Abstract
Objectives.
Air pollution is an increasing global concern, and its effect on allergic inflammation has attracted the attention of many researchers. Particulate matter (PM) is a major component of ambient air pollution, and heavy metals are the primary toxic constituents of PM. As previous studies on the impact of air pollutants on allergic inflammation did not adequately mimic real-world atmospheric exposure, we developed an experimental model to investigate the effects of aerosolized air pollutants on nasal epithelial cells and fibroblasts.
Methods.
We collected particulate matter 2.5 (PM2.5) samples from ambient 24-hour air samples obtained in Seoul from August 2020 to August 2022, and then conducted component analysis for metallic constituents. Primary nasal epithelial cells and nasal fibroblasts, obtained and cultured from the turbinate tissues of human participants, were treated with PM2.5. The associations of heavy metals identified from the component analysis with cytokine expression were investigated. A three-dimensional (3D)-hybrid culture model, consisting of co-culture of an air-liquid interface and nasal fibroblast spheroids, was constructed to observe the impact of aerosolized air pollutants.
Results.
Among the heavy metals, Si was the predominant component of PM2.5, and Zn showed the highest correlation with the concentration of PM2.5 in Seoul. PM2.5, Zn, and Si increased the production of epithelial cell-derived cytokines, and PM2.5 and Zn exhibited similar trends with one another. Exposure of the 3D-hybrid model to aerosolized PM2.5 and Zn resulted in elevated periostin, alpha-smooth muscle actin, and fibronectin expression in fibroblast spheroids, and those without an epithelial barrier exhibited a similar increase in periostin expression.
According to the World Health Organization (WHO), approximately 99% of the global population breathes air that exceeds the WHO guideline limits for pollution, leading to an estimated 7 million premature deaths annually due to air pollution. Air pollution is categorized into two types: ambient (outdoor) pollution and household (indoor) pollution. Despite the different compositions and varying spatial and temporal patterns of these pollution types, particulate matter (PM) is a common component in both. PM consists of a heterogeneous mixture of liquid and solid particles, with heavy metals being among its most toxic constituents [1]. Despite international efforts to reduce air pollution, particulate matter 2.5 (PM2.5) levels have increased by 38% globally from 1960 to 2009, and 55% of the global population was exposed to more PM2.5 in 2016 than they were in 2010 [2,3].
In 2015, the World Health Assembly passed a landmark resolution regarding air quality and health, acknowledging air pollution as a primary contributor to noncommunicable diseases, which are the leading causes of death worldwide [4]. Air pollution poses a threat in terms of both overall mortality and the incidence of specific diseases. It can also increase the risk of stroke or ischemic heart disease, aggravate underlying respiratory diseases, and trigger asthma attacks [5]. Even short-term exposure is known to be related to hospitalization, and numerous epidemiological and experimental studies have demonstrated associations between air pollutants and allergic diseases [1,6].
A comparative study conducted in South Korea found that children from industrialized areas had more allergic rhinitis (AR) or asthma-related symptoms than those from non-industrialized areas, and a time-series analysis from Beijing reported a strong association between daily levels of air pollutants and the number of outpatients with AR [7,8]. A cross-sectional study of two European cohorts including 1,408 adults also concluded that patients with rhinitis residing in highly polluted areas experienced more severe nasal symptoms [9]. Many laboratory studies have supported these findings. Elevated concentrations of air pollutants increase the deposition of allergens in the airways, and oxidative injury from air pollutants may increase epithelial permeability [10]. PM is thought to serve as an adjuvant that stimulates persistent allergic inflammation in the respiratory tract synergistically with other known pollutants. Heavy metal exposure is known to alter macrophage-related cytokines and surface markers [11-13].
The adverse effects of air pollution on allergic diseases are well-recognized, but extant laboratory models do not reflect the actual atmospheric environment to which the upper respiratory tract is exposed. Therefore, in this study, we collected and analyzed PM2.5 samples from Seoul and identified the major heavy metals that exhibited the strongest correlation with the overall concentration of PM2.5. We then constructed a physiologically realistic experimental model and exposed our model to PM2.5 collected from Seoul, to observe the effects of PM2.5 and its components on allergic inflammation.
A high-capacity air sampler was employed to capture particulate matter with a diameter of 2.5 μm or less in Seoul. The collected PM2.5 with phosphate-buffered saline (PBS) was filtered through a 2.5 μm pore filter, effectively sieving out particles with a diameter of 2.5 μm or less. The particles smaller than 2.5 μm were separated through centrifugation. Subsequently, the collected particulate matter was transferred to a tube, subjected to a 15 minute heating at 121 °C, and then stored at –20 °C. Quantification of 19 trace elements (PM2.5, Al, Ti, V, Mn, Fe, Ni, Co, Cu, Zn, As, Sr, Mo, Cd, Ba, Pb, P, Cr, Si) was performed using atomic absorption spectroscopy (GBC Avanta PM, Australia). The entire process was designed to ensure the accurate measurement of trace elements associated with PM2.5 in the air quality assessment in Seoul.
The inferior turbinate tissues were harvested from individuals who exhibited no indications of inflammation, allergic reactions, asthma, or sensitivity to aspirin. during rhinoplasty. Patients were recruited from the Department of Otorhinolaryngology at Korea University Medical Center, following the principles outlined in the Declaration of Helsinki, with prior informed consent obtained. This study received approval from the Institutional Review Board for Bioethics at Korea University Medical Center (No. 2023GR0179). Ethical handling of patients and their tissue specimens followed established protocols and guidelines.
Air-liquid interface (ALI) culture in 12 well transwell plates were placed on VITROCELL Cloud12 (VITROCELL Systems) and treated with the exposure system as an aerosol that exposes cells to 200 µL of PM2.5 and ZnCl2 (Sigma-Aldrich) (Fig. 1A). The exposure to aerosol was conducted single time, and real-time measurements of the deposited particles were performed using a quartz microbalance.
Primary nasal epithelial cells were collected using a brush and incubated with PneumaCult-Ex Plus medium (Stemcell Technologies) on plates coated with collagen type 1 (Corning). After cells were adherent, red blood cells were removed by washing with PBS. When cells reached >90% confluence, cells were isolated using an animal-free cell isolation kit (Stemcell Technologies) and cultured in 75T flasks.
Human nasal epithelial cells (HNECs) were expanded, washed with Dulbecco’s Phosphate-Buffered Saline, and dissociated using the Animal Component-Free Cell Dissociation Kit. For ALI culture, 0.5 mL of PneumaCult-Ex medium (Stemcell Technologies) containing HNECs is seeded into the upper chamber of a 0.4 μm Transwell insert (Corning) and only medium is added to the lower chamber. The media in both the upper and lower chambers were refreshed every 2 to 4 days. Upon reaching 100% cellular confluence, the medium in both the upper and lower chambers was aspirated. Subsequently, 1 mL of PneumaCult-ALI Maintenance Medium (Stemcell Technologies) was added only to the lower chamber, with subsequent medium changes every 2 days.
The inferior turbinate, obtained from the patient, are finely chopped and placed in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Invitrogen). The chopped tissue is then evenly distributed onto a plate to isolate. The cells around the tissue are dissociated using 0.05% trypsin and cultured to isolate fibroblasts. When the fibroblasts reach 100% confluence, they are detached from the plate using trypsin and cultured at a density of 5×104 cells per well in a 96-well round-bottom ultra-low attachment plate (Corning). Each well formed a single sphere and was cultured for 7 days, with the medium changed every 3 days. For co-culture with ALI and nasal fibroblast spheroids, spheroids were transferred to Transwell bottom plates, inserted into the upper chamber containing ALI culture, and incubated at 37 °C with 5% CO2 (Fig. 1B). RPMI 1640 medium (Hyclone) was added only to the bottom plate.
Cellular cytotoxicity induced by PM2.5, ZnCl2, and SiO2 (Sigma-Aldrich) was evaluated using a WST-1 assay. Nasal epithelial cells or nasal fibroblasts were plated in 96-well plates and exposed to various concentrations of PM2.5, ZnCl2, or SiO2 for 72 hours. WST-1 solution is then added to the medium. Incubation at 37 °C facilitated the enzymatic conversion of WST-1 to formazan by metabolically active cells. The absorbance at 450 nm (with reference to 650 nm) was measured using a microplate reader (Bio-Rad).
PM2.5, ZnCl2, and SiO2 were treated with epithelial cells and fibroblasts for 24 hours. RNA was isolated from the samples using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Concentration and purity of the extracted RNA were determined using a NanoDrop spectrophotometer. For complementary DNA (cDNA) synthesis, 2 μg of total RNA was reverse transcribed into cDNA using a mixture of M-MLV reverse transcriptase (Invitrogen), oligo dT primers, and a ribonuclease inhibitor. The synthesized cDNA was used for gene expression analysis by real-time polymerase chain reaction (RT-PCR). Power SYBR Green PCR Master Mix (Applied Biosystems) was mixed with the target-specific primers and a cDNA template. The target-specific primers are described in Table 1. RT-PCR was performed using Quantstudio3 to measure PCR amplification. Gene expression levels were quantified using the ΔΔCt method. The Ct values obtained from target gene amplification were normalized to the expression of the reference gene, GAPDH. The relative fold change in gene expression was calculated by comparing the normalized target gene Ct values between experimental and control samples.
PM2.5, ZnCl2, and SiO2 were treated with epithelial cells and fibroblasts for 72 hours. The concentrations of IL-6, IL-25, IL-33, thymic stromal lymphopoietin (TSLP), and periostin in the culture media were determined using enzyme-linked immunosorbent assays (R&D Systems). Standards and samples were added to the wells and incubated at room temperature for 2 hours. After washing the wells three times, the appropriate conjugate (IL-6, IL-25, IL-33, TSLP, or periostin) was added and incubated for 2 hours at room temperature. The reaction was then stopped with a stop solution, and the optical densities of the standards and samples were measured at 450 nm using a microplate reader (Bio-Rad).
PM2.5, ZnCl2, and SiO2 were treated with epithelial cells and fibroblasts for 72 hours. Proteins extracted using radioimmunoprecipitation assay (RIPA) buffer were measured for concentration using the Bradford assay. Samples were prepared by mixing equal amounts of proteins with 5×sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and resolved by electrophoresis on 10% SDS-PAGE gels. The proteins were transferred to polyvinyl difluoride membranes and then blocked in 3% skim milk. The membranes were probed with anti–alpha-smooth muscle actin (α-SMA; Abcam), anti-E-cadherin, anti-fibronectin, anti-GAPDH (Santa Cruz Biotechnology), and anti-vimentin (Cell Signaling Technology) primary antibodies overnight at 4 °C. The horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Vector Laboratories) were diluted in 3% skim milk for 1 hour before measurement using an ECL system (Pierce). Subsequently, acquired images were analyzed with ImageJ software (National Institutes of Health) to quantify the observed signals.
Cellular fixation was achieved by treating cells with 4% paraformaldehyde for 30 minutes, followed by permeabilization using 0.1% Triton X-100 for 10 minutes. Subsequently, a blocking step with 5% BSA for 1 hour was implemented before incubating the cells with primary antibodies against E-cadherin, α-SMA, or periostin (Santa Cruz Biotechnology, 1:100 dilution in blocking buffer) at 4 °C overnight. Goat anti-mouse IgG (H+L) Alexa 488 or goat anti-rabbit IgG (H+L) Alexa 555 (Invitrogen) was diluted 1:200 in blocking solution and cells were incubated in this mixture for 1 hour at room temperature. Nuclei were counterstained with DAPI (4´,6-diamidino-2-phenylindole, dihydrochloride; Invitrogen) for 10 minutes. After washing with PBS, the coverslips were mounted on glass slides using a mounting medium. A confocal laser scanning microscope LSM700 (Zeiss) was used to capture images of the stained cells.
Epithelial electrical resistance (transepithelial electrical resistance [TEER]) measurements were performed by inserting sterile electrodes of an EVOM2 epithelial volt-ohmmeter (World Precision Instruments) into both the upper and lower chambers of the transwell system. The background resistance of the cell-free insert membrane devoid of cells was subtracted from each measurement. TEER values were recorded in ohms (Ω), to provide insight into the barrier integrity of the epithelial cell layer.
The data are presented as the mean±standard deviation of at least three experiments performed in duplicate. Statistical analysis was performed by unpaired t-test or one-way analysis of variance followed by Tukey’s test using GraphPad Prism 5 (GraphPad). Statistical significance was set at P<0.05.
Correlations were observed between concentrations of PM2.5 and concentrations of heavy metals. Among the elements comprising PM2.5, the most abundant was Si, followed by aluminum (Al) (Fig. 1C). The correlation coefficient between PM2.5 and heavy metal concentrations was the highest for Zn, followed by iron (Fe) and nickel (Ni). The correlation coefficients between PM2.5 and Zn and Si concentrations were 0.8 and 0.54, respectively, which were statistically significant (Fig. 1D and E). Considering these results, Zn and Si were selected as target heavy metals for further research.
The primary nasal epithelial cells treated with different concentrations of PM2.5 showed no cytotoxicity until 1,000 μg/mL (Fig. 2A). We evaluated the mRNA and protein expression of markers associated with allergic inflammation, including IL-6, IL-25, IL-33, TSLP, and periostin. The results showed that IL-6, IL-25, IL-33, and TSLP levels significantly increased in a concentration-dependent manner for both mRNA (Fig. 2B-E) and protein expression (Fig. 2G-J) in response to PM2.5. The expression of periostin mRNA and protein slightly increased at a PM2.5 concentration of 100 μg/mL (Fig. 2F and K). Under ALI culture conditions (Fig. 2L), the expression levels of IL-6, IL-25, IL-33, and TSLP increased in response to PM2.5 exposure, whereas periostin expression did not change (Fig. 2M-V).
Primary nasal epithelial cells treated with various concentrations of ZnCl2 showed no cytotoxicity at concentrations <100 μM (Fig. 3A). We evaluated the mRNA and protein expression of markers associated with allergic inflammation, including IL-6, IL-25, IL-33, TSLP, and periostin. Significant increases were observed for IL-6, IL-25, IL-33, and TSLP in both mRNA (Fig. 3B-E) and protein expression (Fig. 3G-J) in a concentration-dependent manner in response to ZnCl2 exposure. Periostin expression weakly increased at a ZnCl2 concentration of 50 μg/mL, but significantly increased at a concentration of 100 μg/mL (Fig. 3F and K). In ALI culture conditions, ZnCl2 did not lead to cytotoxicity at concentrations less than 100 μM (Fig. 3L). The expression of IL-6, IL-25, IL-33, and TSLP increased in response to ZnCl2 exposure, while periostin expression remained unchanged (Fig. 3M-V).
Primary nasal epithelial cells treated with various concentrations of SiO2 showed no cytotoxicity until a concentration of 100 μM (Fig. 4A). We evaluated the mRNA and protein expression of markers associated with allergic inflammation, including IL-6, IL-25, IL-33, TSLP, and periostin. The results showed that IL-6, IL-25, IL-33, and TSLP exhibited significant increases in both mRNA (Fig. 4B-E) and protein expression (Fig. 4G-J) in a concentration-dependent manner in response to SiO2 exposure. However, the periostin mRNA and protein expression levels did not change (Fig. 4F and K). Consistently, in ALI culture conditions, SiO2 did not lead to cytotoxicity at concentrations <40 μM (Fig. 4L), and the expression levels of IL-6, IL-25, IL-33, and TSLP increased in response to SiO2 exposure, whereas periostin expression remained unchanged (Fig. 4M-V).
The effects of PM2.5 and Zn on nasal fibroblast spheroids from the perspective of allergic inflammation were examined. Fibroblast spheroids were exposed to PM2.5 and ZnCl2 by aerosolization under two conditions (with and without ALI on top) to examine the expression of alpha-smooth muscle actin (α-SMA), a marker associated with myofibroblast differentiation, and fibronectin, an extracellular matrix (ECM) component. In the presence of ALI, ALI-cultured cells were exposed to aerosolized PM2.5 (24.2 μg/cm2) and ZnCl2 (46.3 ng/cm2) to investigate their effects on nasal fibroblast spheroids and allergic inflammatory responses. Both the mRNA and protein expression of α-SMA and fibronectin significantly increased following exposure to PM2.5 or ZnCl2 aerosols (Fig. 5A-C). Additionally, both mRNA and protein levels of periostin, an important factor in allergic inflammatory responses, dramatically increased after exposure to PM2.5 or ZnCl2 aerosols (Fig. 5D and E). Immunofluorescence staining confirmed the increased expression of α-SMA and periostin, with increased periostin expression in α-SMA-positive cells (Fig. 5F). Furthermore, direct aerosol exposure to PM2.5 or ZnCl2 significantly elevated periostin mRNA and protein expression in nasal fibroblast spheroids without ALI (Fig. 5G and H). These results suggest that exposure to PM2.5 and ZnCl2, whether directly or indirectly, increases the activation of nasal fibroblast spheroids and, consequently, upregulates periostin expression.
Experiments conducted in ALI culture and nasal fibroblast spheroids examined the effect of exposure to aerosolized PM2.5 and ZnCl2 on the expression of epithelial cell-derived cytokines and epithelial-mesenchymal transition (EMT). TEER, which was measured to quantify the barrier integrity of the cells, decreased upon exposure to PM2.5 and ZnCl2 (Fig. 6A). Both mRNA and protein expression levels of IL-6, IL-25, IL-33, and TSLP significantly increased in response to PM2.5 and ZnCl2 exposure (Fig. 6B-E and G-J). Furthermore, periostin mRNA expression was upregulated by exposure to PM2.5 and ZnCl2 (Fig. 6F). Notably, exposure to PM2.5 and ZnCl2 reduced the expression of E-cadherin, a marker of epithelial cells, and increased the expression of mesenchymal cell markers, including vimentin and fibronectin (Fig. 6K-N). Immunofluorescence staining supported these findings and revealed decreased E-cadherin and increased periostin expression (Fig. 6O). Aerosol treatment with PM2.5 or ZnCl2 showed similar trends in their effect on ALI in the presence of nasal fibroblast spheroids, but with increased reactivity.
Over the past few decades, the global prevalence of allergic diseases has significantly increased. Given that this is a relatively short period for the natural properties of the immune system to evolve, researchers have been investigating external factors that could be responsible for this trend. Air pollution has emerged as a key area of interest for many scientists. The rise in allergic diseases was initially noted in Europe and North America, coinciding with rapid urbanization and industrialization. This pattern is now being observed in the rapidly developing countries of Southeast Asia. A meta-analysis conducted in 2021 provided evidence that exposure to air pollutants may heighten the risk of immunoglobulin E (IgE)-mediated allergic diseases [6].
PM is a pollutant, and compelling evidence supports its impact on public health [4]. PM is classified based on the diameter of the particles, and its toxicity depends on its size and chemical composition. Because smaller particles tend to remain longer in the atmosphere, PM2.5, the major chemical constituents of which are heavy metals, has a greater impact on respiratory diseases than PM10 [1,14]. We found that Si was the most abundant heavy metal in PM2.5, and the concentration of Zn showed the highest correlation with the level of PM2.5. Therefore, we conducted further experiments using PM2.5, Zn, and Si.
Allergic inflammation of the upper airway is caused by IgE-mediated reactions to inhaled allergens. In addition to type 2 inflammatory cells, nasal epithelial cells and fibroblasts play important roles in this process. The nasal epithelium is the first line of defense against inhaled allergens, and it releases inflammatory cytokines such as IL-6, IL-25, IL-33, and TSLP, thereby triggering Th2-mediated inflammation [15]. These cytokines subsequently stimulate nasal fibroblasts, leading to the production of inflammatory mediators and ECM proteins with eosinophil infiltration [16]. In our experiment on primary nasal epithelial cell (PNEC) and ALI cultures, treatment with PM2.5, Zn, and Si solutions increased the expression of epithelial cell-derived cytokines. Overall, Zn more closely paralleled PM2.5 in terms of trends in the increase of cytokine expression than Si.
Periostin, a matricellular and ECM protein involved in tissue remodeling, plays an important role in inflammation and allergy, and it is known to be induced by various stimuli such as transforming growth factor-beta, angiotensin II, and other cancer-derived factors [17]. In the context of allergies, it is mainly produced by fibroblasts stimulated by IL-4 or IL-13, and it is also expressed by some endothelial or epithelial cells [18,19]. In 2011, it was first reported that the serum periostin level could be utilized to predict the efficacy of a monoclonal antibody for severe asthma, and its role as a potential biomarker for type 2 inflammation in allergic diseases has been investigated ever since [20]. Hoshino et al. [21] reported its utility as a marker of response to sublingual immunotherapy, and Krasilnikova et al. [22] found that the exacerbation of AR was associated with increased periostin levels in nasal secretion.
In our experiment with PNEC and ALI cultures, treatment with PM2.5, Zn, and Si solutions at high concentrations led to elevated expression of periostin. In an experiment using our three-dimensional (3D)–hybrid model, PM2.5 and Zn aerosol treatment also led to a dramatic increase in periostin expression in fibroblast spheroids compared to the control. Notably, fibroblast spheroids directly exposed to PM2.5 and Zn aerosols without the upper epithelial cell barrier also exhibited similar results. The pollutant itself may easily penetrate the epithelial barrier and reach the submucosa, or it may impair the epithelial barrier, easing its path to the submucosal layer. In a further experiment with the 3D-hybrid culture model, upper ALI culture showed reduced TEER and E-cadherin expression after exposure to PM2.5 and Zn aerosol, whereas vimentin, a marker of the EMT, increased after exposure. Altogether, it can be inferred that the upper ALI culture was damaged and lost its function as an epithelial barrier, with the reduction and collapse of tight junctions after atmospheric exposure to air pollutants.
To the best of our knowledge, most previous in vitro studies demonstrating associations between PM and allergic inflammation have used nasal epithelial cells and fibroblasts submerged in PM solutions [23,24]. Additionally, the effect of heavy metals on allergies has been mostly investigated by measuring the concentrations of heavy metals in serum or urine [25]. However, in the human body, the nasal epithelium is directly exposed to pollutants through inhaled air, whereas fibroblasts in the submucosal layer are indirectly affected by various cytokines from the epithelium. In addition, whether atmospheric exposure to heavy metals directly affects their concentrations in body fluids remains unclear. Therefore, we built a model containing ALI cells co-cultured with fibroblast spheroids underneath—namely, a 3D-hybrid culture model—which is more anatomically and physiologically realistic than any other experimental method used to date. Furthermore, air pollutants can be directly sprayed over this model, such that the epithelial cytokines would spread to the fibroblast spheroids in the lower chamber through the solution, which reflects the real-world effects of air pollutants on the upper respiratory tract.
In this study, we confirmed that Si and Zn were the most predominant and highly correlated heavy metals with PM2.5 in air samples from Seoul, and that PM2.5, Zn, and Si increased the expression of inflammatory cytokines in nasal epithelial cells and fibroblasts. In PNEC and ALI cultures, we observed the increased production of epithelial cell-derived and proinflammatory cytokines, as well as impaired barrier function, as shown by decreased TEER and elevated EMT markers. In an experiment on fibroblast spheroids, fibroblast activation and increased production of ECM and periostin were observed after exposure to PM2.5. In conclusion, we demonstrated the effect of ambient air pollutants on allergic inflammation in nasal epithelial cells and fibroblasts and confirmed these findings through both physiologically and anatomically realistic experimental models. As air pollution worsens worldwide, it is important to restrict exposure to air pollutants to prevent and manage allergic diseases. Further in vivo studies are required to investigate the clinical effects of air pollutants on allergic inflammation.
▪ Particulate matter 2.5 (PM2.5) was collected from ambient 24-hour air samples taken in Seoul, and a component analysis was conducted for metallic constituents.
▪ We built a three dimensional-hybrid culture model to investigate the effects of aerosolized air pollutants on nasal epithelial cells and fibroblasts.
▪ Ambient air pollutants in the form of aerosols increased the expression of allergic inflammatory cytokines in both nasal epithelial cells and fibroblasts.
▪ Regulations on air pollution will help reduce the burden of allergic diseases worldwide in the future.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: DJS, IHP. Data curation: JHP, HWY. Funding acquisition: IHP. Investigation: JHP, JWM. Methodology: JHP, HWY. Project administration: DJS, IHP. Software: JHP. Supervision: IHP, DJS. Visualization: JHP. Writing–original draft: JHP, JWM. Writing–review & editing: IHP.
ACKNOWLEDGMENTS
This work was supported by the Korea Environmental Industry & Technology Institute (KEITI) through the Core Technology Development Project for Environmental Disease Prevention and Management, funded by the Korea Ministry of Environment (MOE) (RS-2022-KE-002339).
REFERENCES
1. Nguyen TT, Higashi T, Kambayashi Y, Anyenda EO, Michigami Y, Hara J, et al. A longitudinal study of association between heavy metals and itchy eyes, coughing in chronic cough patients: related with nonimmunoglobulin E mediated mechanism. Int J Environ Res Public Health. 2016; Jan. 13(1):110.

2. Butt EW, Turnock ST, Rigby R, Reddington CL, Yoshioka M, Johnson JS, et al. Global and regional trends in particulate air pollution and attributable health burden over the past 50 years. Environ Res Lett. 2017; Oct. 12(10):104017.

3. Shaddick G, Thomas ML, Mudu P, Ruggeri G, Gumy S. Half the world’s population are exposed to increasing air pollution. NPJ Clim Atmos Sci. 2020; Jun. 3(1):23.

4. World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. World Health Organization;2021.
5. Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014; Jun. 30(2):71–5.

6. Lee KI, Chung YJ, Mo JH. The impact of air pollution on allergic rhinitis. Allergy Asthma Respir Dis. 2021; Jan. 9(1):3–11.

7. Jeong SH, Kim JH, Son BK, Hong SC, Kim SY, Lee GH, et al. Comparison of air pollution and the prevalence of allergy-related diseases in Incheon and Jeju City. Korean J Pediatr. 2011; Dec. 54(12):501–6.

8. Zhang F, Wang W, Lv J, Krafft T, Xu J. Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Beijing, China. Sci Total Environ. 2011; Jun. 409(13):2486–92.

9. Burte E, Leynaert B, Marcon A, Bousquet J, Benmerad M, Bono R, et al. Long-term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J Allergy Clin Immunol. 2020; Mar. 145(3):834–42.

10. Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022; Mar. 31(163):210112.

11. Czarnobilska E, Bulanda M, Bulanda D, Mazur M. The influence of air pollution on the development of allergic inflammation in the airways in Krakow’s atopic and non-atopic residents. J Clin Med. 2021; May. 10(11):2383.

12. Lu X, Gong C, Lv K, Zheng L, Li B, Zhao Y, et al. Impacts of combined exposure to formaldehyde and PM2.5 at ambient concentrations on airway inflammation in mice. Environ Pollut. 2022; Dec. 315:120234.

13. Li CH, Tsai ML, Chiou HC, Lin YC, Liao WT, Hung CH. Role of macrophages in air pollution exposure related asthma. Int J Mol Sci. 2022; Oct. 23(20):12337.

14. Jung HJ, Han DH. Effects of particulate matter on allergic rhinitis and possible mechanisms. Korean J Otorhinolaryngol-Head Neck Surg. 2023; Feb. 66(2):75–84.

15. London NR Jr, Ramanathan M Jr. The role of the sinonasal epithelium in allergic rhinitis. Otolaryngol Clin North Am. 2017; Dec. 50(6):1043–50.

16. Shin JM, Yang HW, Park JH, Kim TH. Role of nasal fibroblasts in airway remodeling of chronic rhinosinusitis: the modulating functions reexamined. Int J Mol Sci. 2023; Feb. 24(4):4017.

17. Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017; Dec. 74(23):4293–303.

18. Sonnenberg-Riethmacher E, Miehe M, Riethmacher D. Periostin in allergy and inflammation. Front Immunol. 2021; Aug. 12:722170.
19. Liu AY, Zheng H, Ouyang G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014; Jul. 37:150–6.

20. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; Jul. 118(1):98–104.

21. Hoshino M, Akitsu K, Kubota K, Ohtawa J. Diagnostic utility of serum periostin for house dust mite sublingual immunotherapy response in allergic rhinitis. European Respiratory Society;2021.
22. Krasilnikova SV, Tush EV, Frolov PA, Ovsyannikov DY, Terentyeva AB, Kubysheva NI, et al. Periostin as a biomarker of allergic inflammation in atopic bronchial asthma and allergic rhinitis (a pilot study). Sovrem Tekhnologii Med. 2021; 12(5):37–45.

23. Hong Z, Guo Z, Zhang R, Xu J, Dong W, Zhuang G, et al. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J Exp Med. 2016; Jun. 239(2):117–25.

Fig. 1.
Schematic drawing of aerosolized particulate matter 2.5 (PM2.5) and ZnCl2 treatment of a co-culture of air-liquid interface (ALI) and fibroblast spheroids. (A) Aerosol exposure of epithelial cells to PM2.5 and ZnCl2, (B) co-culture of ALI and nasal fibroblast spheroids. Illustration of the correlation between PM2.5 and the heavy metals Zn and Si. (C) The list of the order of abundance of heavy metals in PM2.5. (D) Heatmap of correlation coefficients between PM2.5 concentrations and heavy metal concentrations. (E) The correlation coefficients between Zn concentrations and PM2.5 concentrations and between Si concentrations and PM2.5 concentrations. SD, standard deivation.
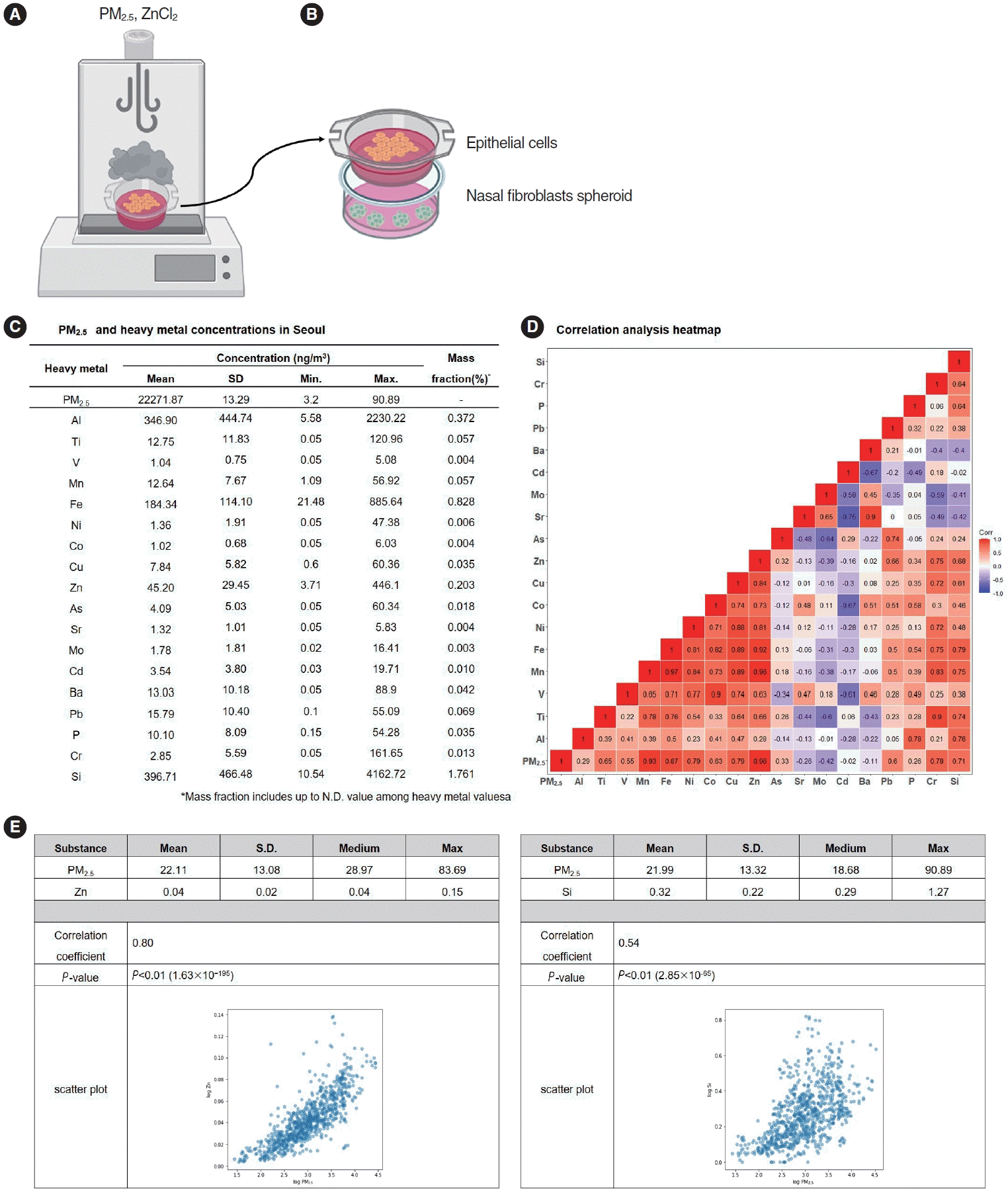
Fig. 2.
The effects of particulate matter 2.5 (PM2.5) on allergic inflammation in primary nasal epithelial cells. (A) Primary nasal epithelial cells were treated by different concentrations of PM2.5 (0–1,000 μg/mL) and cytotoxicity was measured using WST-1. (B-E) The mRNA levels of allergic inflammation markers, including interleukin (IL)-6, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), were measured by real-time polymerase chain reaction after treatment with PM2.5. (G-J) Protein expression of these markers was measured by enzyme-linked immunosorbent assays. (F, K) The expression of periostin mRNA and protein was measured. (L) Air-liquid interface (ALI) culture treated with different concentrations of PM2.5 (0–1,000 μg/mL) was analyzed for cytotoxicity using WST-1. (M-V) IL-6, IL-25, IL-33, TSLP, and periostin mRNA and protein expression levels were measured after treatment with PM2.5. Values are presented as mean±standard deviation of three independent experiments. *P<0.05 compared to control.
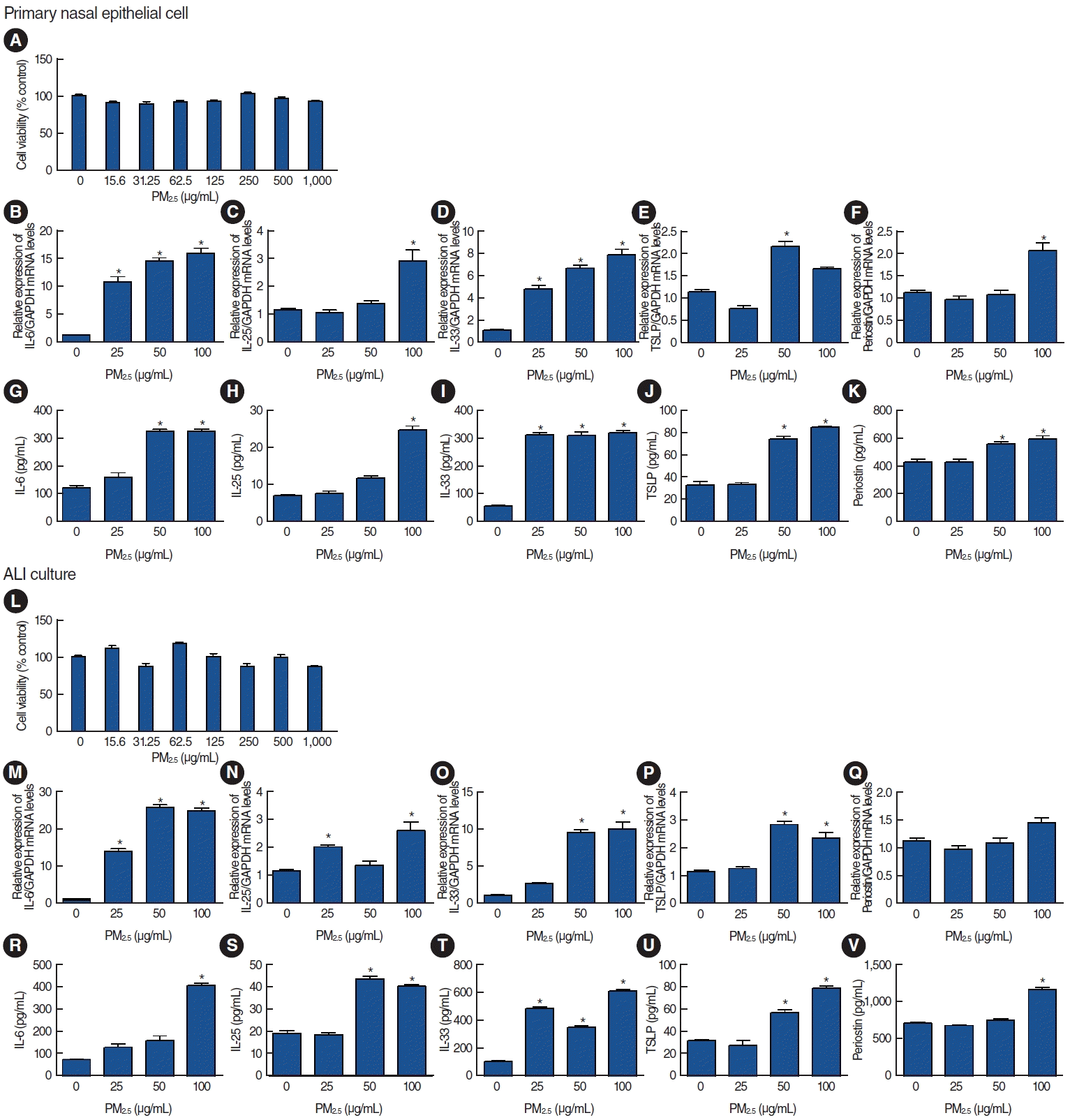
Fig. 3.
The effects of ZnCl2 on allergic inflammation in primary nasal epithelial cells. (A) Primary nasal epithelial cells treated with different concentrations of ZnCl2 (0–1,000 μM) were measured for cytotoxicity using WST-1. (B-E) The mRNA levels of allergic inflammation markers including interleukin (IL)-6, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) were measured by real-time polymerase chain reaction after treatment with ZnCl2. (G-J) Protein expression of these markers was measured by enzyme-linked immunosorbent assays. (F, K) The expression levels of periostin mRNA and protein were measured. (L) Air-liquid interface (ALI) culture treated with different concentrations of ZnCl2 (0–1,000 μM) were measured for cytotoxicity using WST-1. (M-V) IL-6, IL-25, IL-33, TSLP, and periostin mRNA and protein expression levels were measured after treatment with ZnCl2. Values are presented as mean±standard deviation of three independent experiments. *P<0.05 compared to control.
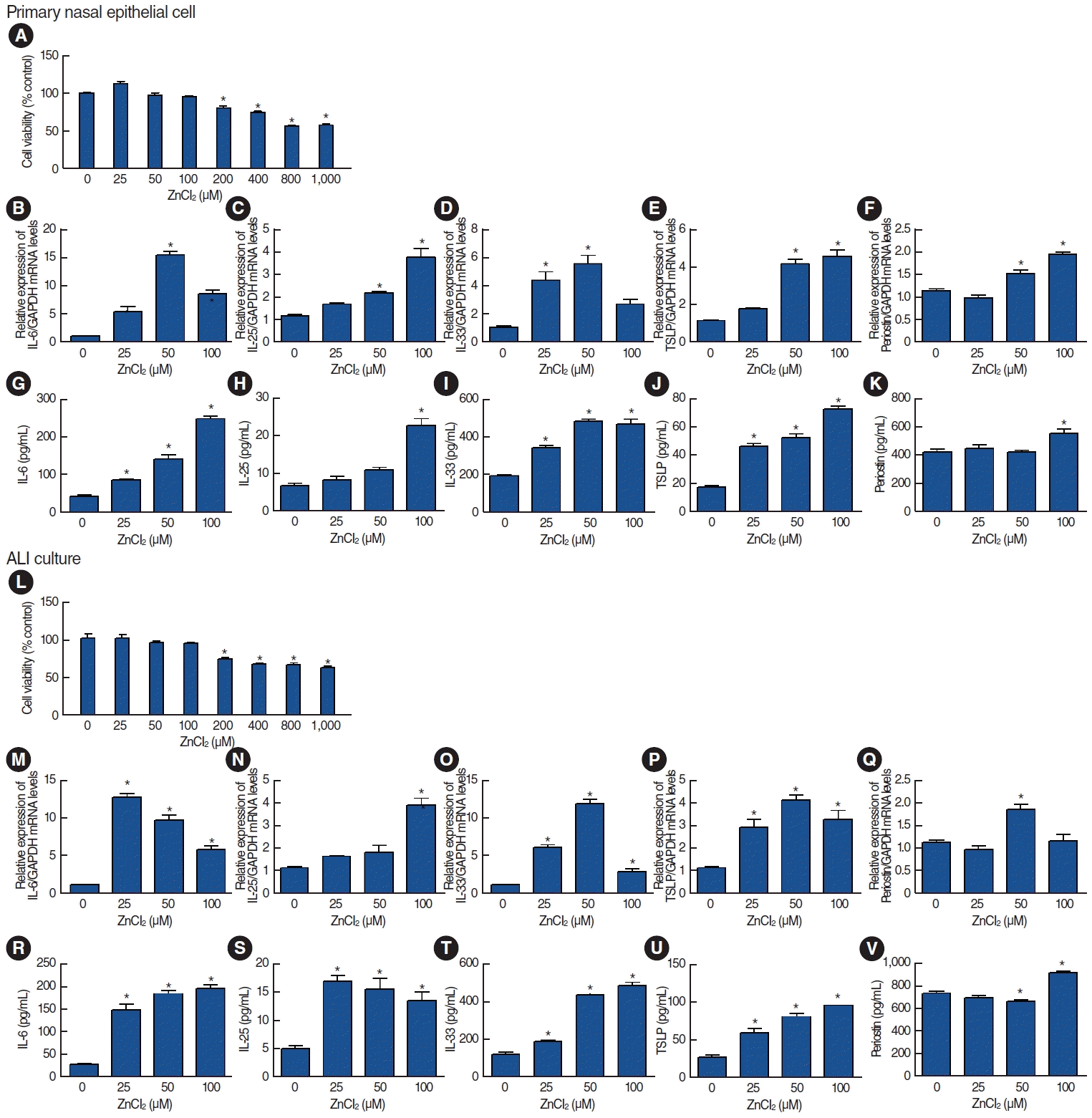
Fig. 4.
Effects of SiO2 on allergic inflammation in primary nasal epithelial cells. (A) Primary nasal epithelial cells treated with different concentrations of SiO2 (0–100 μM) were measured for cytotoxicity using WST-1. (B-E) The mRNA levels of allergic inflammation markers including interleukin (IL)-6, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) were measured by real-time polymerase chain reaction after treatment with SiO2. (G-J) Protein expression of these markers was measured by enzyme-linked immunosorbent assays. (F, K) The expression of periostin mRNA and protein was measured. (L) Air-liquid interface (ALI) culture treated with different concentrations of ZnCl2 (0–100 μM) was measured for cytotoxicity using WST-1. (M-V) IL-6, IL-25, IL-33, TSLP, and periostin mRNA and protein expression levels were measured after treatment with SiO2. Values are presented as mean±standard deviation of three independent experiments. *P<0.05 compared to control.
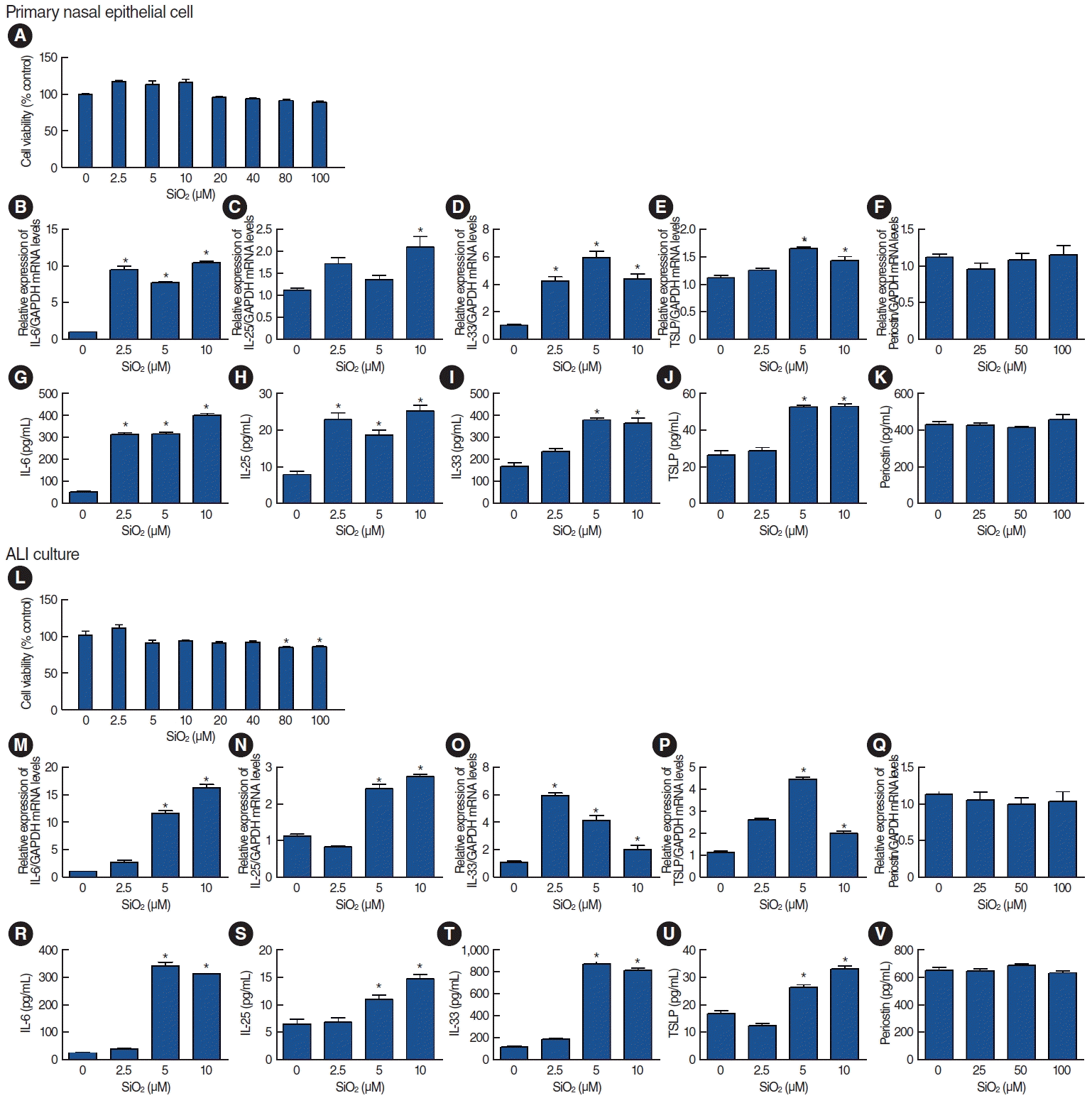
Fig. 5.
Aerosolized particulate matter 2.5 (PM2.5) and ZnCl2 induced fibroblast activation and an allergic inflammatory response in spheroids of nasal fibroblasts co-cultured with air-liquid interface (ALI) cultures. (A-C) The mRNA and protein levels of alpha-smooth muscle actin (α-SMA) and fibronectin were determined after exposure to PM2.5 or ZnCl2 aerosols. (D, E) The mRNA and protein expression of periostin was measured after exposure to PM2.5 or ZnCl2 aerosols. (F) Immunofluorescence staining data confirmed the expression of α-SMA (green) and periostin (red). The nuclei of the cells were stained with DAPI (blue). Scale bar=50 μM. (G, H) Periostin mRNA and protein expression levels were measured in nasal fibroblast spheroids upon direct aerosol exposure to PM2.5 or ZnCl2. Values are presented as mean±standard deviation of three independent experiments. 3D, three-dimensional; DW, distilled water; DAPI, 4´,6-diamidino-2-phenylindole, dihydrochloride. *P<0.05 compared to control.
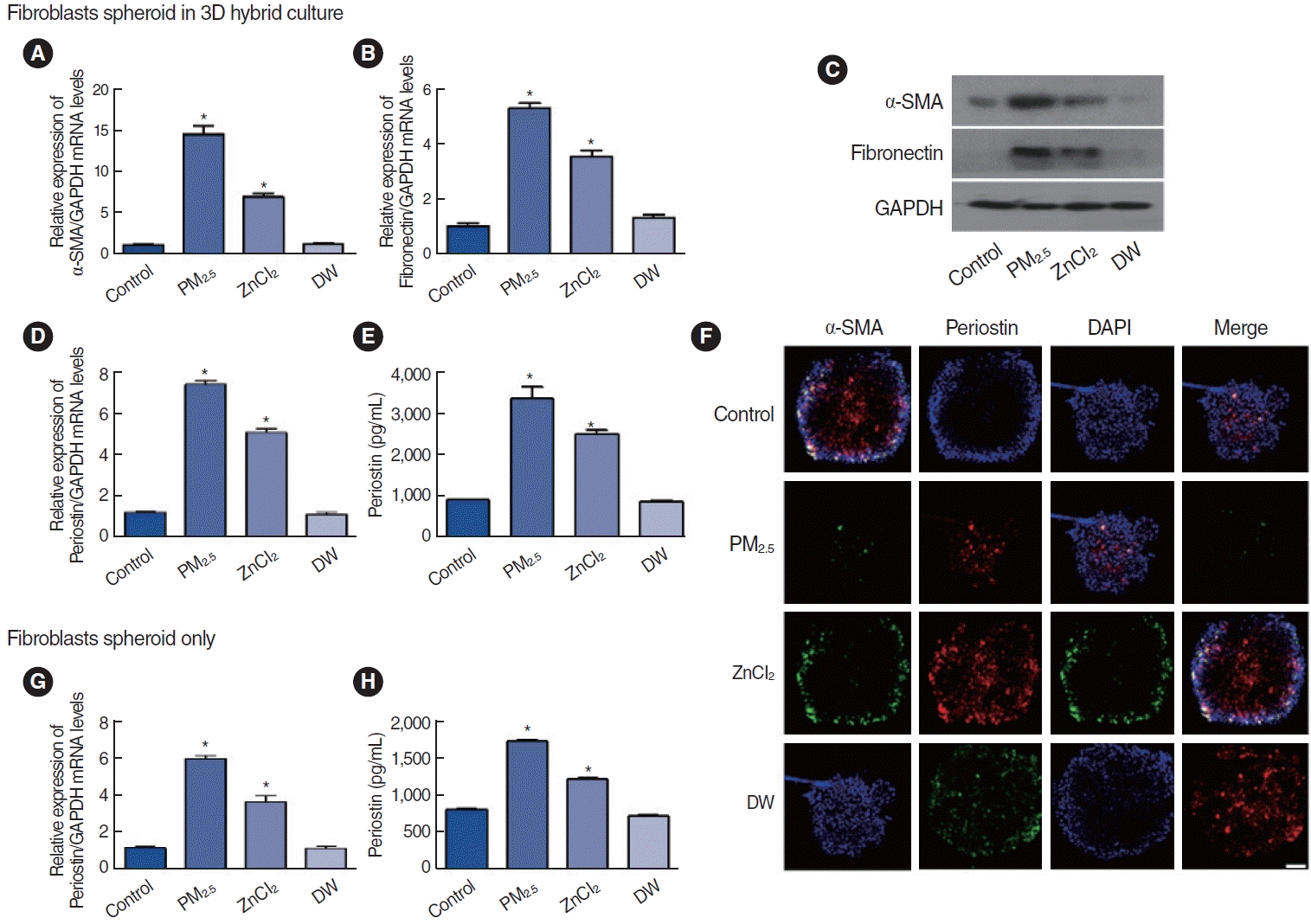
Fig. 6.
Aerosolized particulate matter 2.5 (PM2.5) and ZnCl2 induced an allergic inflammatory response in epithelial cells in co-cultures of air-liquid interface (ALI) and nasal fibroblast spheroids. (A) Transepithelial electrical resistance (TEER), which reflects cell barrier integrity, was measured. Cell exposure to aerosolized PM2.5 or ZnCl2 was examined for (B-E) mRNA and (G-J) protein expression of interleukin (IL)-6, epithelial cell-derived cytokines IL-25, IL-33, thymic stromal lymphopoietin (TSLP), (F) mRNA of periostin. (K-N) The mRNA and protein expression levels of E-cadherin, vimentin, and fibronectin involved in epithelial-mesenchymal transition were measured after exposure to aerosolized PM2.5 or ZnCl2. (O) Immunofluorescence staining data confirmed the expression of E-cadherin (green) and periostin (red). The nuclei of the cells were stained with DAPI (blue). Scale bar=50 μM. Values are presented as mean±standard deviation of three independent experiments. 3D, three-dimensional; DW, distilled water; DAPI, 4´,6-diamidino-2-phenylindole, dihydrochloride. *P<0.05 compared to control.
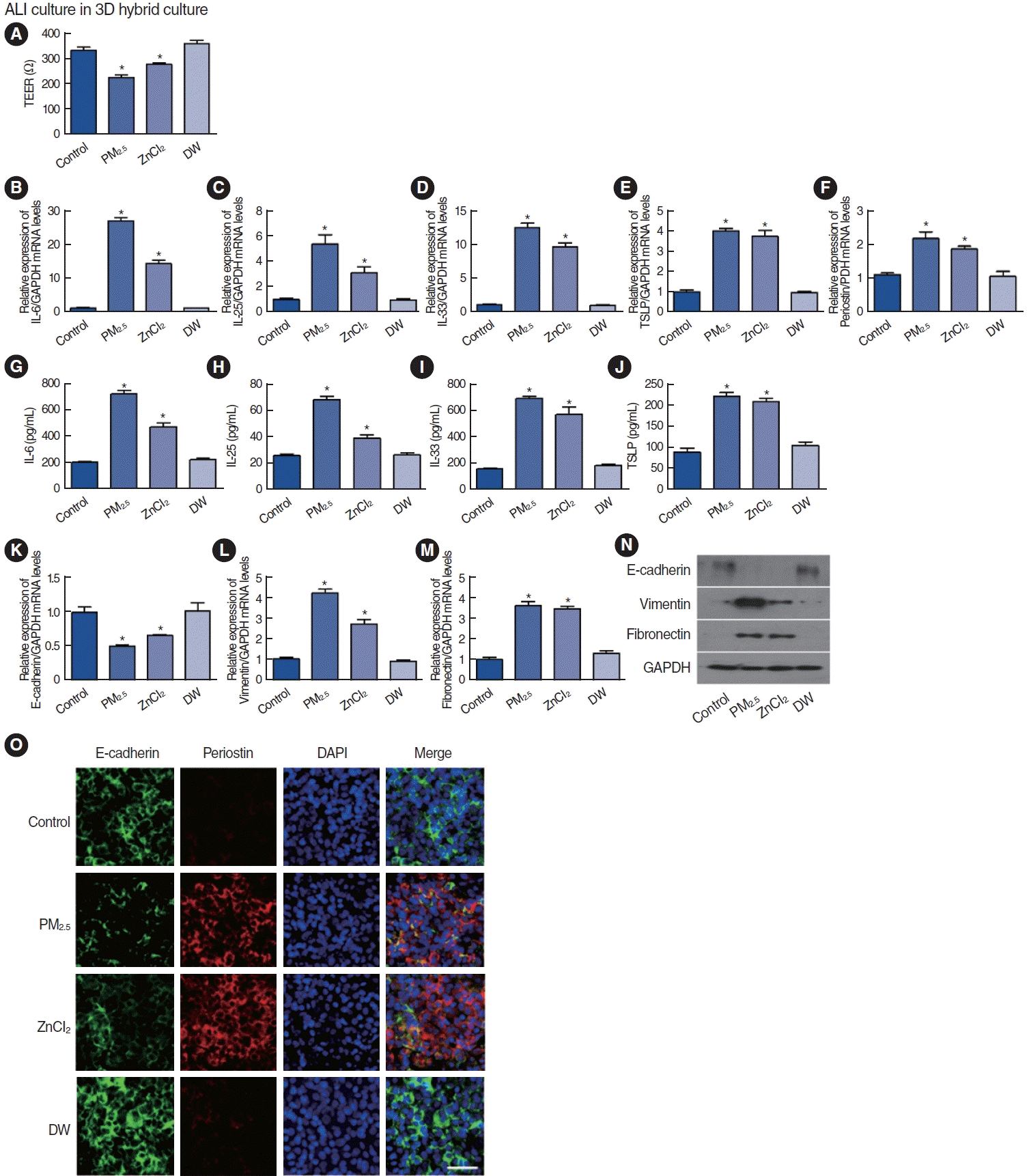
Table 1.
Sequences of PCR primers




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download