Abstract
Objectives.
No study has yet evaluated the degree of contamination after the total disassembly of continuous positive airway pressure (CPAP) devices. We investigated the extent of contamination of CPAP devices used daily by patients with obstructive sleep apnea (OSA) by disassembling the systems and identifying the factors that influenced the degree of CPAP contamination.
Methods.
We conducted a chart review of the medical records of patients with OSA for whom the CPAP devices were disassembled and cleaned. Two skilled technicians photographed the levels of contamination of each component and scored them using a visual analog scale. Patients’ clinical characteristics and records of CPAP device usage were statistically analyzed to identify characteristics that were significantly associated with the degree of CPAP device contamination.
Results.
Among the 55 participants, both the external components, including the mask and tube, and the internal components, such as the humidifier and the interior of the main body, showed a substantial degree of contamination. The total and average daily duration of usage of the CPAP device did not show significant associations with the degree of contamination. Age was most consistently associated with the degree of contamination, such as in masks, humidifiers, and interior and exterior main parts. The degree of contamination of the internal components of the device was significantly correlated with the degree of contamination of the external components.
Obstructive sleep apnea (OSA), a multifactorial disease mediated by multiple genetic, environmental, and developmental factors [1], is highly prevalent and affects an estimated 3% and 9% of women and men, respectively [2]. OSA is associated with a range of medical and social outcomes, such as cardiopulmonary changes, daytime fatigue, irritability, personality changes, and motor vehicle accidents [2,3]. The treatment of OSA often involves the use of nocturnal positive airway pressure (PAP) delivered by continuous PAP (CPAP) devices [4]. A nasal CPAP machine consists of a mask, tubing, and a main body, containing a humidifier and blower motor, and it blows humidified air into the patient’s nose [5]. The National Health Insurance Service (NHIS) in South Korea covers CPAP devices, with one mask and two tubes per year, in patients with OSA. However, standard guidelines for the timing of part replacement or hygiene management of CPAP components are lacking.
Reused instruments, such as nasal steroid spray bottles or nasal irrigation bottles, could be contaminated and may pose an infection risk via the nasal cavity [6,7]. There is a concern that nasal CPAP devices could become contaminated and lead to infections in users. Reports have documented bacterial and fungal colonization on the nasal masks of patients with OSA, yet there have been no cases of associated infections in the upper or lower respiratory tracts [4]. A case of Legionella pneumonia following CPAP device use has been reported [8]. Several studies have attempted to identify microorganisms in CPAP systems; however, no study has disassembled main CPAP units to visually inspect internal contamination.
In this study, we investigated the extent of CPAP device contamination by disassembling systems used daily by patients with OSA and identifying factors that influenced the degree of contamination of each CPAP component.
The research protocol was reviewed in compliance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chung-Ang University Hospital (No. 2312-032-19504), and the requirement for written informed consent was waived.
In South Korea, patients with OSA who have been using CPAP devices are monitored to ensure that they receive replacement masks in accordance with the NHIS guidelines, which qualifies CPAP users for a yearly replacement of one mask and two tubes. We provide our own education to patients on the proper maintenance protocol of the CPAP device at first use and regularly check their condition. The hygiene protocol is as follows: wash the mask and humidifier of the CPAP device with a neutral detergent and air-dry for a smooth finish on the fabric once daily; wash the tube of the CPAP device with a neutral detergent once daily, let it air-dry naturally; clean the main body of the CPAP device as needed; and wipe away any dust during use. Additionally, we independently replaced the tubes at least every 6 months, and the filters at least every 3 months. Furthermore, starting in January 2023, a complimentary disassembly and cleaning service for the CPAP device was provided free of charge to all patients in our institute. Complete disassembly and cleaning of the CPAP device requires highly specialized skills and takes several hours. This process was performed by two skilled technicians (JSK and TJK), who documented the contamination levels of each component through photographs (Fig. 1) and subjectively scored the levels using a visual analog scale (0–10 scale, where 0 represents the cleanest and 10 represents the highest level of contamination) after the disassembly and cleaning process.
This retrospective study was conducted at a single tertiary medical center. Medical records of patients who visited Chung-Ang University Hospital between January 2023 and December 2023 were reviewed. The inclusion criteria were patients who had been wearing a nasal CPAP device for >6 months with a diagnosis of OSA and had consented to and undergone disassembly and cleaning of the nasal CPAP device. The exclusion criteria were: patients who did not undergo disassembly and cleaning services or whose contamination results were not recorded. Medical records, including clinical characteristics, smoking history, underlying medical diseases, apnea-hypopnea index (AHI), information about the usage record of the CPAP device, and the scored degree of contamination, were reviewed and statistically analyzed. The applied CPAP devices are F&P SleepStyle (Fisher & Paykel Healthcare, applied for 14 patients), and prisma SMART (Löwenstein Medical Technology, applied for 44 patients).
We have provided a comprehensive overview of the descriptive statistics. Specifically, details related to continuous variables, such as age and body mass index (BMI) were elucidated by presenting means and variances (indicated in parentheses). For categorical variables, such as sex, we expounded on the relevant information through the presentation of count and frequency (specified within parentheses). Multivariate linear regression models were fitted to the degree of visible contamination of the mask, tube, humidifier, interior, and exterior of the main body. The independent variables were age, sex, BMI, AHI, total duration of usage, and average duration of daily use. We used a stepwise selection procedure based on the Akaike information criterion (AIC) to select the independent variables in our multivariate linear regression models. For the degree of visible contamination of the mask and tube, we categorized the data into two groups: duration of tube usage ≤6 months and >6 months, and duration of mask usage ≤1 year and >1 year. Subsequently, we applied multivariate linear regression to each category by using stepwise selection based on the AIC to fit the models. Lastly, we visualized the correlation between the degree of visible contamination of the mask, tube, humidifier, exterior main body, and interior main body using the “ggpairs” library in R software. Pearson correlation coefficients and statistical significance levels are indicated. All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing).
In total, 55 participants (mean age, 55.38±12.14 years), comprising 44 men and 11 women, were recruited. Fourteen patients had a history of smoking and 27 had been diagnosed with hypertension. Their mean BMI was 26.46±3.13 kg/m2, and the mean AHI was 42.53±22.12. The mean total CPAP usage duration was 932.23±418.50 days, and the mean daily usage duration was 4.93±1.53 hours. The mean values for the degree of contamination of the mask, tube, and filter were 2.94±1.56, 2.60±1.35, and 3.05±2.15, respectively (Table 1). During the complete disassembly and cleaning of the CPAP device, we found that not only the mask and tube, which are easily observed and regularly replaced, but also the interior body and humidifier of the CPAP devices were considerably contaminated (Fig. 1). The mean values for the degree of contamination of the humidifier, interior, and exterior main body were 3.83±2.27, 3.90±2.29, and 3.47±1.68, respectively (Table 1).
We fitted multivariate regression models with independent variables for contamination of the mask, tube, humidifier, interior, and exterior of the main body. Age and AHI were selected as predictors of mask contamination and were statistically significant (P=0.020 for age, P=0.033 for AHI, R2=0.122). The model for tube contamination included only the total usage duration of CPAP as a predictor; however, the coefficients of the selected variables were not statistically significant (R2=0.041). The humidifier contamination model included age and BMI as explanatory variables, and only age emerged as statistically significant (P=0.049, R2=0.093). The model for contamination of the interior of the main body included the total usage duration of CPAP, age, and BMI as explanatory variables, of which age (P=0.020) and BMI (P=0.033) were statistically significant (R2=0.162). For the contamination of the exterior main body, the model considered the total usage duration of the CPAP, age, AHI, and BMI as predictors, of which only age showed statistical significance (P= 0.024, R2=0.176). To summarize, age was statistically significant in the models for contamination of the mask, humidifier, and both interior and exterior main bodies (Table 2).
For further subgroup analysis, we categorized the data on the degree of contamination of the mask and tube according to mask usage duration (≤1 year or >1 year) and tube usage duration (≤6 months or >6 months). For mask contamination, when the mask usage duration was ≤1 year, no variables were fitted to the model. However, when the duration of mask usage was >1 year, age was selected as an explanatory variable and showed statistical significance (P=0.050, R2 =0.265) (Table 3). For the contamination of the tube, when the usage duration was ≤6 months, tube usage duration, sex, BMI, total usage duration of the CPAP device, and average daily usage duration were selected as variables. The tube usage duration (P=0.023) and the average daily usage duration of the CPAP device (P=0.014) showed statistical significance, with the tube usage duration having a negative coefficient. This indicated that, as the duration of usage increased, the degree of contamination decreased (R2=0.624). When the usage duration exceeded 6 months, age, sex, and total duration of usage were selected as variables for tube contamination; however, none of these were statistically significant (R2=0.271) (Table 3).
Finally, we evaluated the correlations between the degree of contamination of the mask, tube, humidifier, interior, and exterior of the main body. Most variables exhibited high correlations of ≥0.7, with a notably higher correlation between the degree of the contamination of the humidifier and exterior main body (correlation coefficient, 0.908) (Fig. 2).
The primary finding of this study was that, despite adhering to the NHIS guidelines, regularly replacing the patients’ masks, and monitoring for proper hygiene management of the CPAP device, a significant level of contamination was visually observed (Fig. 1). The duration of usage was not extensively associated with the degree of contamination of the various CPAP components. A significant correlation was not found between the duration of usage and the level of contamination of the mask and tube, as these parts were regularly replaced in accordance with the national NHIS guidelines. Instead, when we further evaluated the relationship according to the duration of usage of the mask and tube, a negative association was found between the duration of usage of the tube and the degree of tube contamination in cases where the duration was ≤6 months (Table 3). While we cannot provide a conclusive explanation for this, it may be that the visible increase in tube contamination prompted more diligent cleaning by patients. These findings could indirectly suggest that the current our own guidelines for tube replacement every 6 months may be too infrequent, and a shorter replacement interval should be considered. Moreover, the total usage duration of CPAP and the average daily usage duration showed no significant correlations with the degree of humidifier contamination in the interior or exterior of the main body (Table 2). Instead, we found that age was the most consistently associated factor, as it demonstrated statistically significant relationships with the degree of contamination of the mask, humidifier, and interior and exterior of the main body. We hypothesized that elderly patients may be less accustomed to directly handling machinery than younger individuals, potentially leading to a passive approach to hygiene management. Other characteristics, such as the AHI and BMI, were significantly associated with contamination of the mask and the interior of the main body. Therefore, we suggest that clinical characteristics—including, most notably, age—should be considered along with the usage duration for the establishment of guidelines on the hygiene management of CPAP devices and for patient education.
The second finding of the current study was that the humidifier and the internal components of the CPAP device, which are not visible without disassembly and cleaning, may be more prone to contamination than the external parts, such as masks or tubes. The average level of contamination in the humidifier and the interior of the main unit was greater than that found in the mask and filter, suggesting a poor level of hygiene (Table 1). In this country, healthcare is administered through the NHIS, yet there are no specific guidelines for when to replace the humidifier or internal parts of CPAP machines. Therefore, we recommend the development of guidelines for the replacement or regular cleaning of the CPAP device’s internal components, in addition to the masks and tubes.
Finally, the degree of contamination of the parts of CPAP devices showed significant correlations. In particular, the degree of contamination of the humidifier and interior of the main body, which could not be easily accessed by patients, was significantly correlated with the degree of external device contamination (Fig. 2). Therefore, we suggest that the internal compartment of the CPAP device should be checked to determine whether the external part of the CPAP device is contaminated.
The relationship between the cleanliness of CPAP devices and the incidence of inflammatory diseases in patients remains unclear. Given that CPAP is used daily by individuals with OSA and that most of its accessories are designed for repeated use, it is reasonable to suspect that various components of the CPAP system could harbor a range of microorganisms. Christopher et al. explored the possibility of bacterial colonization in CPAP water reservoirs and its potential link to the development of chronic rhinosinusitis (CRS). They hypothesized that air delivered through the CPAP machine could carry pathogenic agents into the patient’s airways. Their findings, however, indicated that even though some atypical bacteria were present in the CPAP reservoir, a positive culture did not correlate with an increase in CRS symptoms [5]. Additionally, no heightened infection risk has been associated with the use of reprocessed CPAP devices when compared to new ones, according to a study involving 120 CPAP devices [9]. In contrast, the rate of infectious diseases was found to be 25% among OSA patients who did not use CPAP, compared to 43% among those who did. Notably, CPAP users who utilized humidifiers experienced a higher incidence of upper airway infections [10]. A recent study employing 16S rRNA gene sequencing to assess the nasal microbiome revealed that CPAP treatment did not result in longitudinal changes in nasal microbiota, with no significant alterations in either α- or β-diversity.
Nasal CPAP is predominantly a home-based treatment for OSA patients. Therefore, maintaining hygiene protocols for CPAP devices presents a significant challenge and necessitates careful consideration. We recommend that additional research be conducted, including 16S rRNA gene sequencing of both CPAP devices and their users, as well as large-scale population-based epidemiological studies.
The current study had some limitations. First, the contamination level was measured subjectively. Although we attempted to locate an objective assessment metric for contamination, we were unable to find one. We suggest that there is a need for objective measurement indicators, beyond specialized methods such as microbial identification, that patients can use to assess contamination levels. In this context, we suggest that it is necessary to establish guidelines for the periodic inspection of the components of CPAP devices and their corresponding maintenance methods. Second, the number of enrolled participants was relatively small. As the disassembly and cleaning service of the CPAP device was provided free of charge by skilled technicians, only a limited number of participants could be recruited. We are continuing this disassembly and clearing service and plan to conduct further studies with a larger dataset. Third, the clinical characteristics of the participants in this study were not evenly distributed. In particular, most participants in this study were men. Although the percentage of female patients with OSA is not high, an additional study on the effect of clinical characteristics, including age, sex, and concurrent medical diseases, should be considered [11]. We could not rule out the possibility that environmental variables, such as humidity and temperature, in which the CPAP devices were daily applied could affect the results. Furthermore, we hypothesize that patient compliance with CPAP maintenance also could influence the contamination level. Finally, this study had a cross-sectional design, and it was not possible to evaluate changes in contamination over time. Therefore, further large population-based observational studies that consider various characteristics are needed to provide confirmative results.
We found that many parts of CPAP devices, including the humidifier and the interior and exterior of the main body, showed considerable levels of contamination. The duration of usage was not uniformly associated with the degree of contamination; among the characteristics that were evaluated, age was most consistently associated with the degree of contamination of CPAP components. We suggest that age-specific guidelines for the hygiene management of components, besides the masks and tubes, of CPAP devices should be prepared.
▪ The total disassembly and cleaning processes of continuous positive airway pressure (CPAP) devices in patients with obstructive sleep apnea were studied.
▪ External (masks/tubes) and internal (humidifiers) parts showed considerable contamination.
▪ The periods of total and average daily usage were not significantly associated with the contamination of CPAP parts.
▪ Age was most consistently associated with the degree of contamination of CPAP device parts.
▪ Age-specific guidelines for hygiene management for CPAP components need to be devised.
ACKNOWLEDGMENTS
This work was supported by a research grant from the Biomedical Research Institute, Chung-Ang University Hospital (2022), and supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1F1A 1063720).
We are very thankful to Jin Sun Kim (HAPPY PAP), and Tae Jong Kim for their valuable CPAP device cleaning service provided to the OSA patients.
REFERENCES
1. Hetzel J, Herb S, Hetzel M, Rusteberg T, Kleiser G, Weber J, et al. Microbiological studies of a nasal positive pressure respirator with and without a humidifier system. Wien Med Wochenschr. 1996; 146(13-14):354–6.
3. Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008; Feb. 5(2):200–6.

4. Lenk C, Messbacher ME, Abel J, Mueller SK, Mantsopoulos K, Gostian AO, et al. The influence of obstructive sleep apnea and continuous positive airway pressure on the nasal microbiome. Eur Rev Med Pharmacol Sci. 2023; Mar. 27(6):2605–18.
5. Chin CJ, George C, Lannigan R, Rotenberg BW. Association of CPAP bacterial colonization with chronic rhinosinusitis. J Clin Sleep Med. 2013; Aug. 9(8):747–50.

6. Aydin E, Hizal E, Akkuzu B, Azap O. Risk of contamination of nasal sprays in otolaryngologic practice. BMC Ear Nose Throat Disord. 2007; Mar. 7:2.

7. Lee JM, Nayak JV, Doghramji LL, Welch KC, Chiu AG. Assessing the risk of irrigation bottle and fluid contamination after endoscopic sinus surgery. Am J Rhinol Allergy. 2010; May-Jun. 24(3):197–9.

8. Stolk JM, Russcher A, van Elzakker EP, Schippers EF. Legionella pneumonia after the use of CPAP equipment. Ned Tijdschr Geneeskd. 2016; 160:A9855.
9. Steinhauer K, Goroncy-Bermes P. Investigation of the hygienic safety of continuous positive airways pressure devices after reprocessing. J Hosp Infect. 2005; Oct. 61(2):168–75.

Fig. 1.
Photographs of a continuous positive airway pressure (CPAP) device before and after complete disassembly and cleaning service. The exterior main body of the CPAP device before (A) and after cleaning (B). The humidifier of the CPAP device before (C) and after cleaning (D). The interior main body of the CPAP device before (E) and after cleaning (F).
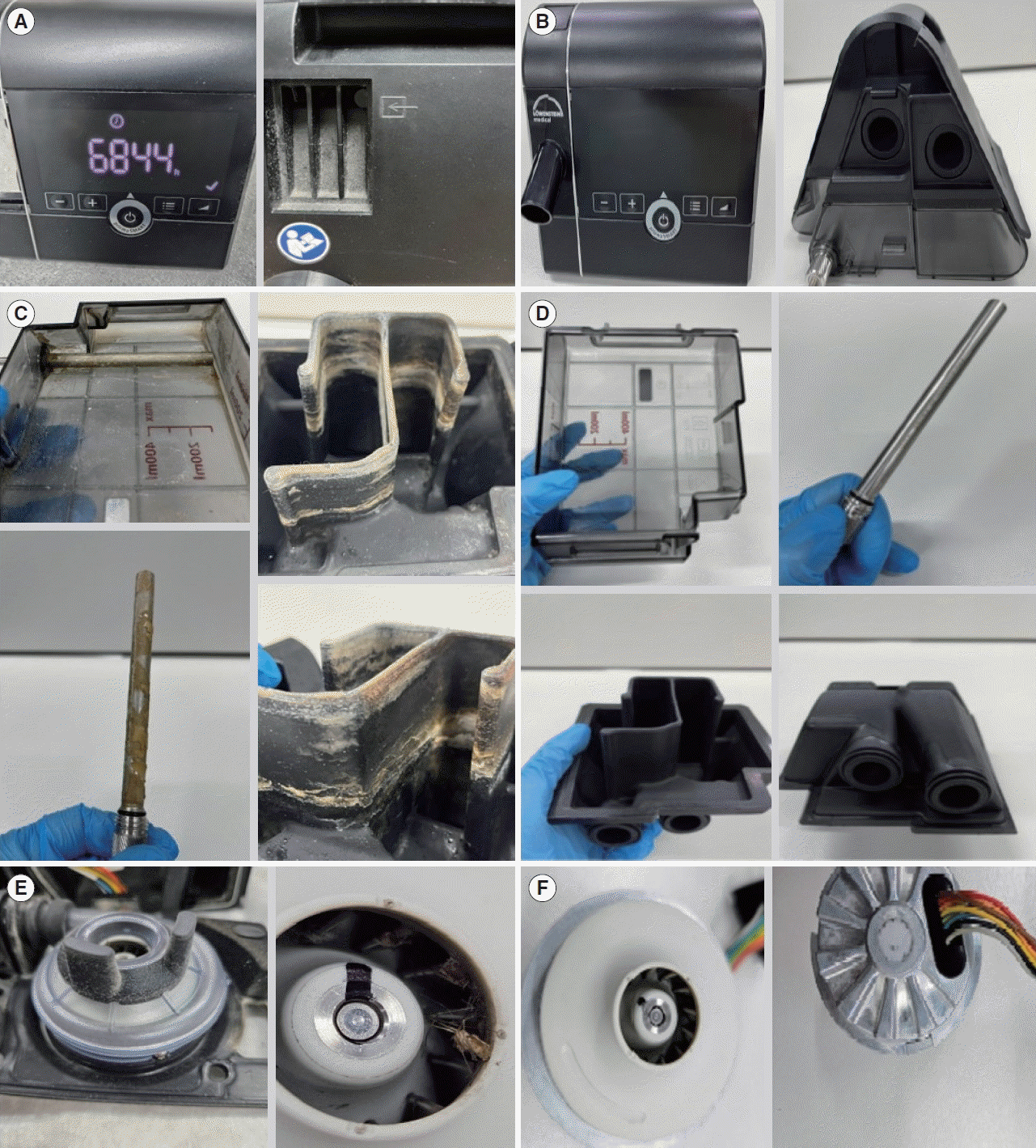
Fig. 2.
Correlation (Corr) among the degree of contamination of the mask, tube, humidifier, interior and exterior main body of the continuous positive airway pressure devices. The upper-right triangular elements illustrate Pearson correlations, the lower-left triangular elements portray two-dimensional (2D) density mapping with jittered data, and the diagonal elements depict variable density through kernel density estimation. A contour plot visually represents a 3D surface by plotting constant Z-level lines (contours) on a 2D plane. ***Significance of the correlation, indicating P<0.001.
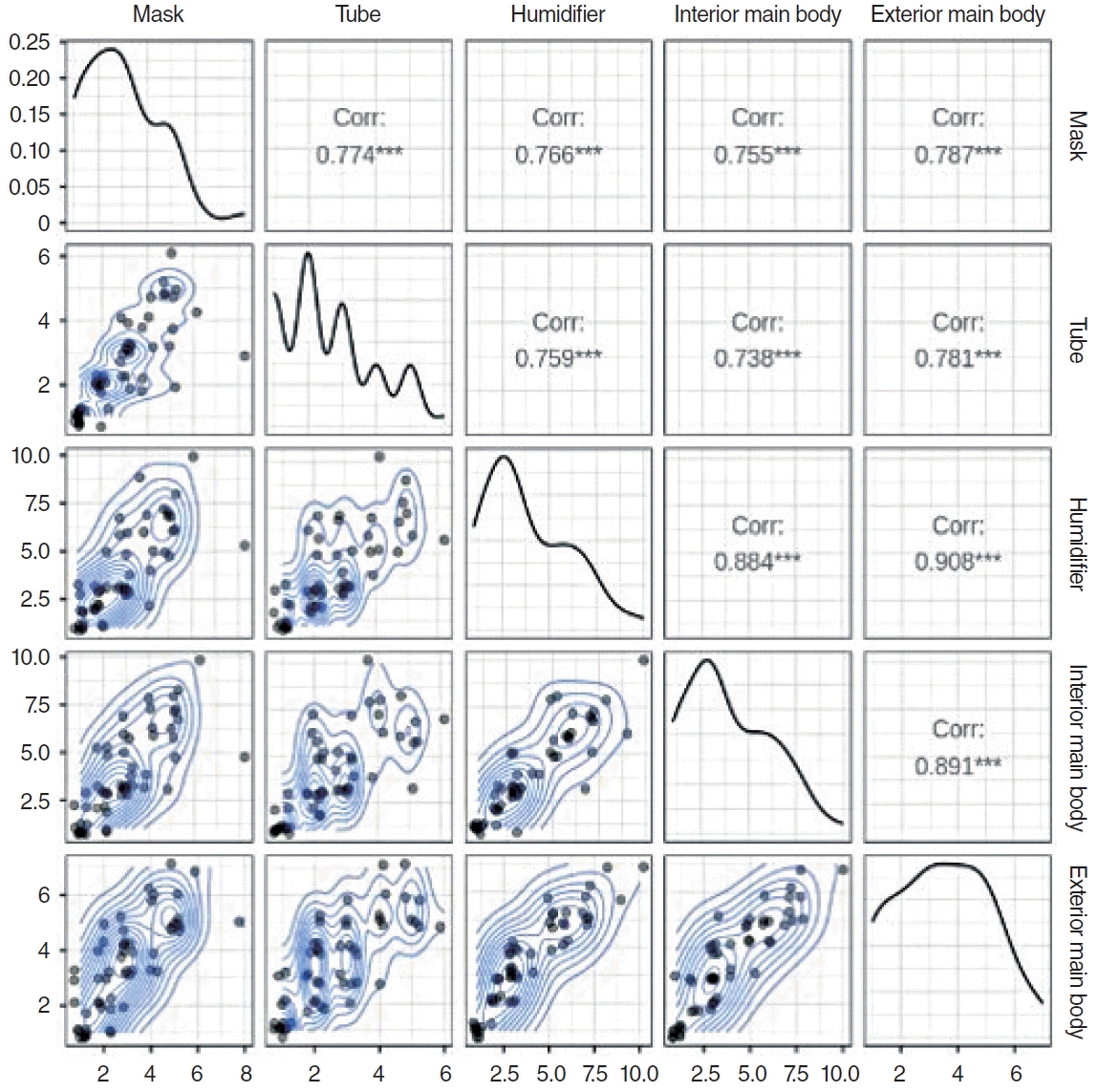
Table 1.
Characteristics of the participants
Table 2.
Multivariate linear regression models with independent variables on the degree of contamination of the mask, tube, humidifier, interior and exterior main body of CPAP devices
| Parameter | Estimate (SE) | 95% CI | P-value |
|---|---|---|---|
| Mask (R2/R2 adjusted: 0.122/0.088) | |||
| (Intercept) | −0.146 (1.177) | −2.508 to 2.216 | 0.074 |
| Age | 0.042 (0.018) | 0.007 to 0.077 | 0.020* |
| AHI | 0.018 (0.010) | −0.002 to 0.037 | 0.033* |
| Tube (R2/R2 adjusted: 0.041/0.023) | |||
| (Intercept) | 1.986 (0.445) | 1.094 to 2.877 | <0.001 |
| Total duration of CPAP usage | 0.001 (0.000) | −0.000 to 0.002 | 0.136 |
| Humidifier (R2/R2 adjusted: 0.093/0.059) | |||
| (Intercept) | −4.629 (3.765) | −12.190 to 2.930 | 0.225 |
| Age | 0.056 (0.028) | 0.000 to 0.110 | 0.049* |
| BMI | 0.204 (0.107) | −1.035 to 0.418 | 0.062 |
| Interior main body (R2/R2 adjusted: 0.162/0.112) | |||
| (Intercept) | −6.946 (3.804) | −14.582 to 0.690 | 0.074 |
| Total duration of CPAP usage | 0.001 (0.001) | −0.000 to 0.003 | 0.079 |
| Age | 0.065 (0.027) | 0.011 to 0.120 | 0.020* |
| BMI | 0.229 (0.105) | 0.019 to 0.439 | 0.033* |
| Exterior main body (R2/R2 adjusted: 0.176/0.110) | |||
| (Intercept) | −3.756 (2.800) | −9.380 to 1.868 | 0.186 |
| Total duration of CPAP usage | 0.001 (0.001) | −0.000 to 0.002 | 0.107 |
| Age | 0.047 (0.020) | 0.007 to 0.087 | 0.024* |
| AHI | 0.016 (0.011) | −0.005 to 0.038 | 0.136 |
| BMI | 0.118 (0.081) | −0.044 to 0.281 | 0.151 |
Table 3.
Multivariate linear regression models with independent variables on the degree of contamination of mask, tube, humidifier, exterior and interior main body of CPAP devices
| Parameter | Estimate (SE) | 95% CI | P-value |
|---|---|---|---|
| Mask usage (≤12 mo) | |||
| Intercept | 2.775 (0.230) | 2.309 to 3.241 | <0.001 |
| Mask usage (>12 mo) (R2/R2 adjusted: 0.265/0.208) | |||
| Intercept | −0.406 (1.807) | −4.310 to 3.498 | 0.826 |
| Age | 0.066 (0.030) | 0.0001 to 0.131 | 0.050* |
| Tube usage (≤6 mo) (R2/R2 adjusted: 0.624/0.452) | |||
| Intercept | −1.810 (3.143) | −8.727 to 5.108 | 0.576 |
| Duration of tube usage | −0.397 (0.151) | −0.728 to −0.065 | 0.023* |
| Sex | −0.958 (0.471) | −1.995 to 0.079 | 0.067 |
| BMI | 0.143 (0.120) | −0.122 to 0.408 | 0.259 |
| Total duration of CPAP usage | −0.001 (0.001) | −0.002 to 0.0004 | 0.184 |
| Average daily duration of CPAP usage | 0.575 (0.196) | 0.142 to 1.007 | 0.014* |
| Tube usage (>6 mo) (R2/R2 adjusted: 0.271/0.206) | |||
| Intercept | −0.105 (1.122) | −2.385 to 2.176 | 0.926 |
| Age | 0.033 (0.016) | −0.0004 to 0.066 | 0.053 |
| Sex | 1.015 (0.575) | −0.154 to 2.184 | 0.087 |
| Total duration of CPAP usage | 0.001 (0.001) | −0.0001 to 0.002 | 0.093 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download