Abstract
Recent advancements in organoid technology have led to a vigorous movement towards utilizing it as a substitute for animal experiments. Organoid technology offers versatile applications, particularly in toxicity testing of pharmaceuticals or chemical substances. However, for the practical use in toxicity testing, minimal guidance is required to ensure reliability and relevance. This paper aims to provide minimal guidelines for practical uses of kidney organoids derived from human pluripotent stem cells as a toxicity evaluation model in vitro.
Nephrotoxicity, the adverse effects of substances on kidney function, presents a significant challenge in drug development. Conventional preclinical models, such as animal studies and two dimensional cell cultures, often fail to accurately predict renal responses in humans, resulting in unexpected toxicities in clinical trials (1, 2). In recent years, there has been active research aimed at enhancing the generation and maturity of kidney organoids and these findings have opened up new approaches for research on kidney development, drug screening, and disease modeling. Moreover, they hold the promise of evolving into the generation of more organotypic kidneys for realizing the ambitious goal of generating transplantable synthetic kidneys or regenerative materials in the near future (3-5). Among these applications, kidney organoids has revolutionized nephrotoxicity assessment by offering a sophisticated in vitro model that closely mimics the structural and functional complexity of human kidneys (6-8). However, to harness the full potential of this innovative technology, systematic and standardized guidelines are essential for toxicity assessment using kidney organoids. Therefore, in order to evaluate nephrotoxicity with higher precision and efficiency, an innovative and reliable platform and guidelines for minimal standard criterial for generation of kidney organoid and toxicity evaluation by using them to control it are desperately needed. Therefore, in this paper, we would like to present the essential guidelines on the quality and functionality of the kidney organoid as in vitro renal model and the process the toxicity evaluation using them.
The main purpose of this standardization guideline is to provide reliable production of organoids suitable for toxicity testing and evaluation, along with standardized performance specifications (Fig. 1). This aims to support professionals in the field to easily achieve these standards. To achieve these objectives, these guidelines propose focusing on kidney organoids that comprise the fundamental components of the nephron, which play a crucial role in evaluating renal toxicity tests. These components include glomerular structure with podocytes and tubular structures (proximal/distal tubules) (9, 10). However, although limited to their basic composition, it is important that the structure, composition and function of these organoids are relevant to the human body. Moreover, generation of kidney organoids that closely resemble those of the human body is complicated, and industrial-scale production is often challenging. Therefore, efforts to develop and enhance different types of kidney organoids for specific purposes or objectives may need to continue at the laboratory level. Additionally, whenever new technological advancements applicable to mass production arise, these guidelines can be modified and improved to reflect such advancements. The scope of the guidelines for nephrotoxicity evaluation using kidney organoid is outlined as follows first guidelines on the manufacture of kidney organoids for toxicity evaluation, second guidelines on the functionality and quality of kidney organoids for toxicity evaluation and third guidelines on renal toxicity evaluation methods using kidney organoids (Fig. 2).
∙Pluripotent stem cells (PSCs): stem cells capable of differentiating into almost all types of cells constituting the endoderm, mesoderm, and ectoderm (including both induced pluripotent and embryonic stem cell [ESC] lines).
∙Organoid: a three-dimensional structure exhibiting similar structure, function, and composition to the human organ, created based on the differentiation/self-assembly ability of PSCs.
∙Kidney (nephron) organoid: constituted as the most fundamental unit of the kidney, consisting of glomeruli and tubular segments.
∙Primitive streak (PS): the beginning of gastrulation, marking the onset of mesoderm formation.
∙Intermediate mesoderm (IM): the intermediate layer of mesoderm, the origin of the urogenital system.
∙Metanephric mesenchyme (MM): mesenchymal cells derived from the IM, forming the nephrons of the kidney.
∙Renal toxicity evaluation: a method for quantitatively assessing cell damage (delayed growth, apoptosis) occurring in the kidney.
General recommendations: Manage according to general stem cell handling guidelines: Link to guidelines https://static1.squarespace.com/static/611faaa8fee (11).
Quality management recommendations: Manage according to general stem cell handling guidelines: Link to guidelines https://static1.squarespace.com/static/611faaa8fee 682525ee16489/t/647de42a1a18dd7bfb91e68e/1685972011644/ISSCR_Standards_09_FINAL.pdf (11).
Cell types, characteristics, and quality requirements:
a.PSCs
1) Cell types and characteristics
Including ESCs maintaining pluripotency (12) or induced pluripotent stem cells (iPSCs) (13) maintain pluripotency/multipotency, capable of differentiating into various cell types from three germ layers, autonomously replicating and proliferating through self-renewal, and sustaining overexpression of stem cell-specific genes including OCT4 and SOX2.
2) Quality requirements for cells
(1)Cell line authentication: confirmation of cell line identity through short tandem repeat profiling.
(2)Pluripotency marker expression: verification of the expression of specific pluripotency markers (such as OCT4, NANOG, SOX2) to confirm the cells’ pluripotent capabilities.
(3)Genomic stability: karyotype analysis or other assessments to confirm genomic stability.
(4)Teratoma formation capability: confirmation of the cells’ ability to form teratomas.
(5)Endotoxin and mycoplasma testing: verification to ensure absence of contaminants such as endotoxins and mycoplasma.
3) Cell suppliers
Specify the source of PSCs, including the name of the company, catalog number if purchased, or the name of the institution if obtained from a non-profit organization.
Preparation for kidney organoid generation:
a.Essential elements and reagents
1) Media components
Cell maintenance media including mTeSR1, or Stem-Flex for maintaining PSCs could be utilized. Cell differentiation media such as advanced Dulbecco’s modified Eagle’s medium/F12 for organoid generation could be employed.
2)Essential growth factors and reagents
CHIR99021, retinoic acid, bone morphogenetic protein 4, fibroblast growth factor 9 could be available for differentiation. Extracellular matrix equivalent to matrigel with similar functionality, inhibitors such as rock inhibitor, EMT inhibitor, or GSK inhibitor and detachment solution, antibiotics, fetal bovine serum, and phosphate-buffered saline could be used for differentiation.
Production process and culture environment:
a. Culture protocols
Stem cells are differentiated or induced according to the following sequence:
Maintaining of PSCs>Differentiation into PS>Differentiation into IM>Inducing MM (>nephron progenitor cell [NPC]>) >Organoid formation>Maturation.
The degree of differentiation at each step needs to be confirmed using well-known markers and quantitatively assessed such as TBX6 for PS, WT1, HOXD11, and OSR1 for IM (posterior IM), SIX2, WT1 and SALL1 for MM/NPC (6, 17). The most suitable culture plate or platforms are not specified, and any vessel or platform that allows reproducible production can be utilized including conventional cell culture plate, transwell inserts, spinner flasks, and low-attachment culture dish. Well-established public protocol and its modified version for the production of kidney organoid with ensured reproducibility could be available (Fig. 3) (7, 8, 18-25).
b. Culture environment conditions
Typically cultured at 37℃, 95% humidity, and 5% carbon dioxide, however, floating culture, rotational culture, etc., can be used depending on the purpose. This is just the most commonly used example, and reagents enabling reproducible production of kidney organoids for nephrotoxicity testing are available for use.
Organoid quality requirements:
a.Size and morphology
Typically producible size of mature kidney organoids, which allows for nephrotoxicity assessment and exhibits observable cellular structures, is around 200 micrometers or larger. Morphology of organoids with more than 80% tubular structures should be sufficiently observable on the surface under a microscope.
b.Cell composition
The kidney organoids should include approximately 10%∼20% podocytes, ∼40% proximal tubule cells, and ∼10% distal tubule cells, with qualitative and quantitative verification of specific markers for each cell type required such as PODXL for podocyte, LTL for proximal tubules and CDH1 for distal tubule (2, 26, 27). Quantitative assessment of cell composition should be confirmed using specific markers for each cell type through techniques such as fluorescence activated cell sorting or equivalent methods such as single cell transcriptomic analysis.
c.Confirmation of organ-specific functionality
The kidney organoids should express fictional transpor-ers such as peptide transporters (PEPTs) for apical part and organic cation transporters (OCTs) for basolateral transporters and should be confirmed qualitatively (via immunostaining) and quantitatively (via quantitative polymerase chain reaction (28). Transporter functionality should be confirmed by testing uptake of substances such as glucose. But these markers are a minimal and essential suggestions, other cell specific markers can be added to approve the functionality.
Monitoring of quality assessment results (batch-variation, periodic analysis, etc.):
Quality assessment of produced kidney organoids must satisfy the following criteria:
a.At least one organoid should be formed per unit area (mm2).
b.The coefficient of variation in size between formed organoids should be less than 10%.
c.Coefficient of variation of morphological variations between generated organoids should be less than 10%.
d.Coefficient of variation of functional characteristics between generated organoids should be less than 10%.
e.Quality of actual batches used for toxicity evaluation should be evaluated using optical microscopy or tomography to ensure compliance with the above four criteria.
The objective is to assess damage to podocytes and tubule cells (proximal/distal) using kidney organoids to predict the nephrotoxicity of substances. Equipment necessary to treat toxic test substances concentration-dependently and obtain quantitative results: culture plate or platforms capable of culturing homogeneous kidney organoids in bulk such as 96-well plates or 192-well plates. Equipment or devices capable of quantitatively assessing kidney organoid damage capable of quantitatively measuring cell death, renal toxicity markers like KIM1 (1).
Test protocol (procedure):
The protocol for nephrotoxicity testing using kidney organoids is as follows:
a.Selection of test substances.
b.Preparation of kidney organoids passing quality criteria.
c.Treatment of test substances: Treatment with various concentrations to determine IC50 and standard substance acquisition reacquired.
d.Confirmation of cell damage and survival: Including structural damage to kidney organoids.
e.Confirmation of kidney cell-specific toxicity markers: Detection of changes in podocyte or tubule cell-specific toxicity markers (both protein and gene levels, quantitative assessment results obtained).
f.Ensuring reproducibility through repeated assessments and statistical significance of toxic ranges.
g.Summary of results.
Standard format for analysis results (summary and certification): Evaluation reports should encompass general and specific aspects of the testing procedure. Results should include both qualitative and quantitative outcomes. Results of nephrotoxicity testing using kidney organoids should include structural damage to the organoids (29).
Storage protocol:
a.Freezing and thawing process
There are challenges in freezing the mature kidney organoids derived from iPSCs, but the methods are not still established; however, it is suggested to store them at the NPC stage (30) and proceed with organoid formation thereafter. In this case, standard methods for freezing and thawing cells can be utilized. Equipment and instruments such as freezing solution, and liquid nitrogen tank required for typical cell freezing and thawing.
b.Quality factors
Cell viability after thawed should be over 80%. In addition, absence of bacterial, mycoplasma, and viral infections should be ensured.
While there may be no ethical concerns directly related to the use of organoids, it should be noted that the cells used to generate organoids may originate from human-derived materials, which fall under the purview of bioethics laws regarding the use of human-derived materials. Therefore, any discomfort regarding this issue should be acknowledged.
Acknowledgments
Authors appreciated Organoid Standards Initiative committee members, raised considerable recommends to improve this guideline.
References
1. Kang HM, Lim JH, Noh KH, et al. 2019; Effective reconstruction of functional organotypic kidney spheroid for in vitro nephrotoxicity studies. Sci Rep. 9:17610. DOI: 10.1038/s41598-019-53855-2. PMID: 31772214. PMCID: PMC6879515.

2. Little MH, Combes AN. 2019; Kidney organoids: accurate models or fortunate accidents. Genes Dev. 33:1319–1345. DOI: 10.1101/gad.329573.119. PMID: 31575677. PMCID: PMC6771389.

3. Nishinakamura R. 2023; Advances and challenges toward developing kidney organoids for clinical applications. Cell Stem Cell. 30:1017–1027. DOI: 10.1016/j.stem.2023.07.011. PMID: 37541208.

4. Chae HK, Suh N, Jang MJ, et al. 2023; Efficacy and safety of human bone marrow-derived mesenchymal stem cells according to injection route and dose in a chronic kidney disease rat model. Int J Stem Cells. 16:66–77. DOI: 10.15283/ijsc21146. PMID: 35483715. PMCID: PMC9978839.

5. Kim YS, Aum J, Kim BH, et al. 2023; Therapeutic effect of three-dimensional cultured adipose-derived stem cell-conditioned medium in renal ischemia-reperfusion injury. Int J Stem Cells. 16:168–179. DOI: 10.15283/ijsc22137. PMID: 36310026. PMCID: PMC10226861.

6. Taguchi A, Kaku Y, Ohmori T, et al. 2014; Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 14:53–67. DOI: 10.1016/j.stem.2013.11.010. PMID: 24332837.

7. Freedman BS, Brooks CR, Lam AQ, et al. 2015; Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 6:8715. DOI: 10.1038/ncomms9715. PMID: 26493500. PMCID: PMC4620584.

8. Takasato M, Er PX, Chiu HS, et al. 2015; Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 526:564–568. DOI: 10.1038/nature15695. PMID: 26444236.

9. Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. 1991; Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 64:777–784.
10. Kobayashi A, Valerius MT, Mugford JW, et al. 2008; Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 3:169–181. DOI: 10.1016/j.stem.2008.05.020. PMID: 18682239. PMCID: PMC2561900.

11. The International Society for Stem Cell Research. Standards for human stem cell use in research. 2023. The International Society for Stem Cell Research.
12. Evans M. 2011; Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat Rev Mol Cell Biol. 12:680–686. DOI: 10.1038/nrm3190. PMID: 21941277.

13. Takahashi K, Yamanaka S. 2006; Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.

14. Omole AE, Fakoya AOJ. 2018; Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ. 6:e4370. DOI: 10.7717/peerj.4370. PMID: 29770269. PMCID: PMC5951134.

15. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.

16. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. 2000; Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 18:399–404. DOI: 10.1038/74447. PMID: 10748519.

17. Lindström NO, McMahon JA, Guo J, et al. 2018; Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol. 29:785–805. DOI: 10.1681/ASN.2017080887.

18. Combes AN, Phipson B, Lawlor KT, et al. 2019; Single cell analysis of the developing mouse kidney provides deeper insight into marker gene expression and ligand-receptor crosstalk. Development. 146:dev178673. DOI: 10.1242/dev.178673. PMID: 31118232.

19. Koning M, van den Berg CW, Rabelink TJ. 2020; Stem cell-derived kidney organoids: engineering the vasculature. Cell Mol Life Sci. 77:2257–2273. DOI: 10.1007/s00018-019-03401-0. PMID: 31807815. PMCID: PMC7275011.

20. Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. 2018; Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 23:869–881.e8. DOI: 10.1016/j.stem.2018.10.010. PMID: 30449713. PMCID: PMC6324730.

21. Low JH, Li P, Chew EGY, et al. 2019; Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell. 25:373–387.e9. DOI: 10.1016/j.stem.2019.06.009. PMID: 31303547. PMCID: PMC6731150.

22. Garreta E, Prado P, Tarantino C, et al. 2019; Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater. 18:397–405. DOI: 10.1038/s41563-019-0287-6. PMID: 30778227. PMCID: PMC9845070.

23. Kim JW, Nam SA, Yi J, et al. 2022; Kidney decellularized extracellular matrix enhanced the vascularization and maturation of human kidney organoids. Adv Sci (Weinh). 9:e2103526. DOI: 10.1002/advs.202103526. PMID: 35322595. PMCID: PMC9130892.

24. Yoshimura Y, Taguchi A, Tanigawa S, et al. 2019; Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol. 30:304–321. DOI: 10.1681/ASN.2018070747. PMID: 30635375. PMCID: PMC6362617.

25. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. 2015; Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 33:1193–1200. DOI: 10.1038/nbt.3392. PMID: 26458176. PMCID: PMC4747858.

26. Suhito IR, Kim JW, Koo KM, Nam SA, Kim YK, Kim TH. 2022; In situ detection of kidney organoid generation from stem cells using a simple electrochemical method. Adv Sci (Weinh). 9:e2200074. DOI: 10.1002/advs.202200074. PMID: 35506260. PMCID: PMC9284177.

27. Gupta N, Dilmen E, Morizane R. 2021; 3D kidney organoids for bench-to-bedside translation. J Mol Med (Berl). 99:477–487. DOI: 10.1007/s00109-020-01983-y. PMID: 33034708. PMCID: PMC8026465.

28. Yousef Yengej FA, Jansen J, Ammerlaan CME, et al. 2023; Tubuloid culture enables long-term expansion of functional human kidney tubule epithelium from iPSC-derived organoids. Proc Natl Acad Sci U S A. 120:e2216836120. DOI: 10.1073/pnas.2216836120. PMID: 36724260. PMCID: PMC9963523.

29. Soo JY, Jansen J, Masereeuw R, Little MH. 2018; Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol. 14:378–393. DOI: 10.1038/s41581-018-0003-9. PMID: 29626199. PMCID: PMC6013592.

30. Wiersma LE, Avramut MC, Lievers E, Rabelink TJ, van den Berg CW. 2022; Large-scale engineering of hiPSC-derived nephron sheets and cryopreservation of their progenitors. Stem Cell Res Ther. 13:208. DOI: 10.1186/s13287-022-02881-5. PMID: 35578313. PMCID: PMC9109372.

Fig. 2
Summary of guideline; minimal considerations and requirements of kidney organoid for toxicity test in vitro. PSCs: pluripotent stem cells, PS: primitive streak, IM: intermediate mesoderm, MM: metanephric mesenchyme, NPC: nephron progenitor cell, FGF9: fibroblast growth factor 9, BMP4: bone morphogenetic protein 4, ECM: extracellular matrix, PDC: podocytes, PTC: proximal tubule cells, DTC: distal tubule cell.
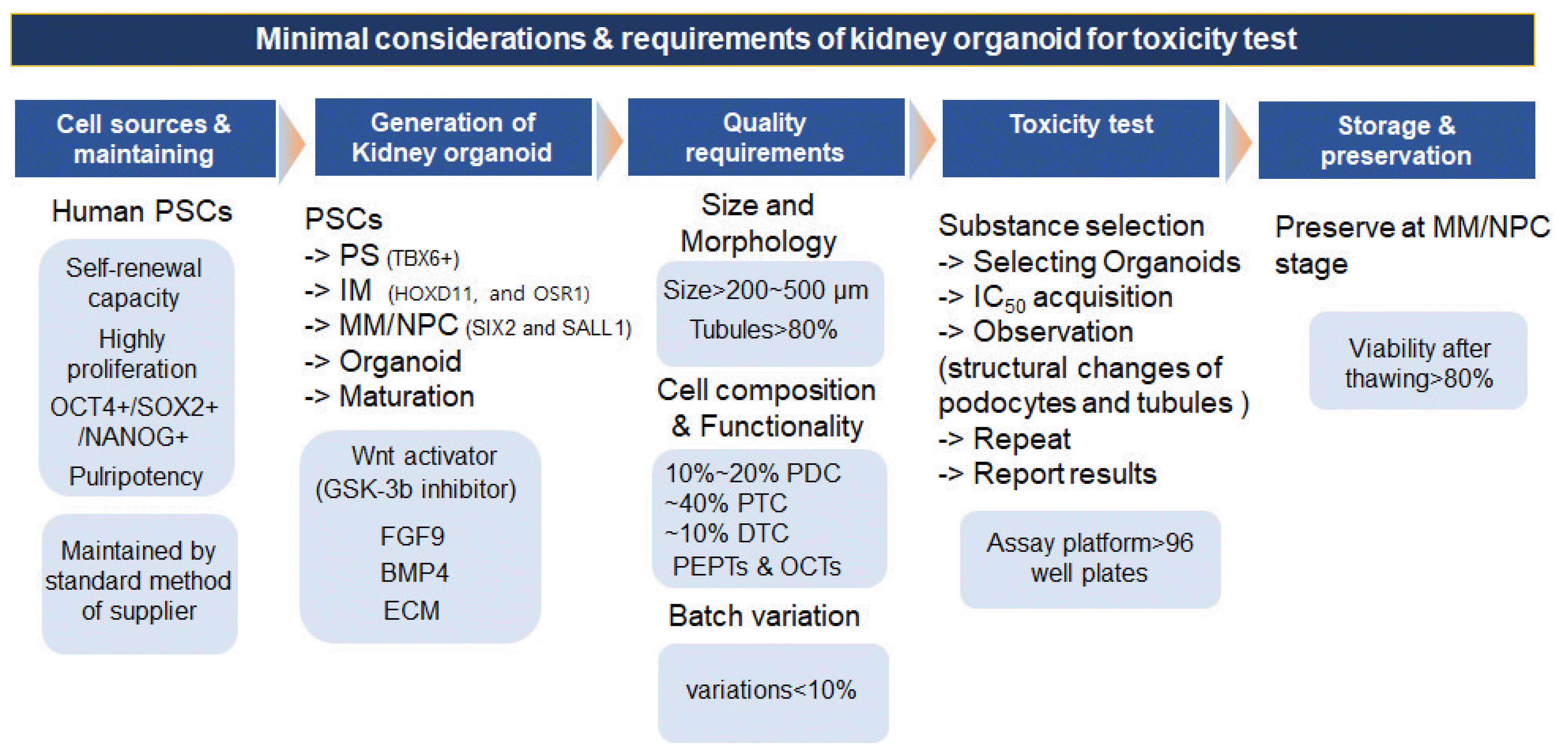
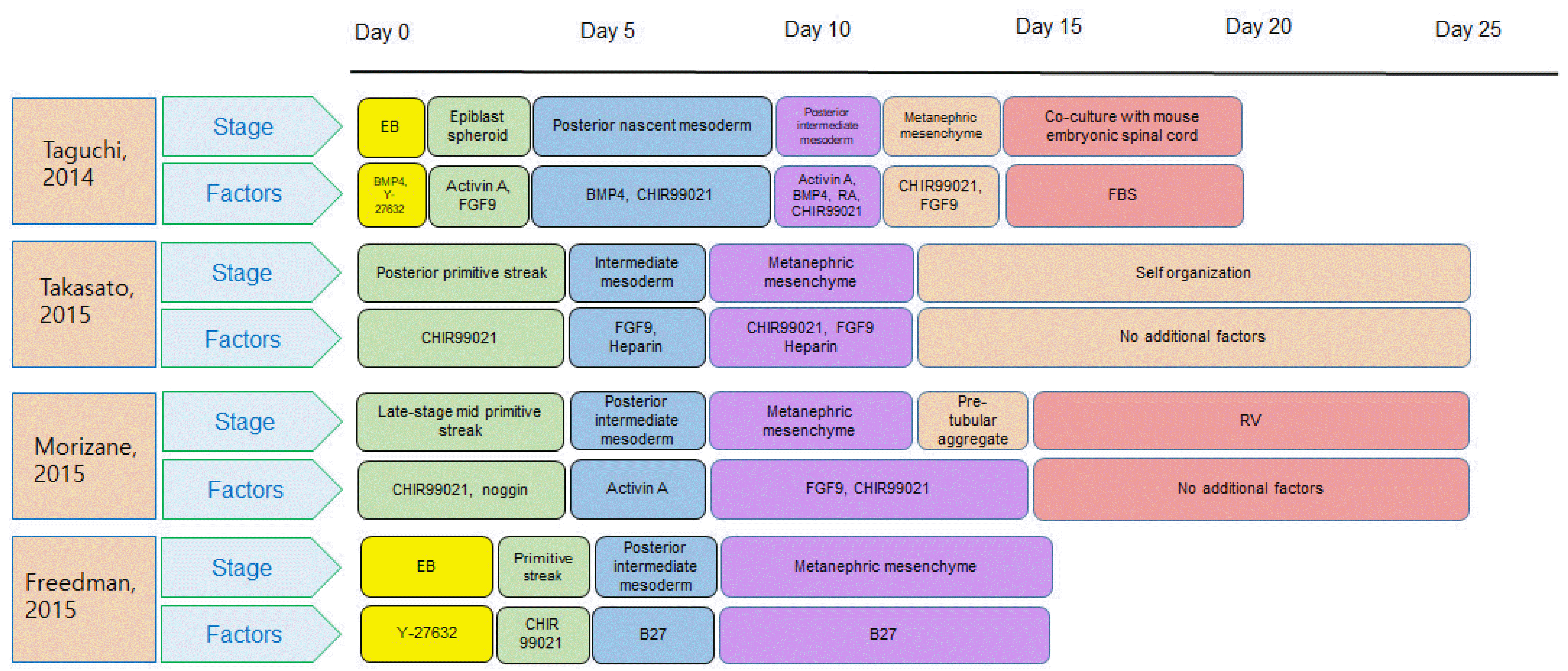
Fig. 3
Representative differentiation protocols for generation of kidney organoids. This serves as an examples of a representative differentiation protocols, but any reproducible differentiation protocol can be utilized for kidney organoids for nephrotoxicity assessments. EB: embryonic body, BMP4: bone morphogenetic protein 4, FGF9: fibroblast growth factor 9, FBS: fetal bovine serum, RV: renal vesicle.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download