Abstract
An organoid is a self-organized three-dimensional structure derived from stem cells that mimics the structure, cell composition, and functional characteristics of specific organs and tissues and is used for evaluating the safety and effectiveness of drugs and the toxicity of industrial chemicals. Organoid technology is a new methodology that could replace testing on animals testing and accelerate development of precision and regenerative medicine. However, large variations in production can occur between laboratories with low reproducibility of the production process and no internationally agreed standards for quality evaluation factors at endpoints. To overcome these barriers that hinder the regulatory acceptance and commercialization of organoids, Korea established the Organoid Standards Initiative in September 2023 with various stakeholders, including industry, academia, regulatory agencies, and standard development experts, through public and private partnerships. This developed general guidelines for organoid manufacturing and quality evaluation and for quality evaluation guidelines for organoid-specific manufacturing for the liver, intestines, and heart through extensive evidence analysis and consensus among experts. This report is based on the common standard guideline v1.0, which is a general organoid manufacturing and quality evaluation to promote the practical use of organoids. This guideline does not focus on specific organoids or specific contexts of use but provides guidance to organoid makers and users on materials, procedures, and essential quality assessment methods at end points that are essential for organoid production applicable at the current technology level.
Organoids are biologically engineered, self-organized structures derived from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), or neonatal or adult stem cells (ASCs) and can be used as in vitro models to help understand human and disease development processes of various organs (1). Organoids can provide partial transplantation as well as identify drug effectiveness and evaluate toxicity because they recapitulate structures and functions similar to those of real organs or tissues.
Organoids represent a groundbreaking frontier in biomedicine, offering unprecedented opportunities for personalized therapy (2). Although inexact replicas of native organs or tissues, they are capable of mimicking human physiological responses. This compatibility positions organoids as instrumental in advancing the principles of Replacement, Reduction, and Refinement, signaling a significant shift away from traditional animal models towards more human-relevant research models. Particularly, organoids have been employed in unraveling the pathophysiology of diseases impacting the brain and heart, highlighted by investigations into Zika virus and coronavirus disease 2019 (COVID-19), treating liver diseases using hepatocytes from organoids, showing the emergence of eye structures in human embryos in miniature organoid brains with optic cups, precursors to the retina. These findings highlight the potential of organoids in developing personalized healthcare solutions by enhancing our comprehension of disease mechanisms and drug responses based on individual genetic profiles. Regulatory bodies have been adapting to these scientific advancements, with leading health authorities, such as the U.S. Food and Drug Administration (FDA) in the United States and the European Medicines Agency, adopting New Approach Methodologies (NAMs) (3), which range from cell-based assays and microphysiological systems (organ chips) to bioprinting and in silico models, moving away from traditional animal testing. This transition is reflected in legislative changes, such as the FDA Modernization 2.0 Act, which emphasizes nonclinical over conventional testing.
However, replicating complex in vivo environments in vitro remains a challenge (4), requiring stringent quality control (QC), standardization, and efforts to incorporate organoids into regulatory frameworks. In response, the Ministry of Food and Drug Safety in Korea, in partnership with Sungkyunkwan University, initiated the Organoid Standards Initiative (Fig. 1) in September 2023. This effort seeks to define standard benchmarks for organoids, positioning them as a fundamental component of future biomedical innovations.
The guidelines aim at promoting the practical application of organoids, an emerging alternative in testing methodologies, by setting standards for researchers involved in the production or utilization of organoids, industry professionals engaged in the manufacture, distribution, and commercialization of organoids, and regulatory experts assessing organoids’ safety and efficacy. The key benefits of the guidelines include:
-Enhanced transparency, reproducibility, and reliability: recommendations for source cell management, culture, differentiation methods, characteristics analysis, and quality evaluation metrics will improve organoid experiment transparency, reproducibility, and reliability
-Consistency and quality assurance: ensures organoid production and QC adhere to high standard.
-Increased reliability for drug evaluation: boosts the reliability of organoid technologies in evaluating drug safety and efficacy.
-Guided practical application: aims at high reproducibility and the safe and efficient use of organoids in non-clinical/clinical applications.
Organoid research spans several organs, including the liver, intestines, heart, kidneys, lungs, brain, and skin, with applications in drug screening, safety and efficacy evaluation, disease modeling, regenerative medicine, and cell differentiation research. The guidelines seek to standardize minimum quality requirements for organoids to increase their utility in safety and efficacy evaluations. Developed through collaboration among academic, industrial, and governmental organoid experts, the guidelines promote organoid practical application by defining organoid characteristics and technical requirements for cell culture, quality maintenance, testing, and evaluation. The guidelines support the development of patient-specific organ-mimicking platforms for such applications as in pharmaceuticals, cosmetics, and cell therapies. Despite organoids’ potential, the varied manufacturing methods, and lack of standards for ensuring reproducibility pose challenges in quality evaluation and validation. Establishing standard guidelines is crucial for ensuring the safety, reproducibility, reliability, and ethical use of organoids, offering more reliable results in drug toxicity evaluation, and improving time and cost efficiencies. The guidelines set standards for creating organoids from stem and primary cells, focusing on research and clinical uses. They detail protocols for cell quality, culture conditions, and QC, including size, shape, and function metrics. Procedures for organoid storage and testing methods for toxicity and drug screening are also specified, ensuring uniformity across applications (Fig. 2).
-Primary cell: cells directly isolated from tissues or organs extracted from an organism using enzymatic or mechanical methods.
-Subculture or passage: process of further culturing cells in a new culture vessel to provide a higher surface area/ volume, allowing for cell growth.
-Cryopreservation: process of maintaining cells at ultra-low temperatures in an inactive state so they can be revived later.
-Multipotency/multipotent: ability of a part of the embryonic germ layer to differentiate into several types of cells, similar to stem cells, mainly apparent early in the developmental process.
-Microenvironment: location where molecules or complex factors, such as nutrients and growth factors, which play a crucial role in determining the properties of cells, exist.
-ESCs: undifferentiated cells derived from an embryo about 5 days post-fertilization, before implantation, capable of developing into the initial three germ layers.
-Transforming growth factor-β (TGF-β): Factor synthesized in various tissues, acting cooperatively with TGF-α to induce transformation and can also act as a negative autocrine growth factor. TGF-β plays a potential role in embryonic development, cell differentiation, hormone secretion, and immune function, mostly found in homodimeric forms of separate gene products, TGF-β1, TGF-β2, or TGF-β3. heterodimers composed of TGF-β1 and 2 (TGF-β1, 2) or TGF-β2 and 3 (TGF-β2, 3) have also been isolated, and TGF-β proteins are synthesized as precursor proteins.
-Differentiation: process of directing a cell to a defined specific cell state.
-Viability: characteristics of survival according to purpose (e.g., metabolic activity, reproductive ability).
-Fibroblast growth factor (FGF): family of small polypeptide growth factors sharing several common features, including a central barrel-shaped core region composed of 140 amino acids with high homology among family members and strong affinity for heparin. Initially studied as proteins that stimulate the growth of fibroblasts, this distinction is no longer a prerequisite for belonging to the FGF family.
-Growth factors: substances that stimulate the growth, proliferation, and differentiation of cells.
-ASCs: undifferentiated cells found in various organs or differentiated tissues with limited self-renewal and differentiation abilities.
-Cell line: cell population with designated characteristics, created by sequentially subculturing primary cells, which can be managed by creating a cell bank.
-Organoid: organization of cells into structures resembling organs. Organoids can be formed in culture, mainly representing self-organized three-dimensional tissue structures. These are tissue structures formed by cells organizing themselves in vitro, primarily derived from stem cells, and can also include three-dimensional structures of cells derived from microphysiological systems and found in certain neoplasms.
-Cell source: basic units of structure and function in an organism, comprising various types of cell organelles, including the cytoplasm and cell membrane boundary, and are the smallest units that can proliferate independently, used for therapeutic and research purposes.
-iPSCs: pluripotent stem cells (PSCs) derived from adult cells.
-Gene expression: process in which a gene is activated within a cell to produce RNA and proteins.
-Pluripotency/pluripotent: state of being able to differentiate into various types of cells from the initial three germ layers.
-PSCs: stem cells that can differentiate into all cell types of the body and have the capability for indefinite self-renewal in vitro.
-Proliferation: increase in cell numbers through cell division.
-Fetal bovine serum (FBS): bovine serum albumin commonly used in ex vivo biological research.
-Epidermal growth factor (EGF): protein that promotes the growth and division of epidermal cells, exerting various biological effects, including the stimulation of proliferation and differentiation of mesenchymal and epithelial cells, synthesized as a transmembrane protein that can be cleaved to release a soluble active form.
-Thawing: process of activating cells from an inactive frozen state to an active state.
Organoids use human-derived ASCs, iPSCs, and ESCs derived from blastocysts as source cells. The quality and characteristics of the cells are crucial in the efficient differentiation, maturity, and functional integrity of organoids. Donor-derived stem cells are cultured in a three-dimensional environment with scaffolds that replicate tissue microenvironments. iPSC-derived organoids employ reprogrammed iPSCs, capable of sustained pluripotency and maintenance in an undifferentiated state across generations, either on feeder cells or in feeder-free conditions like extracellular matrix (ECM). While this document provides QC recommendations for source cells for organoid development, it excludes recommendations for cell line providers.
General and QC recommendations: The quality verification of cell lines for organoid development involves genetic validation, chromosomal analysis, evaluation marker evaluation, differentiation potential assessment, viral contamination and sterility tests, and biological characteristics evaluation. These techniques include short tandem repeat (STR) profiling, karyotyping for chromosomal aberrations detection, differentiation marker expression analysis, and contaminant testing (e.g., mycoplasma, mycobacterium, and viruses). Furthermore, monitoring growth rates and morphological features and ensuring batch-to-batch consistency are important. These multifaceted assessments are instrumental in maintaining cell line quality and increasing organoid reliability.
a.Organoid development requires a material transfer agreement that respects supplier specific requirements, and each cell line from a donor should be assigned a unique identifier.
b.Upon acquiring source cells, establishing a source cell bank with cells having undergone minimum passaging is recommended. Post-thaw quality assessment of these cells is mandatory.
c.Validation and QC are crucial. STR profiling (5, 6) or karyotype analysis (7) is recommended for PSCs, while ASCs require detailed documentation of donor demographics and cell origin for traceability. Genetic validation, particularly through STR profiling, along with single nucleotide polymorphism/comparative genomic hybridization arrays (8) for chromosomal analysis, ensures cell line integrity. Immunohistochemistry (9), flow cytometry (10), and polymerase chain reaction (PCR) (11) are vital for assessing cellular characteristics, with markers like Oct4 (12, 13), SSEA-3/4 (14), and TRA-1-60/81 essential for PSCs.
d.The culture of source cells requires sterility evaluations to detect bacterial, viral, fungal, and mycoplasma contamination. Suppliers often provide contamination analysis data. For human-specific viruses, PCR techniques are recommended for confirmation. Additionally, the use of animal-derived materials, such as FBS, requires specific virus screening to ensure the safety and integrity of the cell lines.
e.Proper cell line storage, including liquid nitrogen preservation, adherence to recommended storage durations, initial and maximum recommended passage numbers (e.g., passage 2 and not exceeding passage 8, respectively), and precise freezing and thawing methods, is critical.
f.Regular QC of cell lines should be performed. Following initial quality validation, regular assessments should be conducted to monitor chromosomal morphology, growth rates, and contamination levels.
Cell-based quality requirements for organoids:
a.ASC-based organoids
ASCs, present in adult tissues or organs, hold the capacity for differentiation and self-replication. The undifferentiated ASCs are expected to display functional characteristics related to the target tissues of the organ for organoids.
1)ASC quality requirements
Quality criteria for ASCs include: (a) ASCs should be pure and homogeneous without unrelated cell types that may hinder organoid formation and functionality; (b) ASCs should maintain a high degree of viability to ensure proliferation and differentiation into requisite cell types for organoid development; (c) ASCs should be free from contamination by bacteria, fungi, viruses, mycoplasma, or other similar agents; (d) ASCs should have a robust proliferative capacity to produce sufficient numbers of cells for organoids; and (e) ASCs should be maintain genetic stability, devoid of mutations or chromosomal abnormalities that may compromise the quality and functionality of organoids.
2)ASC source
The acquisition of ASCs for organoid culture varies depending on the target tissue or organ and its specific application. Commonly employed methods include tissue biopsy (e.g., intestinal stem cells isolated from biopsy samples of the small intestine) or primary cell culture.
b.PSC-based organoids
PSCs include ESCs and iPSCs and are characterized by their capacity for unlimited self-replication and pluripotency (which allows them to differentiate into endoderm, mesoderm, and ectoderm cells). These cells typically form monolayer colonies and are cultured either with feeder cells, such as fibroblasts, or in feeder-cell–free conditions using specific culture media.
1)PSC quality requirements
Quality criteria for PSCs include (a) PSCs should be viable, capable of ATP production; (b) PSCs should be free from non-stem cells and stem cell-specific surface markers should be verified; (c) PSCs should be free from bacterial, fungal, viral, and mycoplasma contaminants; (d) PSCs should be genetically stable with as few mutations or generic aberrations as possible; (e) PSCs should be capable of self-replication and differentiation into diverse tissues and organs; and (f) PSCs should be identified and characterized accurately through marker analysis.
2)PSC source
ESCs can be obtained from the inner cell mass of the blastocyst. iPSCs are generated by inducing pluripotency in somatic cells (e.g., skin, blood, bone marrow, urine) through the introduction of reprograming factors (e.g., Yamanaka factors).
Essential culture elements for tissue-derived organoids:
a.Culture medium
The medium for tissue-derived (e.g., ASCs) organoids varies depending on the target organ and tissue. Essential components include:
1)Basal medium constitutes the core of the culture medium, providing vital nutrients, sodium, and buffers. Representative examples are Dulbecco’s modified Eagle medium (DMEM) and advanced DMEM/F12, with the latter containing essential nutrients, vitamins, sodium pyruvate, and osmotic pressure necessary for cellular proliferation and functionality.
2)Supplements such as R-spondin, vitamins, amino acids, glucose, and other nutrients promote cell growth and metabolic activity.
3)Supplemental hormones tailor physiological conditions for specific organoid cultures.
4)Serum or serum equivalents provide proteins for cellular growth, with a trend towards serum-free or serum-reduced cultures for reproducibility.
The medium composition adapts to the target organ, research objectives, and culture methods, requiring adherence to established standard guidelines.
b.Growth factors and signaling molecules
Essential for proliferation and differentiation, tissue-specific growth factors include:
1)EGF is a protein that binds to the EGF receptor on the epidermis, facilitating cell generation and differentiation (15).
2)FGF supports stem cell self-renewal, regulating cell proliferation and differentiation.
3)Wnt ligands are involved in controlling cell fate determination, proliferation, migration, and polarity (16, 17).
4)Bone morphogenetic protein (BMP) inhibitors (e.g., Noggin, Gremlin, LDN-193189) block BMP signaling, helping to maintain stem cell undifferentiated state.
5)Nerve growth factor is a protein that regulates growth and survival of nerve cells and promotes the growth of sympathetic and peripheral sensory nerve cells.
6)Hepatocyte growth factor (HGF) is a mitogen for hepatocytes and other cells such as endothelial cells, epithelial cells, and melanocytes.
7)Glial cell line-derived neurotrophic factor is a protein that supports the survival and growth of various types of neurons and plays a role in the maintenance of neural stem cells.
8)Retinoic acid assists in the development of various tissue types, including neural tissue, and lung and intestinal epithelium, and promotes differentiation of organoids, such as intestine or lung tissue.
9)Thyroid-stimulating hormone (18) is a pituitary hormone that stimulates the thyroid to release thyroxine and triiodothyronine.
10)Platelet-derived growth factor (19) is a mitogen for connective tissues, required for lung growth.
11)Small molecules regulate signaling pathways or induce specific differentiation processes, such as CHIR99021 to activate Wnt-signaling pathway.
c.ECM components
The ECM in culture is a dynamic and complex assembly that influences cell behavior and function. Composed of various proteins such as collagen, fibronectin, laminin, and vitronectin, the ECM provides not only essential structural support for cells but also initiates crucial biochemical and biomechanical cues required for tissue morphogenesis, cell proliferation, and differentiation (20). It serves as a scaffold that promotes more differentiated phenotypes and generally supports longer cell survival, sometimes even allowing cells to be cultured in the absence of serum and growth factors. The specific composition of the ECM, including the presence of growth factors and cytokines, mediates cell adhesion to biomaterials, crucial for tissue engineering applications. Through its complex interactions with cells, the ECM regulates a wide array of cellular processes such as survival, growth, migration, and physiological responsiveness, making it indispensable in both natural and artificial cellular environments (21).
Essential culture elements for PSC-based organoids:
a.Culture medium
The medium composition should mirror the characteristics of the target organ and tissue. The essential components include:
1)Basal medium includes DMEM, Roswell Park Memorial Institute 1640, and mTeSRTM Plus.
2)Nutritional supplements: vitamins, amino acids, glucose, glutamine, and insulin-transferrin-selenium to foster cellular growth and metabolism.
3)Hormones: hormones are integrated to provide physiological conditions conducive to tissue organoid-specific differentiation and maturation.
4)Niche-specific supplements: tailored components are added based on organoid types (e.g., Wnt3a and R-spondin for intestinal organoids, brain-derived neurotrophic factor, and neurotrophin-3 for neural organoids).
b.Growth factors and signaling molecules
The following shows a classification of organoids based on their origin: endoderm-derived, mesoderm-derived, and ectoderm-derived organoids. Each category lists specific types of organoids, the growth factors required for their development.
1)Endoderm-derived organoids
(1)Intestinal organoids
-Growth factors: Activin A, Wnt3, EGF, FGF4, Noggin, R-spondin 1, BMP4 (22)
(2)Liver organoids
-Growth factors: Activin A, FGF, TGF-α, HGF, BMP, Rspo1, Nicotinamide, Gastrin I, N-acetylcysteine, EGF, Y-27632 (23)
(3)Lung organoids
2)Mesoderm-derived organoids
(1)Kidney organoids
-Growth factors: Acvitin A, BMP4, Wnt, FGF
(2)Cardiac organoids
-Growth factors: TGF-B1, FGF-2, Activin A, BMP-4
3) Ectoderm-derived organoids
(1)Brain organoids
-Growth Factors: Wnt, BMP4, OCT4, SOX2, KLF4, C-MYC (22)
(2)Skin organoids
-Growth Factors: EGF, AMP4
Consistency and reproducibility in organoid quality is critical but challenging due to variations in protocols, reagents, equipment, and research environments. Organoids should meet established quality criteria at endpoints for reliable evaluations of drug toxicity and efficacy. The guidelines below outline quality assessment categories and QC recommendations to improve organoid quality.
General concerns and quality of organoids:
-Structural maturity (morphological observation): This involves examining the physical characteristics of organoids, such as their shape, size, and the arrangement of cells within them. Imaging techniques like microscopy are used to capture detailed images, allowing researchers to document and compare the structural development of organoids over time. The aim is to ensure that the organoids accurately replicate the structure of the organ they are modeled after.
-Cellular composition: This refers to analyzing the types of cells present in the organoid and their molecular features, including the proteins and other biomolecules they express. Specific biomarkers can indicate whether the organoid contains the types of cells found in the original organ and whether these cells are functioning correctly. Techniques such as immunostaining, where antibodies are used to detect specific proteins, and gene expression analysis are commonly employed.
-Functional maturity: This aspect focuses on assessing whether the organoid is not just structurally but also functionally like the organ it represents. Organ-specific functional assessments are tests designed to evaluate the specific functions of the organoid; for example, if the organoid models the liver, researchers might assess its ability to metabolize drugs. These assessments help to determine how closely the organoid’s function matches that of the real organ.
Morphological requirements: The dimensions and form of organoids serve as indicators of cell viability, proliferation rates, and structural or morphological irregularities. The following are recommendations for morphological requirements.
a.Qualitative inspection
1)Microscopy: utilize bright field, phase contrast, and fluorescence microscopy for observation of organoid structure, cellular organization, and differentiation state, with documented findings.
2)Transmission electron microscopy: provide high-resolution insights into cellular organelles and ultrastructures within organoids.
3)Confocal microscopy: offer three-dimensional visualization of fluorescent-labeled organoid sections, enabling examination of cell arrangements and interactions.
4)Immunofluorescence/H&E staining: confirm the presence of specific cell types and distribution within organoids and identify cell and tissue structures.
b.Quantitative inspection
1)Size and diameter measurement: measure organoid sizes and diameters to assess growth dynamics and developmental progression.
2)Organoid count: count viable organoids per culture medium to gage viability.
3)Proliferation analysis: employ Ki-67 and BrdU staining for quantitative cellular proliferation analysis within organoids.
4)Differentiation assessment: evaluate cellular diversity and distribution within organoids using specific marker expression and cellular population proportions.
5)Growth uniformity: measure surface area and volume of organoids for monitoring growth uniformity.
6)Maturation indictor: analyze nucleus/cytoplasm ratio as an indicator of cell differentiation and maturation.
7)Structural integrity: assess organoid proportionality and symmetry to evaluate morphological fidelity and developmental integrity.
8)Differentiation pathways: perform quantitative analysis of differentiation patterns within organoids to understand lineage-specific differentiation.
Compositional requirements:
a.Qualitative inspection
1)Immunofluorescence staining: visualize specific proteins/biomarkers within organoids to analyze molecular presence and spatial distribution.
2)Immunohistochemistry: examine protein distribution and volume, providing detailed protein localization and concentration within organoids.
3)Western blotting: quantitatively measure protein expression levels within organoids to assess protein presence and relative abundance.
4)In situ hybridization: identify the presence and location of specific RNA/DNA sequences within organoids, informing on genetic characteristics.
b.Quantitative inspection
1)Enzyme-linked immunosorbent assay (ELISA): quantify specific antigens to measure concentration within organoids.
2)Quantitative PCR: quantify RNA molecules within organoids to assess gene expression levels.
3)Flow cytometry: evaluate presence and quantity of cell-surface markers within organoids, aiding in characterization of cellular populations.
4)Mass spectrometry: identify and quantify proteins and biomolecules within organoids, providing a molecular composition overview.
5)RNA sequencing: conduct transcriptome profiling via RNA sequencing for comprehensive gene expression analysis.
6)Proteomics analysis: perform quantitative analysis of organoid protein composition to uncover protein profile.
Functional requirements:
a.Organ-specific functional assessment requirement
The following outlines four types of organoids, the specific functions they are designed to mimic and the methods used to assess these functions. It is important to note that these are examples, not an exhaustive list. The assessment methods are critical for demonstrating that these organoids accurately model the biological and physiological processes of the organs they represent.
1)Brain organoids
(1)Target function: electrophysiological properties of neurons, which are essential for understanding how neurons communicate, process information, and contribute to the overall function of the brain (27).
(2)Assessment methods:
-Patch-clamp: a technique used to measure action potentials and other intracellular electrophysiological features of neurons. This allows researchers to study the electrical properties of neurons in detail.
-Microelectrode array (MEA) recording: This method records extracellular electrophysiological features. MEA can monitor the electrical activity of neurons over time, providing insights into neural networks and brain organoid function.
-Calcium imaging: a method to directly indicate neural activity by detecting changes in intracellular calcium levels, which fluctuate with neuron activation.
2)Heart organoids
(1)Target function: Contractile properties, which are central to the heart’s ability to pump blood (28).
(2)Assessment methods:
-Contraction recording and analysis (MEA): Similar to its use in brain organoids but focused on measuring the contractile activity of heart cells. This assesses how well the heart organoid can mimic the heart’s pumping action.
3)Kidney organoids
(1)Target function: Protein uptake properties, specifically reflecting the function of proximal tubules in the kidney, which are responsible for the reabsorption of proteins such as albumin from the urine (28).
(2)Assessment methods:
-Albumin uptake assay: This tests the organoid’s ability to take up albumin, typically using fluorescently labeled albumin (e.g., TRITC-albumin), mimicking the reabsorption process.
4)Liver organoids
(1)Target function: Extrahepatic metabolism and clearance, key liver functions that include metabolizing substances and producing proteins necessary for blood clotting and immune function (29).
(2)Assessment methods:
-Indocyanine green (ICG) uptake and release: ICG is a dye used in medical diagnostics, and its uptake and release can be used to assess the organoid’s metabolic function.
-Albumin, α1-antitrypsin, and urea quantitation: measured typically through ELISA tests to evaluate the organoid’s ability to perform essential liver functions such as protein synthesis and nitrogen waste metabolism.
-Cytochrome P450 (CYP) enzyme measurements: CYP enzymes (e.g., CYP450, CYP3A4, CYP1A2) are involved in drug metabolism. Measuring these can validate the organoid’s ability to metabolize pharmaceuticals.
-Bile acid quantification: bile acids are produced by the liver to help digest fats. Their quantification can assess the organoid’s functionality in bile production.
To preserve organoids, freezing arrests cellular growth and prevents structural and genetic alterations due to cell passage. A detailed storage protocol should be followed to ensure the retention of organoid quality post-thaw, maintaining structure, cellular integrity, and preventing contamination. The cryopreservation protocol may vary depending on the cell type and the cryopreservation medium used. The guidelines provided by the cell and cryopreservation medium manufacturers should be consulted. Similarly, the thawing protocol may differ based on the cell type, and adherence to the supplier-provided protocols is recommended. For a broad spectrum of cell types, the “General Cryopreservation Protocol of Stem Cells” outlined below serves as a guideline. The cryopreservation medium, similar to the raw materials, should be verified to not contain any substances derived from animals or animal cells.
Freezing and thawing processes:
a.Cryopreservation
Before cryopreservation, ensure that organoids are in optimal health; avoid organoids that are overgrown or deteriorating as this impacts post-thaw viability. Organoids with a vesicular structure are less amenable to cryopreservation than their healthy counterparts because of the potential for epithelium detachment and the formation of cytoplasmic membrane protrusions, which indicates organoid deterioration. Cryopreservation at −80℃ to −196℃ is crucial for halting cellular metabolism. Cryopreservation, however, introduces risk of solute imbalances, and cellular and structural damage due to intracellular ice formation. Therefore, cryopreservation should ensure cell integrity and optimize viability post-thaw. While a short-term storage at −80℃ is possible, it is advisable to limit the storage period for stability. Key factors in stem cell cryopreservation include:
-Sterility: using 70% ethanol or isopropanol is essential for preserving sterility throughout the cryopreservation process.
-Cell health: prior to freezing, assess cell health and contamination, e.g., via mycoplasma testing. Media clarity or turbidity, color, and cell morphology should be examined, removing any contaminated cells.
-Cell confluency: cells should be in their maximum growth phase, exhibiting >80% confluency to ensure post-cryopreservation integrity.
-Cell concentration in vials: optimal concentration varies for freezing varies by cell type, generally ranging from 1×103 to 1×106 cells/mL, balancing between low survival rates at sparse concentrations and clumping at high concentrations.
-Cryopreservation medium: employ GMP-compliant, verified cryopreservation medium.
-Freezing rate: controlled cooling rates, typically slow freezing, are recommended to minimize quality loss, with adjustments based on cell types.
For organoid cryopreservation, aiming for storing 100∼200 organoids for small size (<300 μm) is advised, accounting for potential loss and decreased viability during freezing and thawing cycles. If organoids are to be refrozen, they should undergo at least two subculturing rounds to mitigate quality degradation from medium exposure. Cryopreservation of mature organoids may face challenges like disintegration and stem cell component loss. Optimal methods and timing for cryopreservation are organ-dependent.
Some organ-specific considerations:
-Kidney organoids: specific cell types within kidney organoids may exhibit sensitivity to freezing and thawing, inducing apoptosis and functional impairment. Storage at the renal precursor cell stage following standard cell cryopreservation methods is recommended.
-Brain organoids: neuronal and other brain cells are sensitive to temperature changes, and cells and neural networks may be damaged during freezing and thawing. Cryopreservation can reduce oxygen supply to organoids, causing oxidative stress and cellular damage.
-Intestine: cryopreservation of human intestinal organoids is possible from the fifth day post-passage.
-Liver organoids: liver organoids can be effectively cryopreserved for long-term storage at the expandable stage before reaching full differentiation. It is recommended to use organoids exhibiting a viability of over 70% upon thawing and to allow a recovery period of 1∼2 weeks following thawing to enable the organoids to regain stability in growth and promote further differentiation.
-Cardiac organoids: before freezing, confirm the quality of cardiac organoids by PCR using culture media to check for the absence of fungi and microbes and select only cardiac organoids maintaining a beating rate of 0.5∼1.5 Hz. Analyze the recovery of beating function during a stabilization period of 1 week after thawing the cardiac organoids, using a platform that allows for analysis under a microscope or of cardiac beating characteristics.
b.Thawing
General thawing protocol of stem cells:
1)Pre-warm stem cells in a 37℃ water bath, gently rotate the cryogenic vial in one direction to ensure even thawing, minimizing cell damage. The process should be completed within approximately 1∼2 minutes.
2)Sterilize vial exteriors with 70% ethanol, proceeding under aseptic conditions.
Organoid technology holds promise in disease modeling, drug testing, organ transplantation, and regenerative medicine, offering the potential to reduce animal testing and provide research outcomes more directly relevant to human health. However, ethical concerns arise from the use of biological materials from humans. These concerns include safeguarding donor privacy, establishing concrete consent procedures, and addressing the commercialization of research findings. Organoid research should be conducted in international cooperation and regulation frameworks, fostering dialogue and collaboration between scientists and policymakers, to align scientific progress with human dignity and societal values.
Informed consent: Effective informed consent involves educating donors about organoid research and its implications in an accessible manner. Consent should include comprehensive understanding and agreement on the use of their cells, including any potential for commercialization. Key points for donor consent include: (1) the purpose of organoid use in research and development; (2) personal data protection and management; (3) the possibility of additional tissue use without further consent; (4) organoid preservation, management, disposal protocols; (5) consent withdrawal procedures, and rights regarding organoids after withdrawal; (6) potential for organoid cryopreservation for future use; and (7) consent for linking organoids to medical, clinical, and genetic data.
Commercialization: The commercialization of organoid technology presents ethical challenges: (1) intellectual property-the development of organoids may lead to patent applications on the technology, organoids, or manufacturing methods, raising intellectual property issues related to tissue donors; (2) Accessibility-commercialization risks creating inequalities in access to medical advancements due to cost barriers. Strategies to ensure equitable access, regardless of socioeconomic status, are important; and (3) consent for commercial use-donors should be informed that their tissues could be used to develop commercial products, with explicit consent obtained for such purposes.
In this report, we discuss the organoid manufacturing and the quality assessment criteria at their endpoints. The culture conditions for organoids derived from human stem cells are under constant refinement and corroboration. The integrity and pluripotency level of stem cells, which serve as the source materials for organoids, significantly dictate the subsequent organoid characterization. The organoid development strategy varies based on the type of stem cell (e.g., ASC and PSC); hence the combination of essential growth factors, maintenance of characteristics, differentiation stages, and QC of organoids at their endpoints guide the production of organoids with a high degree of similarity to the mimicked organs. Furthermore, verifying the morphological, functional, and physiological characteristics through imaging and immunological assays allows organoid developers to validate the applicability and efficacy of organoids for disease modeling, toxicity assessment, and the development of new treatments. The recommendations presented facilitate a comprehensive understanding of the production and quality evaluation of human-derived organoids, offering a scalable framework to organ-specific organoid manufacturing and their diverse application fields.
Acknowledgments
We thank those who contributed to the successful completion of this manuscript. We would like to thank first the Ministry of Food and Drug Safety, OSI committee chairs and members, and the Institute of Quantum Biophysics, especially, Professor Luke Lee for his invaluable insights.
Notes
Authors’ Contribution
Conceptualization: SJA, SO, SL. Data curation: SO, SL. Formal analysis: SO. Funding acquisition: SJA. Investigation: SO, SL, SJ, DK. Project administration: IUO, TSK, JHL. Supervision: IUO, TSK, JHL. Validation: IUO, TSK, JHL. Visualization: CP, DK. Writing – original draft: SJA, SL. Writing – review and editing: SJA, SL, DK.
References
2. Derouet MF, Allen J, Wilson GW, et al. 2020; Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep. 10:14514. DOI: 10.1038/s41598-020-71589-4. PMID: 32884042. PMCID: PMC7471705.

3. Schmeisser S, Miccoli A, von Bergen M, et al. 2023; New approach methodologies in human regulatory toxicology - not if, but how and when! Environ Int. 178:108082. DOI: 10.1016/j.envint.2023.108082. PMID: 37422975.

4. Griffith LG, Swartz MA. 2006; Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 7:211–224. DOI: 10.1038/nrm1858. PMID: 16496023.

5. Nims RW, Sykes G, Cottrill K, Ikonomi P, Elmore E. 2010; Short tandem repeat profiling: part of an overall strategy for reducing the frequency of cell misidentification. In Vitro Cell Dev Biol Anim. 46:811–819. DOI: 10.1007/s11626-010-9352-9. PMID: 20927602. PMCID: PMC2995877.

6. Korch CT, Hall EM, Dirks WG, et al. 2022; ANSI/ATCC ASN-0002-2022. Human cell line authentication: standardization of short tandem repeat (STR) profiling - revised 2022. American National Standards Institute (ANSI).
7. Pasquier L, Fradin M, Chérot E, et al. 2016; Karyotype is not dead (yet)! Eur J Med Genet. 59:11–15. DOI: 10.1016/j.ejmg.2015.11.016. PMID: 26691665.

8. Wiszniewska J, Bi W, Shaw C, et al. 2014; Combined array CGH plus SNP genome analyses in a single assay for optimized clinical testing. Eur J Hum Genet. 22:79–87. DOI: 10.1038/ejhg.2013.77. PMID: 23695279. PMCID: PMC3865406.

9. Sukswai N, Khoury JD. 2019; Immunohistochemistry innovations for diagnosis and tissue-based biomarker detection. Curr Hematol Malig Rep. 14:368–375. DOI: 10.1007/s11899-019-00533-9. PMID: 31338668.

10. Givan AL. 2001. Flow cytometry: first principles. 2nd ed. Wiley‐Liss;DOI: 10.1002/0471223948. PMCID: PMC37243.
11. Innis MA, Gelfand DH, Sninsky JJ, White TJ. 1990. PCR protocols: a guide to methods and applications. Academic Press.
12. Zhang Q, Han Z, Zhu Y, Chen J, Li W. 2020; The role and specific mechanism of OCT4 in cancer stem cells: a review. Int J Stem Cells. 13:312–325. DOI: 10.15283/ijsc20097. PMID: 32840233. PMCID: PMC7691851.

13. Na J, Plews J, Li J, et al. 2010; Molecular mechanisms of pluripotency and reprogramming. Stem Cell Res Ther. 1:33. DOI: 10.1186/scrt33. PMID: 20974014. PMCID: PMC2983446.

14. Evron A, Goldman S, Shalev E. 2011; Human amniotic epithelial cells cultured in substitute serum medium maintain their stem cell characteristics for up to four passages. Int J Stem Cells. 4:123–132. DOI: 10.15283/ijsc.2011.4.2.123. PMID: 24298345. PMCID: PMC3840962.

15. Wang X, Wang S, Guo B, et al. 2021; Human primary epidermal organoids enable modeling of dermatophyte infections. Cell Death Dis. 12:35. DOI: 10.1038/s41419-020-03330-y. PMID: 33414472. PMCID: PMC7790817.

16. Mikels AJ, Nusse R. 2006; Wnts as ligands: processing, secretion and reception. Oncogene. 25:7461–7468. DOI: 10.1038/sj.onc.1210053. PMID: 17143290.

17. Liu J, Xiao Q, Xiao J, et al. 2022; Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 7:3. DOI: 10.1038/s41392-021-00762-6. PMID: 34980884. PMCID: PMC8724284.

18. Sarapura VD, Gordon DF, Samuels MH. Melmed S, editor. 2011. Thyroid-stimulating hormone. The pituitary. 3rd ed. Academic Press;p. 167–203. DOI: 10.1016/B978-0-12-380926-1.10006-9.
19. Boström H, Gritli-Linde A, Betsholtz C. 2002; PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn. 223:155–162. DOI: 10.1002/dvdy.1225. PMID: 11803579.

20. Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC. 1987; Use of extracellular matrix components for cell culture. Anal Biochem. 166:1–13. DOI: 10.1016/0003-2697(87)90538-0. PMID: 3314585.

21. Kleinman HK, Philp D, Hoffman MP. 2003; Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 14:526–532. DOI: 10.1016/j.copbio.2003.08.002. PMID: 14580584.

22. Kim J, Koo BK, Knoblich JA. 2020; Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 21:571–584. DOI: 10.1038/s41580-020-0259-3. PMID: 32636524. PMCID: PMC7339799.

23. Harrison SP, Baumgarten SF, Verma R, Lunov O, Dejneka A, Sullivan GJ. 2021; Liver organoids: recent developments, limitations and potential. Front Med (Lausanne). 8:574047. DOI: 10.3389/fmed.2021.574047. PMID: 34026769. PMCID: PMC8131532.

24. Kong J, Wen S, Cao W, et al. 2021; Lung organoids, useful tools for investigating epithelial repair after lung injury. Stem Cell Res Ther. 12:95. DOI: 10.1186/s13287-021-02172-5. PMID: 33516265. PMCID: PMC7846910.

25. Dye BR, Hill DR, Ferguson MA, et al. 2015; In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 4:e05098. DOI: 10.7554/eLife.05098. PMID: 25803487. PMCID: PMC4370217.

26. McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. 2017; Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell. 20:844–857.e6. DOI: 10.1016/j.stem.2017.03.001. PMID: 28366587. PMCID: PMC5457392.

27. Kim J, Sullivan GJ, Park IH. 2021; How well do brain organoids capture your brain? iScience. 24:102063. DOI: 10.1016/j.isci.2021.102063. PMID: 33554067. PMCID: PMC7856464.

28. Gabbin B, Meraviglia V, Angenent ML, et al. 2023; Heart and kidney organoids maintain organ-specific function in a microfluidic system. Mater Today Bio. 23:100818. DOI: 10.1016/j.mtbio.2023.100818. PMID: 37810749. PMCID: PMC10550812.

29. Mun SJ, Ryu JS, Lee MO, et al. 2019; Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. DOI: 10.1016/j.jhep.2019.06.030. PMID: 31299272.

Fig. 1
Organoid Standards Initiative (OSI) organoid guidelines development scope (2023∼2024) (2023.09.∼2024.02.) The OSI developed the first version of the guidelines for promoting organoid practical use, which presented general requirements and considerations for organoid production and quality evaluation with extensive evidence analysis and expert consensus. These guidelines comprise two overall guidelines that consider the fabrication of organoids from human-derived cells and seven guidelines for organoid use with specific organs (liver, intestines, heart, kidney, brain, lung, skin).
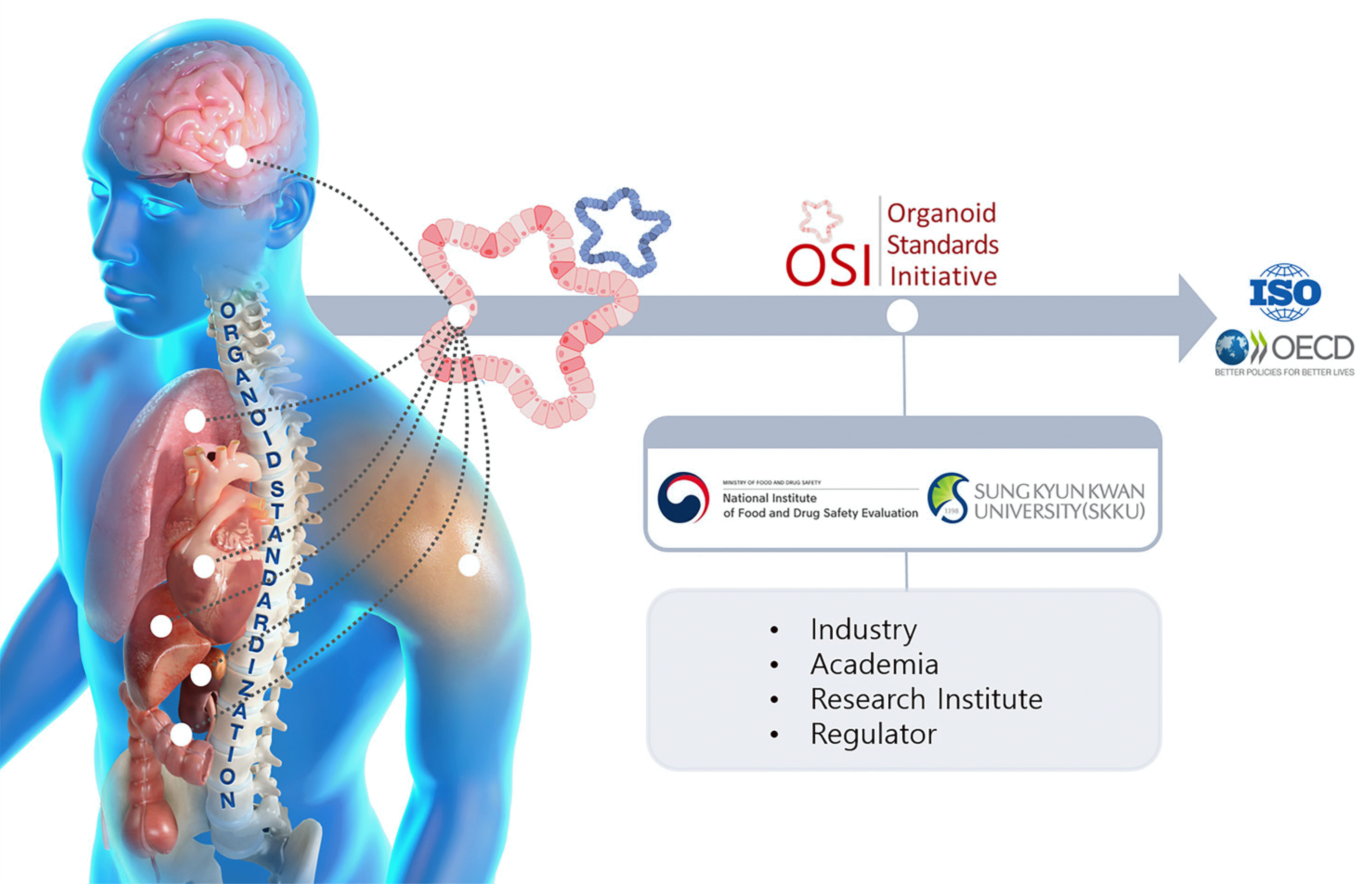
Fig. 2
Research scope. The guidelines cover cell source, culture, organoid quality control, and storage and preservation. ASCs: adult stem cells, iPSCs: induced pluripotent stem cells, ESCs: embryonic stem cells, DMEM: Dulbecco’s modified Eagle medium, RPMI: Roswell Park Memorial Institute, IMDM: Iscove‘s modified Dulbecco‘s medium, EGF: epidermal growth factor, FGF: fibroblast growth factor, BMP: bone morphogenetic protein, ELISA: enzyme-linked immunosorbent assay, qPCR: quantitative polymerase chain reaction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download