain organoid models: The human CNS is not simply a collection of neurons but has a complex structure consisting of numerous neuron subtypes, as well as glial cells, such as astrocytes and oligodendrocytes, blood vessels, the blood–brain barrier, and immune cells. The interactions between these cell types result in various physiological functions. For example, the cerebral cortex is composed of various neuron subtypes arranged in a continuous six-layered structure, and the complex neuronal network is maintained by interactions with glial cells that support the functions of the neurons. This structural complexity is an essential element for maintaining the functionality of cerebral tissue (28-30). As a result, disease model research conducted in conventional two-dimensional cultures using a single cell type will inevitably show incomplete functionality, leading to limited results.
Over the last several decades, organoids have been developed with the characteristics of various types of brain tissue (cerebral cortex, midbrain, retina, hippocampus, cerebellum, pituitary gland, etc.) using rat and hPSCs, and research to refine and commercialize brain organoid technology are ongoing (31-41). Cerebral organoids, which account for most studies, have been demonstrated to almost perfectly recreate the layered structure and developmental process of the cerebral cortex, and have been used to model various developmental disorders caused by anatomical structural abnormalities, including deformity of the cortical layers (31-33, 42-46). Models of ASD, Alzheimer’s disease (AD), and ZIKA virus-induced microcephaly have been reported using cerebral organoids (47-64), allowing the inspection of three-dimensional phenotypes that cannot be observed in two-dimensional culture models, such as structural deformities, and imbalanced development of excitatory and inhibitory neurons. Midbrain organoids have been demonstrated to be an adequate ex vivo culture model that strongly reflects the physiological characteristics of the midbrain, including the formation of specific dopaminergic neurons, dopamine secretion, and neuromelanin production (39-41, 65), and are being used as models for midbrain-specific intractable diseases, such as Parkinson’s disease (PD) (66, 67). The positive outcomes of these disease model studies support the need for the development of next-generation ex vivo culture models, such as brain organoids, as well as the refinement and commercialization of these technologies, in order to study intractable brain diseases.
In summary, the promising findings of these studies lead to the conclusion that we must model the human brain more precisely to study intractable brain diseases. Moreover, to develop systematic, refined brain organoids, it is crucial that we first develop next-generation culture models, and to refine and commercialize these technologies.
Therefore, in these guidelines, we have presented various recommendations that should be considered for the research and development of healthy brain organoids at the culture stage.
As described above, brain organoids are typically cultured from PSCs over a long culture duration. Since the specific culture methods differ between laboratories, outcomes may also differ depending on the culture methods, conditions, and duration. Furthermore, there are various conditions to consider, such as cell death, which depends on the type of supporting structure, and the morphology and size of the cultured cells. Herein, we provide a guide for the standardized evaluation of the characteristics and quality of brain organoids of different regions, for toxicity and efficacy testing of drugs. We do not provide a guide for the storage and preservation of brain organoids since this is more difficult than for other organoid types.
As mentioned, brain organoids are typically cultured from PSCs over a long culture duration. Since the specific culture methods differ between laboratories, outcomes may also differ depending on the culture methods, conditions, and duration. Furthermore, there are various other important conditions to consider, such as cell death, which depends on the type of supporting structure, and the morphology and size of the cultured cells. Herein, we provide a guide for the standardized evaluation of the characteristics and quality of brain organoids of different regions, for toxicity and efficacy testing of drugs. This document was created to provide a basic guide for more effective testing. In this document, we describe the characteristics of whole-brain organoids and the following region-specific brain organoids: (1) dorsal forebrain, (2) ventral forebrain, (3) hypothalamus, (4) pituitary gland, (5) midbrain, (6) cerebellum, and (7) spinal cord.
PSCs: pluripotent stem cells
TGF-β: transforming growth factor-β
WNT: wingless-type MMTV integration site family
ECM: extracellular matrix
FGF: fibroblast growth factor
EGF: epidermal growth factor
EB: embryoid body
ULA: ultra-low attachment
CNS: central nervous system
PNS: peripheral nervous system
GFAP: glial fibrillary acidic protein
S100β: S100 calcium-binding protein-β
VGLUT: vesicular glutamate transporter
SYT1: synaptotagmin-1
NRXN: neurexin
NLGN1: neuroligin-1
GDNF: glial cell line-derived neurotrophic factor
BDNF: brain-derived neurotrophic factor
MEA: multi-electrode array
BMP: bone morphogenetic protein
SHH: sonic hedgehog
ASD: autism spectrum disorder
NMP: neuromesodermal progenitor
HTS: high throughput screening
vRG: ventricular radial glia
oRG: outer radial glia
General recommendations: The method for generating brain organoids can be summarized as follows. Phase 1: creating PSCs, such as ESCs or iPSCs, into three-dimensional aggregates called embryoid bodies; Phase 2: inducing differentiation by applying environmental factors that stimulate specification into neuroectoderm and brain tissue; Phase 3: maturation of differentiated brain tissue to exhibit the functionality of the nervous system (Fig. 2).
Differentiation induction (Phase 2) can be broadly divided into two categories depending on whether patterning factors are used, which can regulate the regional specificity of the brain organoid. Un-guided methods, in which brain organoids are created via the stem cells inherent self-organizing ability, using minimal differentiation culture medium without patterning factors, are used to produce whole-brain organoids, which exhibit the diverse characteristics of multiple brain regions.
In these guidelines, we describe methods for generating brain organoids based on the literature to date. We aim to describe approaches to region-specific organoids based on parts that are being applied to various studies.
General characteristics: Whole-brain organoids are used for basic science studies, including the development of the entire brain tissue, and the structural and physiological characteristics of individual brain regions. Brain organoids generated using an unguided method show differentiation according to the stem cells’ inherent self-organizing ability, and they have the advantage of developing into brain regions that constitute a very small portion of the tissue, such as the choroid plexus. However, whole-brain organoids demonstrate limitations relating to the high heterogeneity of the brain, showing significantly different characteristics in different brain regions. Whole-brain organoids have very low reproducibility for investigators seeking to generate organoids with specific, desired properties (31, 68).
Brain organoids fabricated using the unguided method described above do not aim to control the stem cell patterning process during differentiation induction (Phase 2). This allows development into various regions observed throughout human brain tissue but has the disadvantage of producing brain organoids with very heterogeneous structural characteristics. Indeed, brain organoids generated using common unguided methods, in addition to cortical neuroepithelium, often contain a mixture of various brain tissues, including the hippocampus, prefrontal lobe, choroid plexus, and retina (68, 69). Although these models are highly suitable for studies on the development of brain tissue from an embryological perspective or on the interactions between different brain regions, they usually have critical limitations when it comes to modeling or studying treatments for intractable disease occurring in specific brain tissues. Thus, generating region-specific brain organoids using a guided method can provide better homogeneity and reproducibility. Moreover, it is important to use brain organoids that are well-suited to the study purpose.
Dorsal forebrain organoids: Dorsal forebrain (pallium, cerebral cortex) organoids are region-specific brain organoids that mimic the cortical neuroepithelium, and can be induced by inhibiting WNT signaling, which forms a concentration gradient because it is mostly produced in the caudal end of the neural tube (Fig. 3). Although there are differences between specific patterning methods, dorsal forebrain organoids are being made efficiently using WNT inhibitors, and should clearly show the characteristic stratified structure of the cerebral cortex, as well as the various cells comprising this structure (vRG, oRG, neural progenitor cells, excitatory neurons, astrocytes, and oligodendrocytes) (32, 70-73).
Ventral forebrain organoids: Ventral forebrain (subpallium, gangrionic eminances) organoids mimic the ganglionic eminence, which develops into the basal ganglia and corpus striatum. Ventral forebrain organoids are fabricated by inhibiting WNT signaling, like dorsal forebrain organoids, while also activating SHH signaling pathways (Fig. 3). During development, the SHH protein is expressed from the ventral direction in the inferior part of the neural tube and spreads dorsally, forming a concentration gradient, which helps to pattern the dorsoventral axis of the neural tube. Ventral forebrain organoids show high levels of FOXG1 expression, and expression of the characteristic marker NKX2.1. Upon maturation, they show distinctive development of GABAergic inhibitory neurons (74-76).
Hypothalamus organoids: For the fabrication of hypothalamus organoids, patterning to ventral diencephalon tissue is very important, which can be induced by activating specific intensities of WNT and SHH signaling at precise time intervals (Fig. 3). Embryoid bodies that have been patterned in this way can be made into mature hypothalamus organoids using a maturation medium containing FGF2 and ciliary neurotrophic factor. Hypothalamus organoids manufactured in this manner can be verified to express well known hypothalamus-specific neuron markers, such as NPY, SST, ISL1, and PV (34).
Pituitary gland organoids: The pituitary gland is a brain region that secretes various hormones, including growth hormones (GHs). Unlike other brain regions that develop from neuroectoderm, the pituitary gland develops from oral ectoderm. To make this specialized region, considering that the pituitary gland is anatomically connected to the hypothalamus, it is typical to induce development of pituitary gland organoids based on the patterning method for hypothalamus organoids (Fig. 4). One of the most important aspects of this patterning method is that embryoid bodies undergoing differentiation to neuroectoderm should be exposed to SHH and BMP proteins in order to induce simultaneous development of neuroectoderm and oral ectoderm. In this case, two characteristic phenomena are observed: the neuroectoderm part shows characteristics of the hypothalamus, while the oral ectoderm part shows characteristics of the pituitary gland. Pituitary gland organoids contain corticotropic and somatotropic cells and show distinctive secretion of adrenocorticotropic hormone and GH from these tissues (35).
Midbrain organoids: The key mechanism for midbrain organoid generation is patterning to the ventral mesencephalon; this can be induced by controlling patterning in the anteroposterior and dorsoventral directions, similar to the methods above (Fig. 4). The common patterning methods used in many studies are outlined here. By precisely controlling WNT and SHH signaling in the embryoid bodies, it is possible to induce differentiation to midbrain floor plate tissue, and to generate midbrain organoids by treating with FGF8, a growth factor expressed at the isthmus, which is the region where the midbrain and the metencephalon meet. Midbrain organoids are characterized by the presence of midbrain-specific dopaminergic neurons, inhibitory neurons, and various glial cells (39-41, 65).
Cerebellar organoids: The cerebellum develops from the metencephalon, and is mainly comprised of Purkinje cells, which are a characteristic cell type of the cerebellar cortex. The important factor for patterning to produce cerebellar organoids is to introduce two patterning factors, FGF8 and WNT1, which are proteins derived from the isthmus at the boundary between the midbrain and metencephalon, to embryoid bodies undergoing development to neuroectoderm. Furthermore, specific factors are essential for the maturation of cerebellar organoids. In cerebellar organoids that have entered the maturation phase, the balanced development of Purkinje cells and other components can be induced by exposure to FGF19 and stromal cell-derived factor 1 (38). Cerebellar organoids are composed of neurons, mainly Purkinje cells, Golgi cells, and granule cells, and glial cells, such as astrocytes and oligodendrocytes (Fig. 4).
Spinal cord organoids: The key mechanism required to produce spinal cord organoids is patterning to produce bipotential NMPs, which is achieved by inhibiting TGF-β signaling and activating WNT signaling (77, 78). After inducing differentiation to NMPs in two dimensions, the NMPs can be re-aggregated in three dimensions and maturation to spinal cord organoids can be induced using a maturation medium containing FGF2. Spinal cord organoids produced in this manner show expression of the spinal cord-specific HOX gene, and are composed of excitatory neurons, inhibitory neurons, and glial cells once maturity is reached.
Whole brain organoids: The generation protocol of brain organoid model that mimics the whole brain using human PCSs was written with reference to Lancaster’s nature protocol (79).
a.Essential elements
1)Reagents
Matrigel (Corning)
mTeSR1 medium (mTeSR1; STEMCELL Technologies)
Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM-F12; Gibco)
Neurobasal medium (Gibco)
Penicillin Streptomycin (P/S; Gibco)
N-2 Supplement (N2; Gibco)
B-27 Supplement (B27; Gibco)
B-27 Supplement, minus vitamin A (B27 not contain vitamin A; Gibco)
MEM Non-Essential Amino Acids Solution (NEAA; Gibco)
GlutaMAX Supplement (GlutaMAX; Gibco)
β-Mercaptoethanol (Gibco)
KnockOut Serum Replacement (Gibco)
Dulbecco’s Phosphate Buffered Saline (D-PBS; Welgene)
Ethylenediaminetetraacetic acid solution (EDTA; Thermo Scientific)
Accutase (STEMCELL Technologies)
Basic Fibroblast Growth Factor (bFGF; PeproTech)
Heparin (Sigma-Aldrich)
Insulin (Sigma-Aldrich)
Y-27632 (ROCK inhibitor; BioGems)
2)Media components
(1)PSC culture
mTeSR1
(2)Neural induction medium
DMEM-F12
1% P/S
0.5x N2
1x GlutaMAX
1x NEAA
(3)Brain organoid differentiation medium
DMEM-F12
Neurobasal medium
1% P/S
0.5x N2
1x B27
1x B27 (not contain vitamin A)
1x GlutaMAX
1x NEAA
b.Culture process and requirements
1)Culture protocols
Phase 0. Making EBs
1.Grow hESC or iPSC colonies in one well of a six-well plate until they are 70%∼80% confluent. All or some wells produce EBs.
2.Wash the cells with 1 mL of D-PBS without calcium and magnesium and add 600 μL of 0.5 mM EDTA solution in 1 mL of D-PBS without calcium and magnesium for each well of the six-well plate. And place the cells back in the incubator for 4 minutes.
3.Gently aspirate the EDTA solution without disturbing the colonies, and add 1 mL of Accutase. And place the cells back in the incubator for another 4 minutes.
4.Use a 1 mL pipette tip to spray the colonies with 1 mL of mTeSR1 medium to detach them from the dish. Transfer 2 mL to a 15 mL conical tube and triturate the mixture using a 1 mL pipette tip until the solution becomes cloudy with single cells.
5.Take two repetitions of 5 μL for cell counting and then add another 3 mL of mTeSR1 medium and mix.
6.Centrifuge the cells at 270 ×g for 5 minutes at room temperature.
7.Count live cells by adding an equal volume of trypan blue and calculate the average of the two replicates.
8.Resuspend the cells with 1 mL of low-bFGF hESC medium with ROCK inhibitor (final concentration 50 μM). Pipette up and down to ensure a single-cell suspension.
9.Prepare an additional appropriate volume of low-bFGF hESC medium with ROCK inhibitor to obtain 9,000 live cells per 150 μL.
10.Plate 150 μL in each well of a low attachment 96-well U-bottom plate and place it back in the incubator.
Phase 1. Initiation of germ layer differentiation
1.Observe the plate 24 hours later. Small EBs with clear borders should be visible, although many dead cells will decorate the area around the EB. This is completely normal. Continue to culture EBs in the tissue culture incubator at 37℃ and 5% CO2.
2.Feed the EBs every other day by gently aspirating approximately half of the medium without disturbing the EB at the bottom of the well. Add an additional 150 μL of fresh medium including ROCK inhibitor (1:100) and low-bFGF medium (4 ng/mL) until EBs begin to brighten or are >350∼400 μm in diameter. Typically, ROCK inhibitor and low-bFGF medium are included only for the first 4 days.
3.While EBs are 350∼600 μm in diameter, feed EBs every other day, using hESC medium without ROCK inhibitor or bFGF.
Phase 2. Induction of primitive neuroepithelia
1.When EBs are ∼500 to 600 μm in diameter and begin to brighten and have smooth edges, transfer each EB with a cut 200-μL pipette tip to one well of a low-attachment 24-well plate containing 500 μL of neural induction medium.
2.Feed the EBs by adding another 500 μL of neural induction medium 48 hours after transferring them to the 24-well plate.
3.Observe the EBs on the tissue culture microscope after a further 2 days. EBs should be brighter around the outside, indicating neuroectodermal differentiation.
4.Once these regions begin to show radial organization of a pseudostratified epithelium consistent with neuroepithelium formation which should happen after 4∼5 days in neural induction medium, proceed to transfer the aggregates to Matrigel droplets.
Phase 3. Transferring neuroepithelial tissues to Matrigel droplets
1.Thaw Matrigel on ice at 4℃ for 1∼2 hours.
2.Prepare dimpled Parafilm substrate for the generation of Matrigel droplets by layering a square of Parafilm over an empty tip tray for size 200-μL tips. Press your gloved finger into the Parafilm over each hole in the tip tray to create small dimples in the Parafilm.
3.Make a grid of 4×4 dimples (16 total) and trim the Parafilm with sterile scissors to a small square containing this grid. Place the square of Parafilm into a 60-mm tissue culture dish.
4.Use a cut 200-μL tip to transfer the neuroepithelial tissues one by one to each dimple in the Parafilm.
5.Remove excess medium from each tissue by carefully sucking off the fluid with an uncut 200-μL tip.
6.Immediately add droplets of Matrigel to each aggregate by dripping ∼30 μL onto each tissue so that the droplet fills the Parafilm dimple.
7.Position each aggregate in the center of the droplet using a 10-μL pipette tip to move the tissue within the droplet.
8.Place the 60-mm dish containing droplets on Parafilm back into the 37℃ incubator, and incubate it for 20∼30 minutes to allow the Matrigel to polymerize.
9.Add 5 mL of cerebral organoid differentiation medium without vitamin A to the 60-mm dish.
10.Remove the Matrigel droplets from Parafilm by first using sterile forceps to turn the Parafilm sheet over and by agitating the dish until the droplets fall off the sheet.
11.Continue culturing the tissue droplets in a CO2 incubator.
Phase 4. Expanding neuroepithelial buds
1.Observe embedded tissues 24 hours later under the microscope. Tissues should begin forming buds of more expanded neuroepithelium containing fluid-filled cavities within 1∼3 days.
2.Incubate the droplets for a further 24 hours, and then feed the droplets containing neuroepithelial tissues with Brain organoid differentiation medium without vitamin A. Incubate it for a further 48 hours without agitation.
Phase 5. Growth of brain tissue
1.After 4 days in static culture, transfer the embedded organoids to a 125-mL spinning bioreactor by using a cut 1-mL pipette tip with an opening of ∼3 mm. Culture organoids in 75∼100 mL of Brain organoid differentiation medium containing vitamin A. Place the bioreactor on an appropriate magnetic stir plate installed in the incubator. Alternatively, an orbital shaker installed in the incubator, shaking at 85 rpm, can be used. Simply replace the medium in each 60-mm dish with cerebral organoid differentiation medium containing vitamin A, and place the dish on the orbital shaker.
2.Change the medium completely, every 3∼4 days if organoids are on the shaker or every week for the spinner flask, and monitor for morphology.
3.Perform further analysis on the organoids at your preferred time points, depending on the stage of development desired.
Spinal cord organoids: A spinal cord organoid model that mimics secondary neurulation using human iPSCs (77).
a.Essential elements
1)Reagents
Matrigel
mTeSR1
Hank’s Balanced Salt Solution (HBSS; Welgene)
DMEM-F12
Neurobasal medium
P/S
N2
B27
NEAA
GlutaMAX
β-Mercaptoethanol
Acridine Orange/Propidium Iodide (AO/PI) Stain Solution (Nexcelom Bioscience)
ReLeSR (STEMCELL Technologies)
Accutase
SB431542 (Sigma-Aldrich)
CHIR99021 (BioGems)
FGF2 (PeproTech)
EGF (PeproTech)
BDNF (PeproTech)
Ascorbic acid (Sigma-Aldrich)
Y-27632
2)Media components
(1)iPSC culture
mTeSR1
(2)Neural induction
DMEM-F12
1% P/S
1 mM β-Mercaptoethanol (Gibco)
1x NEAA (Gibco)
1x N2 (Gibco)
1x B27 (Gibco)
10 μM SB431542
3 μM CHIR99021 (Sigma-Aldrich)
10 μM Y-27632
(3)Neural differentiation (DM media)
DMEM-F12 medium (Gibco)
1% P/S
1 mM β-Mercaptoethanol
1x NEAA
1x N2
1x B27
20 ng/mL bFGF
20 ng/mL EGF
50 μM Y-27632
(4)Neural maturation (MM media)
DMEM-F12 medium
Neurobasal medium
1% P/S
1 mM β-Mercaptoethanol
0.5x NEAA
1x GlutaMAX
0.5x N2
1x B27
20 ng/mL BDNF
200 μM Ascorbic acid
b.Culture process and requirements
1)Culture protocols
(1)Stem cell maintenance
1.Starting 1 hour before the experiment, mTeSR1 is stored at room temperature and HBSS is stored in a water bath.
2.Matrigel: After diluting to a 1:24 ratio in DMEM/F12, 2.5 mL of Matrigel is prepared in a 15 mL tube. The tube is kept as cold as possible.
3.Using a pipette, at least 600 μL is added to each well of a 6-well plate. After shaking the plate to facilitate even coverage of the Matrigel, the plate is left for 1 hour to ensure coating of wells.
4.After removing the medium from human iPSCs maintained in an incubator, 2 mL of HBSS is added to wash the cells. After removing the HBSS, 2 mL of mTeSR1 is added.
5.Cells are allowed to react with ReLeSR for 30 seconds following the addition of 1 mL ReLeSR. Thereafter, ReLeSR is removed, and cells are incubated for 3 minutes in an incubator.
6.While the cells are being incubated, the Matrigel is removed from the coated plates, and the plates are washed with 2 mL HBSS. Next, HBSS is removed and 2 mL of mTeSR1 is added.
7.After 3 minutes, 1 mL of mTeSR1 is added, and the plate is subject to shaking approximately five times to suspend the colonies. The colonies are collected in an E-tube.
8.The E-tube is inverted 2∼3 times to evenly disperse the colonies, and then 70∼100 μL of the suspension is added to each well. The plate is shaken 3∼5 times to evenly disperse the cells, and then placed in the incubator.
Phase 0. Neural induction
1.Starting 1 hour before the experiment, mTeSR1 is stored at room temperature and HBSS and Accutase are stored in a water bath.
2.Matrigel: After diluting to a 1:24 ratio in DMEM/ F12, 2.5 mL of Matrigel is prepared in a 15 mL tube. The tube is kept as cold as possible.
3.Using a pipette, at least 600 μL is added to each well of a 6-well plate. After shaking the plate to facilitate even coverage of the Matrigel, the plate is left for 1 hour to ensure coating of wells.
4.After removing the medium from human iPSCs being maintained in an incubator, 2 mL of HBSS is added to wash the cells. The HBSS is then removed.
5.After adding 1 mL of Accutase, the cells are placed in an incubator for 5 minutes.
6.During cell incubation, the Matrigel is removed from the coated plates, and the plates are washed with 2 mL of HBSS. After removing the HBSS, 2 mL of mTeSR1 and 2 μL of 10 mM Y-27632 are added. Meanwhile, 2 mL of mTeSR1 is prepared in a 15 mL tube.
7.After 5 minutes, the incubated cells are shaken 2∼3 times, collected, and transferred to a 15 mL tube, before centrifuging for 3 minutes at 200 ×g, and discarding the remaining Accutase.
8.After adding 2 mL of mTeSR1, the mixture is pipetted approximately five times. After preparing 1.2 μL of AO/PI on a cell counting slide, the cell suspension is pipetted approximately five times, before collecting a 9 μL sample from the middle of the tube. The sample is then mixed with AO/PI and placed on the counting slide.
9.The counting slide is placed in the automated cell counter, the GFP/RFP expression level is adjusted, the cell number is counted, and the amount of medium required for 1.0×105 cells is calculated.
10.Following the same procedure as before, the cells are pipetted approximately five times, the calculated amount of medium is added to each well, and the plate is shaken 3∼5 times to ensure the cells are evenly distributed, before placing the plate in an incubator for cell culture. This date is defined as Day −2.
11.On Day −1, the existing mTeSR1 medium is exchanged for 2 mL of fresh mTesR1 to remove the Rock inhibitor. A microscope is used to confirm that the single cells are properly attached.
12.On Day 0, a microscope is used to confirm that the single cells are forming colonies. Next, 2 mL of mTeSR1 is removed and 3 mL of DM medium is added. To promote simultaneous differentiation, 3 μL each of 10 mM SB431542 and 3 mM CHIR99021 are added, and the mixtures are subject to shaking 2∼3 times.
13.On Days 1 and 2, the existing medium is removed, and 3 mL of DM medium with 3 μL each of SB and CHIR is added.
14.On Day 3, three-dimensional conversion is performed after visually confirming differentiation.
Phase 1. Neural differentiation
1.Starting 1 hour before the experiment, HBSS, Accutase, and DM medium are stored in a water bath.
2.On Day 3, 2 mL of HBSS is added to wash the neural stem cells (NSCs). The HBSS is then removed.
3.After adding 1 mL of Accutase, the cells are placed in an incubator for 5 minutes.
4.During incubation, 2.5 mL of DM medium is prepared in a 15 mL tube.
5.After 5 minutes, the incubated cells are shaken 2∼3 times, collected, and moved to a 15 mL tube, before centrifuging for 3 minutes at 200 ×g. The remaining Accutase is then discarded.
6.After adding 2 mL of DM medium, the mixture is pipetted approximately five times. After preparing 1.2 μL of AO/PI on a cell counting slide, the cell suspension is again pipetted approximately five times. Next, a sample is collected (9 μL) from the middle of the tube, mixed with AO/PI, and placed on the counting slide.
7.The counting slide is placed in the automated cell counter, the GFP/RFP expression level is adjusted, the cell number is counted, and the amount of medium required for 2.0×104 cells/1 mL (10 wells) is calculated to reach an ICN of 2,000 cells per well. For a 96-well plate, the calculations are made for a volume of 12 mL (2.4×105).
8.After adding 12 mL of DM medium to a 15 mL tube, 2.4×105 cells are added based on previous calculations, and 12 μL of 50 mM ROCK inhibitor, 24 μL of 10 μg/mL bFGF, and 24 μL of 10 μg/mL EGF are added to prepare the medium.
9.After inverting thrice and pouring into a reservoir, a multi-channel pipette is used to place 100 μL per well into a 96-well plate.
10.The spaces between the wells are filled with 75 μL of distilled water, as shown in blue in the Fig. 5, to prevent drying of the edges. The cells are then incubated in an incubator.
11.The next day, medium is prepared at a ratio of 2x for half-change of the medium.
12.*6 mL volume per plate
13.[6 mL of DM media+24 μL of 10 μg/mL bFGF+24 μL of 10 μg/mL EGF]
14.After tilting the plate at least 45°, the organoids are collected at the bottom by pipetting 2∼3 times.
15.The pipette tip is placed at the top of the tilted well, and 45 μL of medium is removed.
16.After adding 50 μL of the prepared 2x medium, the cells are placed in the incubator.
17.The process in 11∼16 is repeated on Days 5 and 6.
18.On Day 7, medium is prepared for a full medium change.
19.*12 mL volume [12 mL DM media] per plate
20.After tilting the plate at least 45°, the organoids are collected at the bottom by pipetting 2∼3 times.
21.The pipette tip is placed at the top of the tilted well, and 90 μL of medium is removed.
22.*Day 7: −90 μL/Day 9: −85 μL/Day 11: −80 μL/Day 13: −75 μL
23.After adding 100 μL of the prepared 2x medium, the cells are incubated again.
24.The process in 18~23 is repeated on Days 9, 11, and 13.
Phase 2. Neural maturation
1.Starting 1 hour before the experiment, MM medium is stored in a water bath.
2.On Day 15, the organoids from each well of the 96-well plate are suspended by pipetting 3∼4 times using a wide-tip, and then collected in a 60 mm petri-dish. Here, 15 organoids are placed in each dish.
3.After collecting he organoids, 6 mL of MM medium, 6 μL of 20 μg/mL BDNF, and 6 μL of 200 mM ascorbic acid are added. The organoids are cultured on an orbital shaker at 80 rpm.
4.Medium exchange is performed once every four days, and BDNF and ascorbic acid are added.
Quality requirements for organoids:
a.Morphological quality
In case of whole brain organoid generation, EBs cultured on a 96-well ULA plate are observed with a microscope for 5∼6 days until they show well-defined, bright, smooth structures with a diameter of at least 350∼400 mm, showing germ layer specification into neuroectoderm. In the continual maintenance culture, radially organized, optically semi-transparent neuroepithelial structure can be observed.
Mature whole brain organoids show bigger size (1∼3 mm), thick neuroepithelium, and ideal ventricle structure.
The whole brain organoids generated by using am orbital shaker, homogeneously sized, well-defined organoids show a mean size of 2.45±0.42 mm and can reach a maximum sizer of 3.16 mm after 120 days. Although the initial brain organoids measure approximately 350∼400 μm, during maintenance culture on a shaker, organoids have been reported to achieve a size of 3∼5 mm after two months and maintain their size for 5∼6 months.
For culture using a spinning bioreactor, the yield of brain organoids is low, at ∼70%, but the size distribution is more diverse than that using an orbital shaker. The maximum organoid size was reported to be 4.21 and 3.87 mm on Days 60 and 120, respectively, and the mean size on Day 120 was 3.00±0.71 mm.
b.Cell composition
“NSCs” are the key stem cells involved in the self-organization of brain organoids. These NSCs, derived from the initial neural tube, give rise to the diverse tissues of the CNS.
“Rosette” refers to specific structure composed of NSCs at the initial stage of differentiation from PSCs, specifically referring to “neural rosette.” Neural rosettes have a conical radial shape, contain cells that express early neuroectodermal markers, such as PAX6, SOX2 and SOX1, and can differentiate into various types of neurons and neuroglial cells (Fig. 6).
The NSCs are multipotent somatic stem cells and can undergo cell division more than 20∼30 times and maintain the ability to differentiate into multiple types of cells in the nervous system including neurons and neuroglial cells.
In the mature CNS, most NSCs differentiate into neurons and glial cells. Only a small subset of cells retains their stem cell properties and exist in a quiescent state in specific brain regions.
“Neurons” are responsible for signal transmission and information processing via interactions with other neurons. They can be classified as follows, depending on the types of neurotransmitters secreted (Fig. 6).
-Glutamatergic neurons produce and secrete the excitatory neurotransmitter glutamate and can be identified by the expression of vGlut1 and vGlut2.
-GABAergic neurons produce and secrete GABA, an inhibitory neurotransmitter that regulates brain metabolism. They can be identified by the expression of GAT1 and GAD65.
-Dopaminergic neurons produce and secrete the excitatory neurotransmitter dopamine, which is involved in motor function and emotion, and can be identified by the expression of TH, DAT, GIRK2, and FOXA2.
-Serotonergic neurons produce and secrete the neurotransmitter serotonin, which regulates emotions and sleep, and can be identified by the expression of TPH and SERT.
-Cholinergic neurons produce and secrete acetylcholine and perform broad functions throughout the CNS and PNS. They can be identified by the expression of ChAT and VAChT.
“Glial cells” refer to cells in between neurons that help neurons to grow, regulate the concentration of substances in the extracellular space, and can secrete some neurotransmitters. Glial subtypes include ependymal cells, oligodendroglia, astrocytes, and microglia (Fig. 6).
-Ependymal cells play an important role in the production and regulation of cerebrospinal fluid in the ventricles, and, as stem cells in the CNS, make radial glial cells that can differentiate into glial cells or intermediate progenitor cells. They can be identified by the expression of NES, NOTCH1, SOX2, and SOX10.
-Oligodendrocytes are cells that form and maintain myelin sheaths around axons in the CNS. They can be identified by the expression of MBP, MAG, MOG, GalC, PLP, and CNP.
-Astrocytes provide structural support for neurons in the CNS, regulate the concentration of substances in the extracellular space, and supply nutrients. They can be identified by the expression of GFAP, S100B, ALDH1L1, EAAT2, and GLN1.
-Microglia are immune cells in the CNS that deal with waste substances, infected cells, and pathogens. They also play roles in pruning unimportant connections between neurons and regulating inflammation via the secretion of cytokines. They can be identified by the expression of TMEM119, HEXB, and FCRLs.
Organoid quality endpoints:
a.Size and target cell count per size
The growth and size of brain organoids vary significantly depending on the brain region they represent, making it difficult to establish precise standards. However, these guidelines provide reference standards for whole brain organoids. Homogeneously sized, well-defined whole brain organoids are composed with 30∼50 neuron layers after 2∼3 months, demonstrating that mature brain organoids have thick, robust neuronal regions. Moreover, fully mature whole brain organoids show a mean size of 2.45±0.42 mm and can reach a maximum size of 3.16 mm after 120 days.
b.Required and supporting cell composition
Differentiation of NSCs and intermediate progenitor cells to specific nervous tissues can be verified via checking expression level of several marker genes. OCT4 and NANOG are markers of pluripotency. These markers decrease during differentiation to brain organoids, while expression of SOX1 and SOX2 increases as induction to neuroectodermal specification occurs. These markers can be used to verify self-organization and differentiation of brain organoids. The regional specificity of the forebrain and hindbrain regions in the generated brain organoids can also be verified using several genetic markers. The forebrain shows high level of FOXG1 and SIX3, while the hindbrain shows an initial increase and later decrease in EGR2 and ISL1 expression.
To verify whether brain organoids have matured, general neuronal markers can be used, such as class III β-tubulin, neuronal nuclei, and microtubule-associated protein 2. In addition, neurotransmitter transporters, such as GFAP, S100β, VGLUT1 and VGLUT2, and vesicular GABA transporter, synapse proteins, such as AMPA Receptor 1, SYT1, and Shiga toxin, and NRXN and NLGN1 expression have been proposed in several studies, suggesting that they are expressed by mature neurons.
c.Functional assay
Brain organoids fabricated ex vivo have properties that are similar to human brains, including the production of neurons with the functions of actual brain regions, incomplete but normal development of the human cerebral cortex, and action potentials. Although it has not been clearly elucidated how similar brain organoids are to actual brain regions, research is currently ongoing.
In addition to research on immunohistology and gene expression, many studies have used electrophysiological recordings from neurons isolated from organoids or organoid slices as further verification of neuron maturity. Watanabe et al. (2017) (80) made electrophysiological recordings from a three-month-old organoid slice and were able to detect tetrodotoxin-sensitive spike trains in half the recorded neurons, as well as during electrical stimulation of these neurons. Interestingly, when the investigators measured the unique properties of neurons in brain organoids (membrane potential, membrane resistance, capacitance, peak Na+ and K+ current), organoids and in vivo neurons were very similar.
Most cortical organoids showed glutamatergic and GABAergic neurons, while other neuron subtypes were observed in organoids resembling specific brain regions. For example, dopaminergic neurons that were judged to be mature based on immunohistological and electrophysiological data could be obtained from midbrain organoids.
The successful culture of brain organoids containing mature neurons, astrocytes, and neuroglial cells strongly implies that organoids can be used for modeling neurodevelopmental, neurological, and neurodegenerative diseases in humans. Patient-derived iPSCs can be easily obtained compared to patient-derived neurons and differentiated into organoids with the same genetic composition as the patient. These patient-derived organoids can be used to investigate disease-related phenotypes and the effects of genome editing or disease-related mutations on disease phenotype, or to test new drug candidates.
Methods for each quality evaluation metric:
a.Making tissue specimens
For histological analysis, the organoid should be prepared in frozen sections or paraffin-embedded sections. If brain organoids are embedded in ECM droplets such as Matrigel, the ECM should be removed by washing the harvested organoids with cold 1x phosphate-buffered saline (PBS; Welgene). The washed organoid is fixed in 4% paraformaldehyde for 30 minutes on ice, and then washed three times with 1x PBS. For frozen sectioning, the organoid is placed in a mold made of OCT matrix (Tissue-Tek) at −20℃, and continuous frozen sections are made at a thickness of 10∼20 μm. For paraffin-embedded sections, the organoid is dehydrated using different concentrations of ethanol, embedded in paraffin, solidified, and then sectioned into 5 μm slices for staining experiments.
b.Immunofluorescent staining
For immunofluorescent staining, frozen sections are kept at room temperature for 15 minutes and then permeabilized with 0.5% Triton X-100 for 15 minutes. Meanwhile, 1x PBS is prepared at room temperature containing 1% fetal bovine serum (FBS; Gibco), 0.5% bovine serum albumin (BSA; Sigma-Aldrich), 1.15% glycine (Sigma-Aldrich), and 0.1% Tween-20 (Sigma-Aldrich). The primary antibody is diluted in blocking buffer, and the specimen is incubated in the primary antibody for 24 hours at 4℃. After 24 hours, the sample is washed three times in 1x PBS, the Alexa Fluor-conjugated secondary antibody is diluted in blocking buffer, and the specimen is incubated in the secondary antibody for 1 hour at room temperature. After 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) staining to visualize the cell nuclei, the specimens are imaged using confocal microscopy.
c.Immunohistochemical staining
For immunohistochemical staining, paraffin sections are deparaffinized using xylene, and then hydrated while reducing the ethanol concentration. The sections are then stained for 10 minutes at room temperature using hematoxylin (Bio Optica) and 1 minute using eosin (Bio Optica), before incubation in alcohol and xylene, dehydration, and inspection using a light microscope.
d.TUNEL apoptosis assay
The DeadEndTM Fluorometric TUNEL System (Promega) assay is used to test the cytoplasm of mature organoids. First, paraffin-embedded sections are washed twice in 1x PBS for 5 minutes, then permeabilized for 5 minutes in PBS with 0.2% TritonⓇ X-100. After washing again, the sections are equilibrated for 10 minutes with 100 μL of equilibration buffer, labeled, and incubated in TdT reaction mixture in a dark, humid chamber for 60 minutes at 37℃. The sections are thoroughly washed to encourage the reaction, and DAPI staining is performed for 10 minutes at room temperature to visualize all nuclei in the tissue. A fluorescence microscope is used to inspect the portion of apoptotic cells showing green fluorescence. Images are processed using software, such as ImageJ, and the apoptotic zone and nuclei are quantified. GraphPad Prism is used to calculate the data.
e.Western blot quantitative protein analysis
After obtaining the organic solvent using RIP analysis buffer containing a protease/phosphatase inhibitor cocktail, the total protein content of the solvent is isolated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel using bicinchoninic acid, and then transferred to a polyvinylidene fluoride membrane. The membrane is incubated in primary antibody and anti-beta-actin antibody in blocking buffer containing 5% non-fat dry milk on a shaker at 4℃. After washing the primary antibody, the membrane is incubated in horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. The solutions from the Clarity Western ECL Substrate (Bio-Rad) kit are mixed in a 1:1 ratio, and a chemiluminescence imaging system equipped with a CCD camera is used to take measurements at a wavelength of 428 nm.
f.Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
RNA is isolated from the brain organoid and complementary DNA is synthesized. Gene expression levels are measured using real-time PCR with gene-specific primers using the RT SYBR Green qPCR Mastermix and LightCycler 480 Instrument II (Roche). The expression levels of each gene are quantified using HPRT1 and GAPDH and calculated using the two methods (Supp) of the ACTB housekeeping gene and log transformation (ΔΔCt).
g.Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) is a method that can be used for fluorescence-activated classification of constituent cells. Organoids are acquired, washed in 1x PBS, and stored in 1 mL of DMEM/F12. The organoids are incubated for 30 minutes at 37℃ in papain (Sigma-Aldrich) (18.6 U/mL) and DNAse1 (Sigma-Aldrich) (337 U/mL). After adding 2% FBS to stop the enzyme reaction, the resulting single-cell suspension is centrifuged for 5 minutes at 400 ×g. The cells are incubated in PBS buffer (pH 7.4; containing 2 mM EDTA, 1% FBS, and 337 U/mL DNAse 1) for 15 minutes at room temperature, and then single cells are passed through a 70 μm cell strainer. The single cells are then labeled with primary antibody in FACS buffer (filtered 1x PBS at pH 7.4, containing 2% BSA, 50 mM EDTA, and 5 ng/mL stem cell factor; PeproTech) to classify certain cell type. After centrifugation and washing with 1x PBS, the single cells are labeled with Alexa fluor-conjugated secondary antibody in FACS buffer and incubated for 1 hour at room temperature. To detect dead cells, the cells are stained with DAPI after washing with 1x PBS. Thereafter, a 100 μm nozzle tip is used and living cells, single cells, and fluorescence-activated cells are classified using the FACSAria III (BD Biosciences).
h.Patch clamp
Patch-clamp technique is a versatile electrophysiological analysis method for understanding the movement of ion channels. Cell types that can be studied using patch-clamp technique include neurons, muscle fibers, cardiomyocytes, and oocytes that overexpress single ion channels. In the case of organoids, analysis can be performed by measuring membrane potential changes in neurons and glial cells located on the surface. For this purpose, organoids are prepared by attaching them to a dish coated with 100 μg/mL poly-L-ornithine (Sigma-Aldrich) and 10 μg/mL laminin (Sigma-Aldrich).
The organoid for patch clamp assay must be treated with artificial cerebrospinal fluid and extracellular solution for 2 hours, at room temperature, in 5% CO2/95% O2 before the experiment (81-83).
i.MEA
Microelectrode arrays (MEAs) are methods for analyzing electrophysiological signals using devices that contain microelectrodes for acquiring or transmitting neural signals. When an action potential, the basic unit of neural communication, occurs, the flow of ions across the cell membrane generates a rapid voltage change in the extracellular environment. MEAs detect these voltage changes using multiple electrodes. Various commercially available platforms can be used to apply this analysis method to brain organoids. For example, the analysis method is as follows when using an MEA system made in a 12-well plate format: A 12-well MEA plate is prepared. Each well contains sixty-four 30 μm-diameter, low-impedance (0.04 MΩ) platinum microelectrodes at 200 μm intervals. Before starting the experiment, a constant potential of −30 mV is applied to the electrode for 2.5 minutes to reduce noise. In the second week after adding the organoids (the 8th week after organoid differentiation), medium exchange is performed once per week, and the MEA is measured in the 0.1 Hz to 5 kHz frequency range 24 hour later. The plate is placed in the apparatus and, after waiting for 3 minutes, data is recorded over a 4 minutes interval. Electrodes with at least 5 spikes detected per minute are defined as active electrodes. The recorded data from each electrode should show bursts measured at least five times at 100 ms intervals (84, 85).
j.Quality assessment guide
The quality assessment guide is summarized in Table 1.
In this chapter, we describe the progress of research using brain organoids, as well as the characteristics of brain organoids and the latest approaches being used in non-clinical research. Notably, standard guidelines for toxicity and safety assessments using brain organoids have not yet been established, and since there are no techniques to prove the outcomes of comparative studies based on previous animal studies, we have decided not to describe standard experimental methods for this area of research.
Non-clinical research approaches using brain organoids:
a.Research on developmental disorders
Abnormal differentiation, migration, and maturation of NSCs and neuronal precursor cells (NPCs), which play important roles in brain development, are known to be major causes of brain developmental disorders (86). Stru-tural deformity of the brain tissue especially cannot be corrected by surgery or drug therapy after birth, and so most brain developmental disorders are intractable. Brain developmental disorders are severe diseases that not only impair the patient’s own quality of life, but are also more broadly associated with various social issues related to disability. There is growing recognition of the importance of understanding the pathogenic mechanisms of these diseases and developing new treatment approaches.
With the development of disease modeling techniques using brain organoids, there is active research generating organoids with the genetic characteristics of developmental disorders, and constructing ex vivo culture models. These techniques are being used to investigate the causes of developmental disorders and suggest new treatment approaches.
1)Microcephaly
In 2013, Lancaster et al. (31) modeled the genetic disease of microcephaly using whole-brain organoids. According to that study, whole-brain organoids derived from patients with the CDK5RAP2 gene variant, which causes microcephaly, were significantly smaller than normal-type organoids. In 2017, Li et al. (47) modeled microcephaly by generating cerebral cortex organoids from iPSCs with biallelic mutations of the ASPM gene. Organoids with ASPM mutations were characterized by incomplete neuroepithelial structure and reduced numbers of vRG and oRG, which are progenitor cells in developing neuroepithelium. oRG cells are a type of NSC specifically found in the human cerebral cortex. Given that the above traits cannot be observed in animal models, including mice, this study demonstrates the importance of human brain organoids for modeling developmental disorders. There has been continual research from numerous laboratories using brain organoids to model microcephaly associated with genetic variants (48, 49). Interestingly, there have also been efforts to model pathogen-induced microcephaly using cerebral cortex organoids. From 2016 to 2017, several overseas research teams infected cerebrum organoids with ZIKA virus to model the course of pathogen-induced microcephaly in infants whose mothers had been infected with the virus during pregnancy. Additionally, the researchers investigated the pathways by which ZIKA virus proteins induced cell death in fetal NSCs and studied the molecular physiological mechanisms causing microcephaly. Their findings demonstrate that models using human brain organoids can be used to investigate developmental disorders caused not only by genetic mutations, but also those caused by pathogens (50, 51).
2)ASD
ASD is a developmental disorder caused by a combination of many genetic and environmental factors. While the general symptoms of repetitive behavior, impaired social interactions, and impaired communication are similar in all patients, specific problem behaviors can vary greatly between individual patients (16). This makes it very difficult to study the diagnostic criteria and causes of ASD. As such, many researchers in Korea and abroad have been using brain organoids to model different types of ASD, and to try to categorize ASD types depending on the main pathological and pathogenic characteristics.
In 2015, Mariani et al. (52) observed that overexpression of FOXG1 transcription factor in whole-brain organoids derived from ASD patients resulted in faster cell cycles for GABAergic inhibitory neurons compared to normal type organoids. This resulted in excessive production of GABAergic inhibitory neurons, disrupting the excitatory-inhibitory balance of the brain tissue.
Similarly, in 2017, Wang et al. (53) observed abnormal expression of the DLX gene, which is important for the development GABAergic inhibitory neurons, in whole-brain organoids with a deficiency in the chromosomal remodeling factor CDH8. This resulted in the excessive production of GABAergic inhibitory neurons.
In 2020, Hali et al. (54) analyzed whole-brain organoids derived from CNTNAP2-knockout mice and observed impaired production of GABAergic inhibitory neurons. By treating the developing organoids with the anticonvulsant retigabine, they were able to restore normal development of GABAergic inhibitory neurons. Importantly, whereas previous studies showed over-production of inhibitory neurons, this study reported the opposite finding, demonstrating that even among AD models, phenotypes can differ completely. Although this was only a proof-of-concept study, it demonstrates the feasibility of drug validation platforms using brain organoids.
3)Rett syndrome
Rett syndrome (RTT) is a severe developmental disorder mostly observed in female patients. It is accompanied by intellectual disability, motor impairment, and stereotyped behaviors, and is caused by mutations of the MECP2 gene on the X chromosome. RTT is the second most common cause of intellectual disability in females, after Down syndrome, and although various drugs are used to alleviate specific symptoms, there is no effective treatment for the disease itself. There have been many studies describing disease modeling, including animal studies, aiming to investigate the causes of RTT and develop new treatments. Brain organoids are playing an important role in this process.
In 2018, Mellios et al. (55) investigated whole-brain organoids generated using iPSCs from RTT patients, and found that MECP2 mutations are involved in abnormal migration of NPCs due to regulation of miR-199 and miR-214.
In 2020, Xiang et al. (56) manufactured cerebral organoids and medial ganglionic eminence organoids after establishing an ESC line with a MECP2 mutation through genetic engineering. The region-specific brain organoids showed abnormal expression of genes specific to GABAergic inhibitory neurons, abnormal cell morphology, and functionality.
Gomes et al. (57) reported interesting findings in 2020. The investigators modeled RTT using assembloids combining dorsal and ventral forebrain organoids, and recreated pathological phenotypes that could not be reproduced with single-region brain organoids. Intriguingly, in the disease model assembloids, interneurons produced in the ventral brain tissue showed problems with tangential migration towards the dorsal forebrain, and this was shown to be closely related to MECP2 gene mutations.
4)Timothy syndrome
The approach described above, using assembloids combining brain organoids from different regions, has also led to outstanding results in the modeling of another developmental disorder. Timothy syndrome (TS) is a severe genetic disorder caused by mutations in the L-type calcium channel (LTCC). In 2017, aiming to recreate the abnormal migration of interneurons observed in previous animal models of TS, Birey et al. (74) created assembloids consisting of cerebral cortical organoids and ventral forebrain organoids using iPSCs derived from TS patients. The TS assembloids showed abnormal migration of interneurons that was corrected after treatment with an LTCC inhibitor, demonstrating the feasibility of research using disease model assembloids to develop therapeutic agents.
5)Down syndrome
Down syndrome (DS) is a developmental disorder caused by an abnormal number of chromosome 21, which causes microcephaly and intellectual disorders (16). Anatomical characteristics include immature neuron development, small cerebral hemispheres, and cerebellar atrophy. DS is one of the most common developmental disorders, and has been heavily studied, but the development of brain organoid techniques has enabled more in-depth research of the pathogenic mechanisms. In 2019, Xu et al. (58) observed excessive production of OLIG2-expressing progenitor cells in ventral forebrain organoids differentiated from DS patient iPSCs, resulting in over-production GABAergic inhibitory neurons. This shows that the excitatory-inhibitory neuron imbalance known to occur in DS can be recreated in ex vivo culture models.
Tang et al. (59) generated and studied whole-brain organoids generated from DS patient-derived iPSCs and observed decreased neurogenesis. In particular, they observed severely decreased expression of markers for neurons comprising layers 2 and 4 of the cerebral cortex and showed that this was related to the development of microcephaly in DS patients. Notably, the authors demonstrated that the neurogenesis and the size of the organoids could be restored by inhibiting DSCAM/PAK1 signaling. Various models of developmental disorders are continually being studied, including lissencephaly and schizophrenia, helping to elucidate the complex pathogenesis of human brain tissue deformities (60, 61).
b.Degenerative brain disease
There have been consistent efforts to model diseases using neural cells differentiated from patient-derived iPSCs to investigate the causes of degenerative brain diseases, such as AD and PD, and to develop novel treatments. Nevertheless, two-dimensional culture platforms are unable to reproduce the authentic environmental conditions of human tissue. Even if the cells have the same genetic defects as patients, they cannot completely reproduce the physiological phenotypes of these diseases. Accordingly, there has been a continual demand to develop disease model platforms that can recreate the characteristics of brain tissue, including structural characteristics, cell composition, microenvironment, and intercellular interactions.
Refined brain organoid-based research platforms exhibit histological properties that cannot be recreated in two-dimensional cell cultures. These platforms are expected to overcome the limitations of previous degenerative brain disease models, and to produce qualitatively and quantitatively outstanding research outcomes.
1)Alzheimer’s disease
AD is usually a late-onset disease caused by natural aging. Approximately 60%∼80% of dementia patients suffer from memory loss and cognitive impairment due to AD. The pathological characteristics of AD include protein aggregates (such as amyloid beta), tau plaques, and synaptic dysfunction. Brain organoids may not be suitable for modeling AD, as a late-onset disease, because the organoids model the relatively early stages of development. However, since brain organoids recapitulate most properties of human brain tissue, various studies have been conducted, limited to familial AD with genetic cause (Table 2). In fact, brain organoids with genetic mutations are better able to reproduce the representative pathological phenotypes of AD than normal brain organoids.
In 2021, Yin and VanDongen (62) modeled AD by comparing brain organoids with a PSEN2N mutation with healthy brain organoids with normal PSEN2N due to gene correction. When the organoids were treated with a drug that could activate neurons, the brainwaves of the AD model brain organoids recovered to a similar appearance to the normal brain organoids. This demonstrates the feasibility of brain organoid-based disease modeling and drug screening for AD.
In 2020, Zhao et al. (63) succeeded in observing pathological phenotypes related to APOE4 in brain organoids with an abnormal APOE gene. AD brain organoids showed increased apoptosis and synaptic dysfunction, as well as increased levels of amyloid beta and phosphorylated tau protein.
Pérez et al. (2021) (64) used iPSCs lacking the PITRM1 gene (a mitochondrial peptidase) to create brain organoids that model the pathological features of AD. The model brain organoids showed characteristic aggregates of proteins such as amyloid beta, formation of tau plaques, and synaptic dysfunction.
Furthermore, studies have been conducted using brain organoid models of familial AD with various genetic mutations, modeling the representative pathology of AD ex vivo, or suggesting new therapeutic approaches to correct the disease.
2)Parkinson’s disease
PD is a degenerative brain disease caused by selective cell death of dopaminergic neurons in the midbrain. It is a severe, intractable disease that causes various symptoms, such as spasms, loss of motor function, and multiple organ failure, ultimately causing early death due to complications. Although most of the pathogenic mechanisms are closely related to aging, like AD, there are familial forms of PD that can develop at an early age due to genetic mutations, and there has been much research attempting to model familial PD using midbrain organoids (Table 2).
In 2019, Kim et al. (66) used midbrain organoids with a G2019S mutation of the LRRK2 gene to model PD. The authors discovered a correlation between the pathological presentation of PD caused by LRRK2 and thioredoxin-interacting protein. In 2021, Jo et al. (67) used GBA1 knockout ESCs and SNCA-overexpressing ESCs to develop a midbrain organoid model of familial PD. Interestingly, the researchers found that the organoids contained aggregates similar to Lewy bodies, which are a central phenotype of PD, showing that refined midbrain organoid models may be used to study the relationships between PD pathogenesis and Lewy bodies.
Currently, compared to other intractable brain diseases, many of the prominent research findings for PD disease models have come from Korean research teams. These laboratories have established techniques to generate midbrain organoids and use these to study PD. By modeling familiar and idiopathic PD, they are continually discovering new biomolecular phenotypes that are thought to be strongly correlated with the development of PD.
Clinical research approaches using brain organoids:
a.Therapeutic drug screening using brain organoids
Alongside disease modeling studies on the pathogenic mechanisms and causes of disease, one of the clinical areas where brain organoids are expected to be most useful is drug screening studies. Drugs that were proposed from existing drug development platforms for the treatment of intractable brain diseases, based on animal models, have frequently failed efficacy testing at the clinical trial stage. The main cause of this is widely thought to be anatomical and physiological differences between human and animal models.
Studies using organoids as refined models of intractable brain diseases, which can reproduce the anatomical and physiological characteristics of human brain tissue to a high degree, are being conducted to discover and develop drugs that can alleviate these diseases. These therapeutic options are expected to be used in clinical practice in the future.
Indeed, the technological obstacles to implementing these drug screening methods are shrinking. There have been efforts to combine other technologies with brain organoids, such as the development of a HTS technique that can rapidly read diverse biological information from brain organoids, despite their complex three-dimensional structure (87), and MEA measurement techniques that can measure and analyze electrophysiological signals from the numerous neurons in brain organoids (88). These efforts could form the basis for large-scale screening of new drug candidates that can treat or alleviate pathological phenotypes of intractable brain diseases.
b.Use of brain organoids for toxicity testing
Brain organoids generally display early-stage development. Due to this characteristic, there are many difficulties modeling late-onset diseases, such as degenerative brain diseases. However, researchers have aimed to develop toxicity testing platforms, taking advantage of the ability of early brain tissue to respond more sensitively to toxic environments than mature brain tissue (89). We may gain a clinical advantage by using brain organoids to evaluate the neurotoxicity of drugs or environmental substances. Brain organoids may replace conventional animal-based toxicity testing to evaluate and categorize toxic environmental substances that may negatively affect developing brain tissue. They could also be used in pre-clinical research platforms to test the neurotoxicity of newly developed drug candidates. In particular, for new drug candidates that are not based on existing compounds, such as biopharmaceuticals, testing and evaluating safety in humans is very difficult. Thus, the development of next-generation testing platforms, such as refined brain organoid models, is extremely important.
In 2019, with coronavirus disease 2019 (COVID-19) elevating global fears of infectious disease, a new form of mRNA vaccine was supplied to the public without thorough safety testing, leading many people to suffer from adverse effects of the vaccine (90, 91). Through continual epidemiological studies and follow-up studies, it was strongly suggested that the adverse effects of this mRNA vaccine could be due to toxicity of lipid nanoparticles (LNPs) used in the vaccine delivery system.
However, there is no research platform that can test and evaluate the human safety of novel therapeutic agents, such as LNP-based delivery systems, which have been commercialized recently. The problems caused by the COVID-19 mRNA vaccine were observed throughout the human body, including the brain, heart, and gastrointestinal tract, which could not be predicted from conventional animal-based toxicity tests (90, 91). This can be considered powerful evidence of the inadequacy of existing animal and cell culture models for testing the safety of biopharmaceuticals developed in the future.
Since organoids can recreate, to a high degree, the anatomical complexity of in vivo tissues while maintaining human-specific physiological characteristics, the continual research and development of organoid techniques will be essential for the implementation of reliable, refined ex vivo culture models through convergence with techniques in other fields. In the future, research platforms to test the safety and efficacy of pharmaceuticals will need to be constructed based on standardized, refined brain organoid platforms. Given that organoids can overcome the limitations of existing pre-clinical research platforms using animal models and two-dimensional cell culture models, while also providing more accurate, reliable results, the limits of brain organoid research models are unclear.
However, due to the lack of standardization, current methods for the generation of brain organoids have not addressed the problems regarding low yield and reproducibility, and have also shown several limitations, such as incomplete maturation due to the culture environment lacking microenvironmental factors. Moreover, most existing neurotoxicity models are based on evaluating animal behavior, and further research is required in this regard.
We believe that these problems must be addressed by refining brain organoid generation methods and developing cutting-edge and convergent technologies in other fields.
Notes
Authors’ Contribution
Conceptualization: TK, JHY. Data curation: SL, YS. Funding acquisition: SJA. Investigation: TK, SHP, SL. Methodology: SHP, SL. Project administration: SJA, WS, JHY. Supervision: SJA, WS, JHY. Visualization: TK, SHP, YS. Writing – original draft: TK, SHP, SJA, WS, JHY. Writing – review and editing: KJY, SWC, JCP, SHY, HC, HII.
References
1. Vieira de Sá R, Cañizares Luna M, Pasterkamp RJ. 2021; Advances in central nervous system organoids: a focus on organoid-based models for motor neuron disease. Tissue Eng Part C Methods. 27:213–224. DOI: 10.1089/ten.tec.2020.0337.

2. Pacitti D, Privolizzi R, Bax BE. 2019; Organs to cells and cells to organoids: the evolution of in vitro central nervous system modelling. Front Cell Neurosci. 13:129. DOI: 10.3389/fncel.2019.00129. PMID: 31024259. PMCID: PMC6465581.
3. Costamagna G, Comi GP, Corti S. 2021; Advancing drug discovery for neurological disorders using iPSC-derived neural organoids. Int J Mol Sci. 22:2659. DOI: 10.3390/ijms22052659. PMID: 33800815. PMCID: PMC7961877.

4. Giorgi C, Lombardozzi G, Ammannito F, et al. 2024; Brain organoids: a game-changer for drug testing. Pharmaceutics. 16:443. DOI: 10.3390/pharmaceutics16040443. PMID: 38675104. PMCID: PMC11054008.

5. Hong YJ, Lee SB, Choi J, Yoon SH, Do JT. 2022; A simple method for generating cerebral organoids from human pluripotent stem cells. Int J Stem Cells. 15:95–103. DOI: 10.15283/ijsc21195. PMID: 35220295. PMCID: PMC8889334.

6. Kim J. 2022; Lo and behold, the lab-grown organs have arrived! Int J Stem Cells. 15:1–2. DOI: 10.15283/ijsc22026. PMID: 35220287. PMCID: PMC8889329.

7. Chukwurah E, Osmundsen A, Davis SW, Lizarraga SB. 2019; All together now: modeling the interaction of neural with non-neural systems using organoid models. Front Neurosci. 13:582. DOI: 10.3389/fnins.2019.00582. PMID: 31293366. PMCID: PMC6598414.

8. Makrygianni EA, Chrousos GP. 2021; From brain organoids to networking assembloids: implications for neuroendocrinology and stress medicine. Front Physiol. 12:621970. DOI: 10.3389/fphys.2021.621970. PMID: 34177605. PMCID: PMC8222922.

9. Bhattacharya A, Choi WWY, Muffat J, Li Y. 2022; Modeling developmental brain diseases using human pluripotent stem cells-derived brain organoids - progress and perspective. J Mol Biol. 434:167386. DOI: 10.1016/j.jmb.2021.167386. PMID: 34883115.

10. Wang H. 2018; Modeling neurological diseases with human brain organoids. Front Synaptic Neurosci. 10:15. DOI: 10.3389/fnsyn.2018.00015. PMID: 29937727. PMCID: PMC6002496.

11. Wray S. 2021; Modelling neurodegenerative disease using brain organoids. Semin Cell Dev Biol. 111:60–66. DOI: 10.1016/j.semcdb.2020.05.012. PMID: 32513498.

12. Salick MR, Lubeck E, Riesselman A, Kaykas A. 2021; The future of cerebral organoids in drug discovery. Semin Cell Dev Biol. 111:67–73. DOI: 10.1016/j.semcdb.2020.05.024. PMID: 32654970.

13. Sun N, Meng X, Liu Y, Song D, Jiang C, Cai J. 2021; Applications of brain organoids in neurodevelopment and neurological diseases. J Biomed Sci. 28:30. DOI: 10.1186/s12929-021-00728-4. PMID: 33888112. PMCID: PMC8063318.

14. Tang XY, Wu S, Wang D, et al. 2022; Human organoids in basic research and clinical applications. Signal Transduct Target Ther. 7:168. DOI: 10.1038/s41392-022-01024-9. PMID: 35610212. PMCID: PMC9127490.

15. Grenier K, Kao J, Diamandis P. 2020; Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol Psychiatry. 25:254–274. DOI: 10.1038/s41380-019-0500-7. PMID: 31444473.

16. Susaimanickam PJ, Kiral FR, Park IH. 2022; Region specific brain organoids to study neurodevelopmental disorders. Int J Stem Cells. 15:26–40. DOI: 10.15283/ijsc22006. PMID: 35220290. PMCID: PMC8889336.

17. Yadav A, Seth B, Chaturvedi RK. 2021; Brain organoids: tiny mirrors of human neurodevelopment and neurological disorders. Neuroscientist. 27:388–426. DOI: 10.1177/1073858420943192. PMID: 32723210.

18. Muzio L, Consalez GG. 2013; Modeling human brain development with cerebral organoids. Stem Cell Res Ther. 4:154. DOI: 10.1186/scrt384. PMID: 24367992. PMCID: PMC4055082.

19. Qian X, Song H, Ming GL. 2019; Brain organoids: advances, applications and challenges. Development. 146:dev166074. DOI: 10.1242/dev.166074. PMID: 30992274. PMCID: PMC6503989.

20. Zhao HH, Haddad G. 2024; Brain organoid protocols and limitations. Front Cell Neurosci. 18:1351734. DOI: 10.3389/fncel.2024.1351734. PMID: 38572070. PMCID: PMC10987830.

21. Kim SH, Chang MY. 2023; Application of human brain organoids-opportunities and challenges in modeling human brain development and neurodevelopmental diseases. Int J Mol Sci. 24:12528. DOI: 10.3390/ijms241512528. PMID: 37569905. PMCID: PMC10420018.

22. Zhou Z, Cong L, Cong X. 2021; Patient-derived organoids in precision medicine: drug screening, organoid-on-a-chip and living organoid biobank. Front Oncol. 11:762184. DOI: 10.3389/fonc.2021.762184. PMID: 35036354. PMCID: PMC8755639.

23. Chen CC, Li HW, Wang YL, et al. 2022; Patient-derived tumor organoids as a platform of precision treatment for malignant brain tumors. Sci Rep. 12:16399. DOI: 10.1038/s41598-022-20487-y. PMID: 36180511. PMCID: PMC9525286.

24. Korhonen P, Malm T, White AR. 2018; 3D human brain cell models: new frontiers in disease understanding and drug discovery for neurodegenerative diseases. Neurochem Int. 120:191–199. DOI: 10.1016/j.neuint.2018.08.012. PMID: 30176269.

25. Liu S, He Y, Yin J, Zhu Q, Liao C, Jiang G. 2024; Neurotoxicities induced by micro/nanoplastics: a review focusing on the risks of neurological diseases. J Hazard Mater. 469:134054. DOI: 10.1016/j.jhazmat.2024.134054. PMID: 38503214.

26. Casey S, Carter M, Looney AM, et al. 2022; Maternal mid-gestation cytokine dysregulation in mothers of children with autism spectrum disorder. J Autism Dev Disord. 52:3919–3932. DOI: 10.1007/s10803-021-05271-7. PMID: 34505185. PMCID: PMC9349096.

27. Jarmund AH, Giskeødegård GF, Ryssdal M, et al. 2021; Cytokine patterns in maternal serum from first trimester to term and beyond. Front Immunol. 12:752660. DOI: 10.3389/fimmu.2021.752660. PMID: 34721426. PMCID: PMC8552528.

28. Rash BG, Grove EA. 2006; Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 16:25–34. DOI: 10.1016/j.conb.2006.01.004. PMID: 16426837.

29. Berman NE, Johnson JK, Klein RM. 1997; Early generation of glia in the intermediate zone of the developing cerebral cortex. Brain Res Dev Brain Res. 101:149–164. DOI: 10.1016/S0165-3806(97)00060-6. PMID: 9263589.

30. De Juan Romero C, Borrell V. 2015; Coevolution of radial glial cells and the cerebral cortex. Glia. 63:1303–1319. DOI: 10.1002/glia.22827. PMID: 25808466. PMCID: PMC5008138.

31. Lancaster MA, Renner M, Martin CA, et al. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI: 10.1038/nature12517. PMID: 23995685. PMCID: PMC3817409.

32. Paşca AM, Sloan SA, Clarke LE, et al. 2015; Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 12:671–678. DOI: 10.1038/nmeth.3415.

33. Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. 2008; Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 3:519–532. DOI: 10.1016/j.stem.2008.09.002. PMID: 18983967.

34. Huang WK, Wong SZH, Pather SR, et al. 2021; Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell. 28:1657–1670.e10. DOI: 10.1016/j.stem.2021.04.006. PMID: 33961804. PMCID: PMC8419002.

35. Matsumoto R, Suga H, Aoi T, et al. 2020; Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J Clin Invest. 130:641–654. DOI: 10.1172/JCI127378. PMID: 31845906. PMCID: PMC6994153.

36. Nakano T, Ando S, Takata N, et al. 2012; Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10:771–785. DOI: 10.1016/j.stem.2012.05.009. PMID: 22704518.

37. Pomeshchik Y, Klementieva O, Gil J, et al. 2021; Human iPSC-derived hippocampal spheroids: an innovative tool for stratifying Alzheimer disease patient-specific cellular phenotypes and developing therapies. Stem Cell Reports. 16:2838. DOI: 10.1016/j.stemcr.2021.10.003. PMID: 34758331. PMCID: PMC8581187.

38. Ballabio C, Anderle M, Gianesello M, et al. 2020; Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun. 11:583. DOI: 10.1038/s41467-019-13989-3. PMID: 31996670. PMCID: PMC6989674.
39. Jo J, Xiao Y, Sun AX, et al. 2016; Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 19:248–257. DOI: 10.1016/j.stem.2016.07.005. PMID: 27476966. PMCID: PMC5510242.

40. Smits LM, Reinhardt L, Reinhardt P, et al. 2019; Modeling Parkinson's disease in midbrain-like organoids. NPJ Parkinsons Dis. 5:5. DOI: 10.1038/s41531-019-0078-4. PMID: 30963107. PMCID: PMC6450999.

41. Kim H, Xu R, Padmashri R, et al. 2019; Pluripotent stem cell-derived cerebral organoids reveal human oligodendrogenesis with dorsal and ventral origins. Stem Cell Reports. 12:890–905. DOI: 10.1016/j.stemcr.2019.04.011. PMID: 31091434. PMCID: PMC6524754.

42. Mills RJ, Parker BL, Quaife-Ryan GA, et al. 2019; Drug screening in human PSC-cardiac organoids identifies pro-proliferative compounds acting via the mevalonate pathway. Cell Stem Cell. 24:895–907.e6. DOI: 10.1016/j.stem.2019.03.009. PMID: 30930147.

43. Takebe T, Sekine K, Enomura M, et al. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.

44. Takebe T, Enomura M, Yoshizawa E, et al. 2015; Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 16:556–565. DOI: 10.1016/j.stem.2015.03.004. PMID: 25891906.

45. Guan Y, Xu D, Garfin PM, et al. 2017; Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2:e94954. DOI: 10.1172/jci.insight.94954. PMID: 28878125. PMCID: PMC5621886.

46. Takasato M, Er PX, Becroft M, et al. 2014; Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 16:118–126. DOI: 10.1038/ncb2894. PMID: 24335651.

47. Li R, Sun L, Fang A, Li P, Wu Q, Wang X. 2017; Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell. 8:823–833. DOI: 10.1007/s13238-017-0479-2. PMID: 29058117. PMCID: PMC5676597.

48. Omer Javed A, Li Y, et al. 2018; Microcephaly modeling of kinetochore mutation reveals a brain-specific phenotype. Cell Rep. 25:368–382.e5. DOI: 10.1016/j.celrep.2018.09.032. PMID: 30304678. PMCID: PMC6392048.

49. Wang L, Li Z, Sievert D, et al. 2020; Loss of NARS1 impairs progenitor proliferation in cortical brain organoids and leads to microcephaly. Nat Commun. 11:4038. DOI: 10.1038/s41467-020-17454-4. PMID: 32788587. PMCID: PMC7424529.

50. Cugola FR, Fernandes IR, Russo FB, et al. 2016; The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 534:267–271. DOI: 10.1038/nature18296. PMID: 27279226. PMCID: PMC4902174.

51. Qian X, Nguyen HN, Song MM, et al. 2016; Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 165:1238–1254. DOI: 10.1016/j.cell.2016.04.032. PMID: 27118425. PMCID: PMC4900885.

52. Mariani J, Coppola G, Zhang P, et al. 2015; FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 162:375–390. DOI: 10.1016/j.cell.2015.06.034. PMID: 26186191. PMCID: PMC4519016.

53. Wang P, Mokhtari R, Pedrosa E, et al. 2017; CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol Autism. 8:11. DOI: 10.1186/s13229-017-0124-1. PMID: 28321286. PMCID: PMC5357816.

54. Hali S, Kim J, Kwak TH, Lee H, Shin CY, Han DW. 2020; Modelling monogenic autism spectrum disorder using mouse cortical organoids. Biochem Biophys Res Commun. 521:164–171. DOI: 10.1016/j.bbrc.2019.10.097. PMID: 31653345.

55. Mellios N, Feldman DA, Sheridan SD, et al. 2018; MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry. 23:1051–1065. DOI: 10.1038/mp.2017.86. PMID: 28439102. PMCID: PMC5815944.

56. Xiang Y, Tanaka Y, Patterson B, et al. 2020; Dysregulation of BRD4 function underlies the functional abnormalities of MeCP2 mutant neurons. Mol Cell. 79:84–98.e9. DOI: 10.1016/j.molcel.2020.05.016. PMID: 32526163. PMCID: PMC7375197.

57. Gomes AR, Fernandes TG, Vaz SH, et al. 2020; Modeling Rett syndrome with human patient-specific forebrain organoids. Front Cell Dev Biol. 8:610427. DOI: 10.3389/fcell.2020.610427. PMID: 33363173. PMCID: PMC7758289.

58. Xu R, Brawner AT, Li S, et al. 2019; OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of Down syndrome. Cell Stem Cell. 24:908–926.e8. DOI: 10.1016/j.stem.2019.04.014. PMID: 31130512. PMCID: PMC6944064.

59. Tang XY, Xu L, Wang J, et al. 2021; DSCAM/PAK1 pathway suppression reverses neurogenesis deficits in iPSC-derived cerebral organoids from patients with Down syndrome. J Clin Invest. 131:e135763. DOI: 10.1172/JCI135763. PMID: 33945512. PMCID: PMC8203468.

60. Jin M, Pomp O, Shinoda T, et al. 2017; Katanin p80, NuMA and cytoplasmic dynein cooperate to control microtubule dynamics. Sci Rep. 7:39902. DOI: 10.1038/srep39902. PMID: 28079116. PMCID: PMC5228124.

61. Srikanth P, Lagomarsino VN, Muratore CR, et al. 2018; Shared effects of DISC1 disruption and elevated WNT signaling in human cerebral organoids. Transl Psychiatry. 8:77. DOI: 10.1038/s41398-018-0122-x. PMID: 29643329. PMCID: PMC5895714.

62. Yin J, VanDongen AM. 2021; Enhanced neuronal activity and asynchronous calcium transients revealed in a 3D organoid model of Alzheimer's disease. ACS Biomater Sci Eng. 7:254–264. DOI: 10.1021/acsbiomaterials.0c01583. PMID: 33347288.

63. Zhao J, Fu Y, Yamazaki Y, et al. 2020; APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids. Nat Commun. 11:5540. DOI: 10.1038/s41467-020-19264-0. PMID: 33139712. PMCID: PMC7608683.

64. Pérez MJ, Ivanyuk D, Panagiotakopoulou V, et al. 2021; Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer's disease-like pathology in human cerebral organoids. Mol Psychiatry. 26:5733–5750. DOI: 10.1038/s41380-020-0807-4.

65. Kwak TH, Kang JH, Hali S, et al. 2020; Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson's disease modeling. Stem Cells. 38:727–740. DOI: 10.1002/stem.3163. PMID: 32083763.

66. Kim H, Park HJ, Choi H, et al. 2019; Modeling G2019S-LRRK2 sporadic Parkinson's disease in 3D midbrain organoids. Stem Cell Reports. 12:518–531. DOI: 10.1016/j.stemcr.2019.01.020. PMID: 30799274. PMCID: PMC6410341.

67. Jo J, Yang L, Tran HD, et al. 2021; Lewy body-like inclusions in human midbrain organoids carrying glucocerebrosidase and α-synuclein mutations. Ann Neurol. 90:490–505. DOI: 10.1002/ana.26166. PMID: 34288055. PMCID: PMC9543721.

68. Quadrato G, Nguyen T, Macosko EZ, et al. 2017; Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 545:48–53. DOI: 10.1038/nature22047. PMID: 28445462. PMCID: PMC5659341.

69. Renner M, Lancaster MA, Bian S, et al. 2017; Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 36:1316–1329. DOI: 10.15252/embj.201694700. PMID: 28283582. PMCID: PMC5430225.

70. Sloan SA, Darmanis S, Huber N, et al. 2017; Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 95:779–790.e6. DOI: 10.1016/j.neuron.2017.07.035. PMID: 28817799. PMCID: PMC5890820.

71. Marton RM, Miura Y, Sloan SA, et al. 2019; Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 22:484–491. DOI: 10.1038/s41593-018-0316-9. PMID: 30692691. PMCID: PMC6788758.

72. Madhavan M, Nevin ZS, Shick HE, et al. 2018; Induction of myelinating oligodendrocytes in human cortical spheroids. Nat Methods. 15:700–706. DOI: 10.1038/s41592-018-0081-4. PMID: 30046099. PMCID: PMC6508550.

73. Trujillo CA, Gao R, Negraes PD, et al. 2019; Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 25:558–569.e7. DOI: 10.1016/j.stem.2019.08.002. PMID: 31474560. PMCID: PMC6778040.

74. Birey F, Andersen J, Makinson CD, et al. 2017; Assembly of functionally integrated human forebrain spheroids. Nature. 545:54–59. DOI: 10.1038/nature22330. PMID: 28445465. PMCID: PMC5805137.

75. Xiang Y, Tanaka Y, Patterson B, et al. 2017; Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 21:383–398.e7. DOI: 10.1016/j.stem.2017.07.007. PMID: 28757360. PMCID: PMC5720381.

76. Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. 2017; Fused cerebral organoids model interactions between brain regions. Nat Methods. 14:743–751. DOI: 10.1038/nmeth.4304. PMID: 28504681. PMCID: PMC5540177.

77. Lee JH, Shin H, Shaker MR, et al. 2022; Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat Biomed Eng. 6:435–448. DOI: 10.1038/s41551-022-00868-4. PMID: 35347276.

78. Cooper F, Gentsch GE, Mitter R, et al. 2022; Rostrocaudal patterning and neural crest differentiation of human pre-neural spinal cord progenitors in vitro. Stem Cell Reports. 17:894–910. DOI: 10.1016/j.stemcr.2022.02.018. PMID: 35334218. PMCID: PMC9023813.

79. Lancaster MA, Knoblich JA. 2014; Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 9:2329–2340. DOI: 10.1038/nprot.2014.158. PMID: 25188634. PMCID: PMC4160653.

80. Watanabe M, Buth JE, Vishlaghi N, et al. 2017; Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 21:517–532. DOI: 10.1016/j.celrep.2017.09.047. PMID: 29020636. PMCID: PMC5637483.

81. Wickham J, Corna A, Schwarz N, et al. 2020; Human cerebrospinal fluid induces neuronal excitability changes in resected human neocortical and hippocampal brain slices. Front Neurosci. 14:283. DOI: 10.3389/fnins.2020.00283. PMID: 32372899. PMCID: PMC7186381.

82. Hill CL, Stephens GJ. 2021; An introduction to patch clamp recording. Methods Mol Biol. 2188:1–19. DOI: 10.1007/978-1-0716-0818-0_1. PMID: 33119844.

83. Passaro AP, Stice SL. 2021; Electrophysiological analysis of brain organoids: current approaches and advancements. Front Neurosci. 14:622137. DOI: 10.3389/fnins.2020.622137. PMID: 33510616. PMCID: PMC7835643.

84. Schröter M, Wang C, Terrigno M, et al. 2022; Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 47:530–544. DOI: 10.1557/s43577-022-00282-w.

85. Shin H, Jeong S, Lee JH, Sun W, Choi N, Cho IJ. 2021; 3D high-density microelectrode array with optical stimulation and drug delivery for investigating neural circuit dynamics. Nat Commun. 12:492. DOI: 10.1038/s41467-020-20763-3. PMID: 33479237. PMCID: PMC7820464.

86. Telias M, Ben-Yosef D. 2015; Neural stem cell replacement: a possible therapy for neurodevelopmental disorders? Neural Regen Res. 10:180–182. DOI: 10.4103/1673-5374.152361. PMID: 25883606. PMCID: PMC4392655.

87. Durens M, Nestor J, Williams M, et al. 2020; High-throughput screening of human induced pluripotent stem cell-derived brain organoids. J Neurosci Methods. 335:108627. DOI: 10.1016/j.jneumeth.2020.108627. PMID: 32032714.

88. Huang Q, Tang B, Romero JC, et al. 2022; Shell microelectrode arrays (MEAs) for brain organoids. Sci Adv. 8:eabq5031. DOI: 10.1126/sciadv.abq5031. PMID: 35977026. PMCID: PMC9385157.

89. Nickels SL, Modamio J, Mendes-Pinheiro B, Monzel AS, Betsou F, Schwamborn JC. 2020; Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson's disease. Stem Cell Res. 46:101870. DOI: 10.1016/j.scr.2020.101870. PMID: 32534166.
90. Anand P, Stahel VP. 2021; Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 15:20. DOI: 10.1186/s13037-021-00291-9. PMID: 33933145. PMCID: PMC8087878.
91. Brüssow H. 2021; COVID-19: vaccination problems. Environ Microbiol. 23:2878–2890. DOI: 10.1111/1462-2920.15549.
Table 1
Checklist for quality assessment of brain organoids
Table 2
Disease model studies of developmental and degenerative disorders using brain organoids
| Disease | Cell |
Dedifferentiation/ gene editing/ disease induction |
Organoid type | Phenotype | Reference |
|---|---|---|---|---|---|
|
Microcephaly (MCPH) |
Patient-derived fibroblasts | Dedifferentiation using lentiviral OSKM | Whole-brain organoid |
Small organoid size Immature neural differentiation |
Lancaster et al., 2013 (31) |
| Patient-derived fibroblasts | Dedifferentiation using retroviral OSKM | Cerebral cortex organoid |
Incomplete neuroepithelial structure Small organoid size Reduced vRG and oRG cell count Immature neurons |
Li et al., 2017 (47) | |
| Embryonic stem cells | ZIKA virus infection | Whole-brain organoid |
Increased apoptosis Reduced stem cell self-renewal Collapse of the neuroepithelium |
Cugola et al., 2016 (50) | |
| Pluripotent stem cells | ZIKA virus infection | Cerebral cortex organoid |
Small organoid size Increased apoptosis |
Qian et al., 2016 (51) | |
| Autism spectrum disorder (ASD) | Patient-derived fibroblasts | Dedifferentiation using retroviral OSKM | Forebrain organoid |
Increased FOXG1 expression Increased GABAergic inhibitory neurons |
Mariani et al., 2015 (52) |
| Patient-derived fibroblasts | CHD9 knockout by gene editing | Whole-brain organoid |
Abnormal DLX expression Increased GABAergic inhibitory neurons |
Wang et al., 2017 (53) | |
| CNTNAP2- knockout mouse fibroblasts | Dedifferentiation using retroviral OSKM | Whole-brain organoid | Increased GABAergic inhibitory neurons | Hali et al., 2020 (54) | |
|
Rett’s syndrome (RTT) |
Patient-derived fibroblasts | Dedifferentiation using retroviral OSKM | Whole-brain organoid |
Abnormal miR-199 and miR0214 expression Abnormal migration of neural progenitor cells |
Mellios et al., 2018 (55) |
| Embryonic stem cells | MECP2 gene knockout and insertion | Cerebral cortex organoid, medial ganglionic eminence organoid | Deformity and dysfunction of GABAergic inhibitory neurons | Xiang et al., 2020 (56) | |
| Patient-derived fibroblasts | Dedifferentiation using retroviral OSKM | Dorsal forebrain - ventral forebrain assembloid | Abnormal tangential migration of interneurons | Gomes et al., 2020 (57) | |
| Timothy syndrome (TS) | Patient-derived fibroblasts | Dedifferentiation using retroviral OSKM | Cerebral cortex - corpus striatum assembloid | Abnormal tangential migration of interneurons | Birey et al., 2017 (74) |
|
Down syndrome (DS) |
Patient-derived fibroblasts | Sendai virus-based OSKM | Forebrain organoid |
Overproduction of OLIG2-positive progenitors Overproduction of GABAergic inhibitory neurons |
Xu et al., 2019 (58) |
| Patient-derived fibroblasts | Sendai virus-based OSKM | Whole-brain organoid |
Small organoid size Abnormal DSCAM/PAK1 signaling Decreased neurogenesis |
Tang et al., 2021 (59) | |
| Alzheimer’s disease (AD) | Pluripotent stem cells | PSEN2 mutation correction through gene editing | Whole-brain organoid |
Small organoid size High Aβ42/Aβ40 ratio Abnormal Ca2+ homeostasis Excessive neuron activation |
Yin and VanDongen, 2021 (62) |
| Patient-derived fibroblasts | Dedifferentiation using an episomal vector | Whole-brain organoid |
Increased apoptosis Synaptic dysfunction Increased amyloid beta Increased phosphorylated tau |
Zhao et al., 2020 (63) | |
| Pluripotent stem cells | PITRM1 gene knockout through gene editing | Whole-brain organoid |
Synaptic dysfunction Amyloid beta aggregation Tau plaque formation |
Pérez et al., 2021 (64) | |
| Parkinson’s disease (PD) | Patient-derived fibroblasts | Dedifferentiation using a lentiviral OSKM | Midbrain organoid |
Increased sensitivity to toxicity Increased alpha synuclein Increased thioflavin T-positive cells |
Kim et al., 2019 (66) |
| Embryonic stem cells | GBA1 knockout and SNCA overexpression through gene editing | Midbrain organoid | Accumulation of Lew bodies | Jo et al., 2021 (67) |




 PDF
PDF Citation
Citation Print
Print




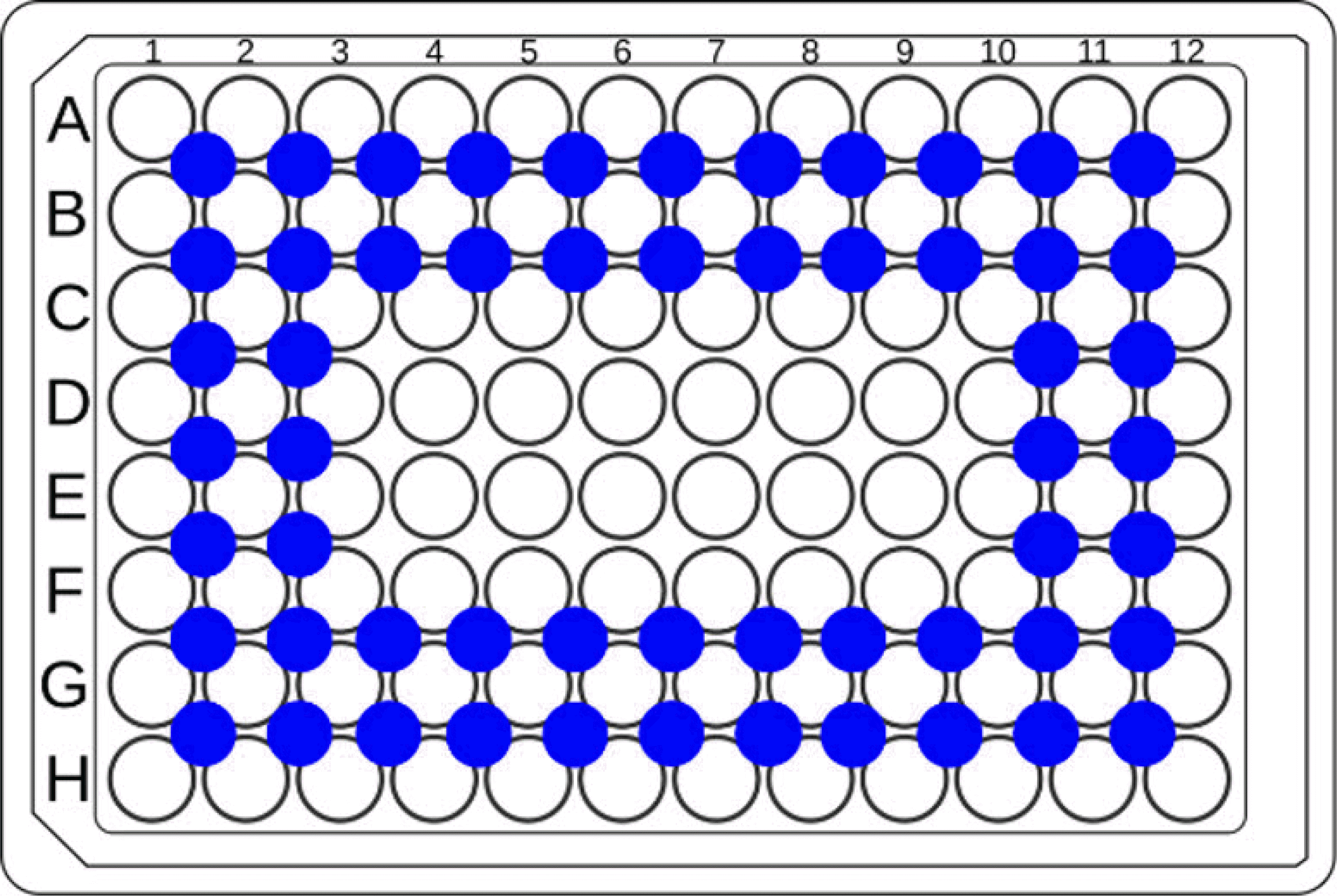
 XML Download
XML Download