Abstract
Background
Although guidelines and protocols are available for central venous access, existing methods lack specificity and sensitivity, especially when placing peripherally inserted central catheters (PICCs). We evaluated the feasibility of catheter detection in the right atrial cavity using transthoracic echocardiography (TTE) during PICC placement.
Methods
This single-center, retrospective study included consecutive patients who underwent PICC placement between January 2022 and March 2023. TTE was performed to detect the arrival of the catheter in the right atrial cavity. Catheter misplacement was defined as an aberrant catheter position on chest x-ray (CXR). The primary endpoint was predicting catheter misplacement based on catheter detection in the right atrial cavity. The secondary endpoint was optimizing catheter placement and examining catheter-associated complications.
Results
Of the 110 patients identified, 10 were excluded because of poor echogenicity and vein access failure. The remaining 100 patients underwent PICC placement with TTE. The catheter was visualized in the right atrial cavity in 90 patients. CXR exams revealed catheter misplacement in seven cases. Eight patients with catheter misplacement underwent the same procedure in the other arm. In two patients, PICC placement failed due to anatomical reasons. Catheter misplacement was detected using TTE with sensitivity, specificity, positive predictive value, and negative predictive value of 97% confidence interval (CI; 91.31%–99.36%), 90% CI (55.50%–99.75%), 99%, and 75%, respectively.
Misplacement of peripherally inserted central catheters (PICCs) is a challenging problem. If the catheter is not in the correct position after placement, the patient may need to be prepared for another puncture site or the other arm may be required for reinsertion. Precise one-time placement of PICCs is crucial, because patients who undergo this type of procedure are mostly older adults or are frail from extended intravascular administration of medication and nutrition. Many patients experience poor vascular conditions when the available central vein is accessed because they have received intravenous fluid peripherally or centrally for extended periods.
Real-time imaging techniques, including fluoroscopy or the C-arm, are routinely used by PICC practitioners during procedures [1,2]. During PICC placement, patients and medical staff are exposed to radiation through fluoroscopy, but fluoroscopy is a valuable imaging tool for real-time functional and anatomical assessments. The cumulative radiation dose may be as high as >950 mGy or the peak skin dose as >760 mGy [3]. Adverse reactions can occur with the use of contrast agents in fluoroscopic procedures, especially in individuals with kidney disease [4], and patients may need to be relocated to another unit for image-guided interventions.
During central venous catheter (C-line) insertion procedures, most C-lines are inserted percutaneously using the Sedlinger technique. In both C-line and PICC procedures, vessel puncture is routinely performed under ultrasonographic guidance. Transthoracic echocardiography (TTE) is a reliable tool for detecting catheter misplacement and optimizing catheter tip positioning during C-line insertion [5]. When inserted correctly, the PICC is long enough to reach the right atrial cavity. Point-of-care ultrasonography (POCUS) is a pervasive and standard technique for verifying PICC placement using TTE [6]. If an echocardiographic probe and a vessel probe are prepared ahead of time, the procedure can be performed by switching the probes without the need to relocate the patient for the procedure.
The methodological differences between PICC and C-line placement lie in the access area and the extent of catheter-length adjustment according to this area [7,8]. In an environment where the ultrasound system is already prepared, the probes can be easily switched: the cardiac probe is placed on the patient’s chest to perform echocardiography. This facilitates the verification of whether the catheter is correctly located on the right side of the heart. Correct guidance prevents the catheter from moving to the opposite arm or neck during PICC placement. Compared to C-arm or fluoroscopy, this method may reduce the total procedure time, patient burden, and health insurance expenses. Additionally, this method uses only ultrasound and no radiation is generated (unlike fluoroscopy or C-arm fluoroscopy) during the procedure.
This retrospective study was conducted at a single center and included consecutive patients who underwent PICC placement. All patients who underwent PICC implantation performed by a single cardiovascular surgeon between January 2022 and March 2023 were eligible for this study. The primary endpoint was predicting catheter misplacement based on catheter detection in the right atrial cavity. The secondary endpoints were optimizing catheter tip placement in the superior vena cava–right atrium (SVC–RA) junction on chest x-ray (CXR) and examining all catheter-associated complications.
The study protocol was approved by the Chungnam University Hospital Institutional Review Board (No. 2023-04-018). Requirement for informed consent was waived by the Institutional Review Board because of the study retrospective design. Approval for publication was obtained from the patients whose photographs are included in this paper.
Vital signs were assessed before each procedure, and real-time monitoring of electrocardiograms and pulse oximetry saturation was performed for each patient. Punctures were conducted under ultrasonographic guidance, and TTE was performed before and during the procedure. Catheter misplacement was defined as an aberrant catheter position (coiled, directed toward the neck or other arm) on peri- or post-procedural CXR.
In this study, two types of catheters were used: (1) 5-Fr polyurethane 3-lumen Pro-PICC (MedComp) and (2) 5-Fr polyurethane 2-lumen Arrowg+ard Blue Advance PICC (Arrow PICC, Teleflex). Multiple ultrasonography devices were employed in various settings, including the intensive care unit, general ward, rehabilitation center, and operating room. These devices included 12L and 3Sc-RS probes (Venue Go Ultrasound System, GE), 11L and 3SP-D probes (Logiq S7 Expert Ultrasound System), UST-5413 linear and UST-5299 cardiac probes (Aloka F31 Diagnostic Ultrasound System, Hitachi), and 12L and 3Sc probes (Vivid S5 Ultrasound System, GE).
The exclusion criteria were as follows: (1) pre-procedural ultrasonography showing no adequate upper extremity veins during rapid peripheral vein assessment (RaPeVA); (2) poor pre-procedural echocardiographic window with difficulty finding the right atrial cavity; (3) signs of severe infection in the upper extremities; and (4) multiple former PICC puncture failures in both the upper extremities [10].
All patients had their arms abducted in the supine position and fixed upward if they could not cooperate. First, the operator measured the patient’s height and sternal length to calculate the catheter length in advance. A tourniquet was tied as high as possible on the shoulder of the arm selected for the procedure. Prior to the sterile drape, a RaPeVA using a linear probe was performed for the right arm [10]. In cases where the patient’s range of arm movement was limited, such as in patients with cerebral infarction, the opposite arm was moved first. The basilic vein in the upper arm was selected as the first target vessel and the brachial vein was identified as the next vessel, considering its size and tortuosity. Blood vessels with diameters ≥2 mm were selected as targets after tying the arm with a tourniquet. When no blood vessel was available in the first arm, the other arm was prepared and draped again. Once the target vessel was determined, the vein access point was pre-marked on the arm to save time and to use in actual procedures (Figure 1A).
TTE was also briefly performed. Each patient was informed that their chest may be pressed before and during the procedure. Similar to the apical four-chamber or subcostal views, the view that best showed the right atrial cavity was selected, and the chest area where the probe was located was marked. This procedure reduced the difficulty of finding a good view of the right atrial cavity (Figure 1B).
Each patient was informed that a wide surgical drape had been applied during the procedure and the possibility of needing to prepare another arm if necessary was explained in advance. The surgical drapes were applied to the arms and chest in a typical manner. For the echocardiographic window, a surgical drape was prepared with a 10×10 cm hole in the area marked in advance and exposed. Probes were wrapped in a sterile probe cover so that both the echocardiographic and linear probes could be used during the procedure.
All PICC procedures started with a puncture in a vein predetermined by RaPeVA, using ultrasonography in real-time. Under observation, 2–3 mL of 1% lidocaine was injected subcutaneously into the puncture site before puncture or after guidewire insertion. The catheter length was predicted and calculated during preparation, but the catheter was not trimmed in advance because when it is trimmed in advance, it may be too short to reach and confirm the right atrial cavity. The PICC was placed in a standard manner using the following catheter detection protocols. (1) If the target vein puncture was successful, a catheter was inserted, with the length calculated in advance, followed by guidewire insertion. (2) When the catheter was fully introduced to the calculated length, whether it was placed correctly in the right atrial cavity was determined using real-time TTE with an echocardiographic probe. (3) If the catheter was placed in the right atrial cavity on TTE, it was withdrawn until the tip was located at the SVC–RA junction. (4) If the catheter was not found in the right atrial cavity, it was retracted and reinserted, and the previous protocol was repeated. (5) If the catheter was not identified in the right atrial cavity again, periprocedural portable CXR was required. (6) If periprocedural portable CXR showed malposition, the procedure was considered to have failed and was then tried in the other arm. (7) If the catheter was placed at the SVC–RA junction on TTE, post-procedural CXR was performed for final confirmation. The practitioner determined the placement of the catheter. The practitioner and patient were kept in the procedure unit until CXR evaluation.
All statistical analyses were performed using IBM SPSS statistics version 26. The sensitivity, specificity, and likelihood ratio (LR) were calculated for TTE and CXR. Positive LR (LR+) was calculated as follows: sensitivity/(1–specificity). Negative LR (LR–) was calculated as follows: (1–sensitivity)/specificity.
We analyzed the records of 110 patients who underwent PICC placement at the Chungnam University Hospital between January 2022 and August 2023. Among these, 103 patients underwent the procedure directly on site without relocation. Seven patients who had previously undergone PICC placement (failed or removed once) were relocated to the operating room. Of the 110 patients who underwent PICC placement, eight were excluded from this study because the right atrial cavity could not be identified clearly on pre-procedural TTE. Two additional patients were excluded because one was not eligible for the predetermined RaPeVA and the other experienced puncture failure. Table 1 presents the basic characteristics of the remaining 100 patients and the materials used. Among these patients, 92 experienced successful punctures in the first trial, while eight exhibited malpositioned catheters on periprocedural CXR and had to undergo the procedure in the other arm. Therefore, punctures were attempted a total of 108 times.
In the first trial, 90 patients were confirmed to have the catheter enter the right atrial cavity on TTE in real-time. Among these, 89 patients had the catheter tip located at the SVC–RA junction on post-procedural CXR (Figure 2); the remaining patient exhibited catheter malposition. However, 10 patients were not confirmed to have had the catheter advance toward the right atrial cavity on TTE. Of these, the PICCs were malpositioned in seven patients, and all were removed immediately.
A second trial was performed using the other arm in the eight patients with malpositioned catheters. In six of these patients, the catheters were confirmed to have entered the right atrial cavity on TTE in real-time, and all were properly positioned at the SVC–RA junction on the final CXR. The remaining two patients were not confirmed to have had the catheter enter the right atrial cavity from the other arm, and the final CXR indicated malposition. Therefore, PICC placement was deemed to have failed, and the PICCs were removed immediately (Figure 3).
The outcomes of the diagnostic tests were calculated by predicting catheter placement for 108 procedures, combining the first and second trials. Catheter misplacement was detected using TTE with sensitivity, specificity, positive predictive value, and negative predictive value of 97% confidence interval (CI; 91.31%–99.36%), 90% CI (55.50%–99.75%), 99%, and 75%, respectively. The LR+ and LR– were 9.69 (95% CI, 1.5–62.3) and 0.03 (95% CI, 0.01–0.1), respectively (Table 2).
Patient data were collected based on the catheter position confirmed using CXR, present illness, history of arrhythmia, patient age, and access location for complications associated with PICCs. Complications associated with PICCs that occurred within 1 month of the procedure were followed up after the procedure. In total, 68 of the 100 analyzed patients had their catheters removed because of treatment termination, self-removal, or death. Catheter-associated complications were communicated to the attending physicians after a formal consultation with all relevant departments.
Repositioning was indicated when the catheter tip did not reach the SVC–RA junction or pass through the right atrial cavity on post-procedural CXR. Four catheters required repositioning (all withdrawn), including three from the right basilic approach and one from the left brachial approach. In one of these four cases, the catheter passed through the tricuspid valve, reached the right ventricle, and was withdrawn in real-time (Supplementary Video 1). Fluoroscopy or C-arm was not performed for catheter repositioning in any cases. Post-procedural CXR findings were the only reference for the length of catheter withdrawal. Two patients developed catheter-related infections, identified through blood culture analyses. Additionally, six patients experienced catheter thrombosis, which explained the ipsilateral arm edema and loss of PICC function in these cases. Of these six cases, five and one involved brachial and basilic approaches, respectively. All complicated PICCs were immediately removed (Table 3).
In this study, we employed a simple diagnostic method for catheter detection using TTE during PICC placement. Our method demonstrated sensitivity, specificity, positive predictive value, and negative predictive value of 97%, 90%, 99%, and 75%, respectively, indicating that the method is reliable. The LR+ was 9.69 and LR– was 0.03. Thus, we had strong evidence for ruling out catheter misplacement in most cases using this method.
PICC placement has been performed at our center by the Department of Cardiovascular and Thoracic Surgery since March 2020. Initially, the method described in this paper was only used for a portion of the patients admitted to the intensive care unit in our department. Gradually, its application expanded to include patients with high frailty who were difficult to move to other units. Previously, our center usually placed PICCs using C-arm in a vascular laboratory or operating room equipped with a fluoroscope. However, considerable effort and risk are involved when transferring critically ill patients to certain procedural units. Furnishing a unit with the equipment necessary for PICC procedures is more useful than relocating the patients to separate procedural units.
POCUS is extensively used in various ward and intensive care unit procedures. The cardiovascular surgeon who performed procedures (YCJ) in this study specializes in treating critically ill patients and is familiar with the percutaneous puncture technique, central catheter insertion, and chest and vascular anatomy, and can promptly and accurately detect artifacts in the heart using TTE. However, this method does not require advanced techniques or detailed interpretation during TTE. Instead, it simply identifies whether catheter artifacts are present in the right atrial cavity. Classical blind techniques (including CXR) are inferior to methods that use several different tools for detecting malposition [12]. Similar to C-arm and fluoroscopy, electrocardiography-electromagnetic guidance is an alternative to the existing methods [13]. However, electromagnetic guidance is not widely utilized in South Korea because of insurance coverage guidelines for PICC procedures.
In a retrospective study at a single institution, Sukyung et al. [14] reported that optimal tip positions were achieved in the first trial in 91.6% of bedside PICC procedures. In 3.5% of the patients, a re-trial PICC placement or failure occurred. Even if multiple punctures are successfully tried in one arm, the arm can develop angioedema. Catheter malposition is dire for both practitioners and patients. In the present study, if basic or brachial vein access failed after a puncture in the first arm, an immediate trial was performed with the other arm rather than with the same arm. Deep or superficial thrombosis frequently occurs in the arm when one or more veins are used in patients with high frailty or severe comorbidities [15]. Among our patients, 18% had stage 1–4 chronic kidney disorders, with seven individuals undergoing dialysis with arteriovenous fistulae in their arms. In these patients, if thrombosis occurred while placing a catheter in an upper extremity vein or if injuries were caused by puncture failure, creating an arteriovenous fistula or accessing a dialysis vessel may be challenging in the future.
However, our method involved several difficulties. Performing the procedure at the bedside may be convenient for the patient, but greater preparation and maintenance are required to achieve readiness at the bedside compared to operating rooms, especially considering the necessity of applying sterile drapes for the arm, chest, and two probes. If patients have a sternotomy wound immediately after heart surgery, sterilizing the drape on the chest can be difficult. In this study, 29% of the patients underwent procedures in the intensive care unit immediately after heart surgery. However, the catheter-related infection complication rate (2%) was consistent with those reported in other studies [7,8,14,16].
One advantage of our method is that while fluoroscopy requires at least two practitioners, this technique usually requires only one practitioner, provided that the patient is cooperative. Importantly, the patients, practitioners, and assistants are not exposed to radiation during the procedure. Additionally, the procedural cost is less than that of fluoroscopy in South Korea.
Lack of familiarity with TTE may hinder identification of the echocardiographic window in the right atrial cavity, and fully identifying the SVC using TTE is challenging. Locating the SVC using transesophageal echocardiography is easy, but the procedure is invasive, causes discomfort to the patient, and requires considerable practice. In this study, pre-procedural TTE failed to visualize the right atrial cavity in seven patients who underwent postcardiac surgery and one patient with cachexia in the rehabilitation unit. Consequently, these eight patients were excluded from this study. The SVC–RA junction can be visualized through the modified apical five-chamber view, modified parasternal short-axis view, and modified subcostal view [17]. However, periprocedural TTE led to false-positive catheter detection in one patient in our study. This patient had a body mass index of 12.43 kg/m2, and the rib shaft was mistaken for a catheter in the TTE apical four-chamber view. POCUS has recently been used at the bedside. Owing to the ease of use of TTE, repetitive training will help practitioners find a good echocardiographic window by enabling simultaneous use of multiple modified views of the right atrial cavity and SVC–RA junction [6].
This study has some limitations. It was single-center and retrospective: numerous patients who underwent cardiovascular surgery and those with high frailty were included, and selection bias inevitably occurred. Therefore, applying our method to all patients requiring PICCs would be premature. This method is intended for catheter detection only and not for PICC placement itself. It is unrelated to the success rates of numerous PICC placement protocols and is not comparable. Moreover, a single cardiovascular surgeon familiar with ultrasound and echocardiography performed the entire procedure for every patient. The various ultrasonography devices used in this study were heterogeneous in performance and resolution. Skilled detection and placement have a learning curve. This method is not comparable with several other detection methods that use electromagnetic guidance or fluoroscopy. Nevertheless, this method could yield better PICC results when POCUS is used in many centers.
In conclusion, bedside TTE is a reliable tool for detecting catheter misplacement and optimizing catheter tip positioning during PICC placement. In the future, we plan to conduct a comparative study using various tools that require central venous access.
▪ For high frailty patients who require critical care, moving patients to other locations equipped with fluoroscopy or C-arm devices for peripherally inserted central catheter (PICC) placement can be dangerous and cumbersome.
▪ When point-of-care ultrasonography is pervasive, bedside transthoracic echocardiography could be a reliable tool for detecting catheter misplacement and optimizing catheter tip positioning during PICC placement.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2024.00150.
REFERENCES
1. Montanarella MJ, Agarwal A, Moon B. Peripherally inserted central catheter (PICC) line placement. StatPearls Publishing;2024. [cited 2024 Mar 1]. Available from: https://pubmed.ncbi.nlm.nih.gov/34424637/.
2. Royce B. Use of C-arm fluoroscopy by nurses for placement of PICC lines. J Assoc Vasc Access. 2009; 14:138–41.

3. Storm ES, Miller DL, Hoover LJ, Georgia JD, Bivens T. Radiation doses from venous access procedures. Radiology. 2006; 238:1044–50.

4. Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int. 2014; 2014:741018.

5. Bedel J, Vallée F, Mari A, Riu B, Planquette B, Geeraerts T, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013; 39:1932–7.

6. Díaz-Gómez JL, Mayo PH, Koenig SJ. Point-of-care ultrasonography. N Engl J Med. 2021; 385:1593–602.

7. Santos FK, Flumignan RL, Areias LL, Sarpe AK, Amaral FC, Ávila RB, et al. Peripherally inserted central catheter versus central venous catheter for intravenous access: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020; 99:e20352.
8. Johansson E, Hammarskjöld F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013; 52:886–92.

9. Greca A, Iacobone E, Elisei D, Biasucci DG, D’Andrea V, Barone G, et al. ECHOTIP: a structured protocol for ultrasound-based tip navigation and tip location during placement of central venous access devices in adult patients. J Vasc Access. 2023; 24:535–44.

10. Brescia F, Pittiruti M, Spencer TR, Dawson RB. The SIP protocol update: Eight strategies, incorporating Rapid Peripheral Vein Assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J Vasc Access. 2024; 25:5–13.

11. Emoli A, Cappuccio S, Marche B, Musarò A, Scoppettuolo G, Pittiruti M, et al. The ISP (Safe Insertion of PICCs) protocol: a bundle of 8 recommendations to minimize the complications related to the peripherally inserted central venous catheters (PICC). Assist Inferm Ric. 2014; 33:82–9.
12. Tomaszewski KJ, Ferko N, Hollmann SS, Eng SC, Richard HM, Rowe L, et al. Time and resources of peripherally inserted central catheter insertion procedures: a comparison between blind insertion/chest X-ray and a real time tip navigation and confirmation system. Clinicoecon Outcomes Res. 2017; 9:115–25.

13. Gullo G, Colin A, Frossard P, Jouannic AM, Knebel JF, Qanadli SD. Appropriateness of replacing fluoroscopic guidance with ECG-electromagnetic guidance for PICC insertion: a randomized controlled trial. AJR Am J Roentgenol. 2021; 216:981–8.

14. Kwon S, Son SM, Lee SH, Kim JH, Kim H, Kim JY, et al. Outcomes of bedside peripherally inserted central catheter placement: a retrospective study at a single institution. Acute Crit Care. 2020; 35:31–7.

15. Ploton G, Pistorius MA, Raimbeau A, Denis Le Seve J, Bergère G, Ngohou C, et al. A STROBE cohort study of 755 deep and superficial upper-extremity vein thrombosis. Medicine (Baltimore). 2020; 99:e18996.

Figure 1.
Before sterile draping: (A) rapid assessment of the peripheral vein by linear probe (12L) and (B) the best point for echocardiography in the apical four-chamber or subcostal view clearly shows the right atrial cavity by the echocardiographic probe (3Sc-RS) (GE Vivid S5 Ultrasound System).
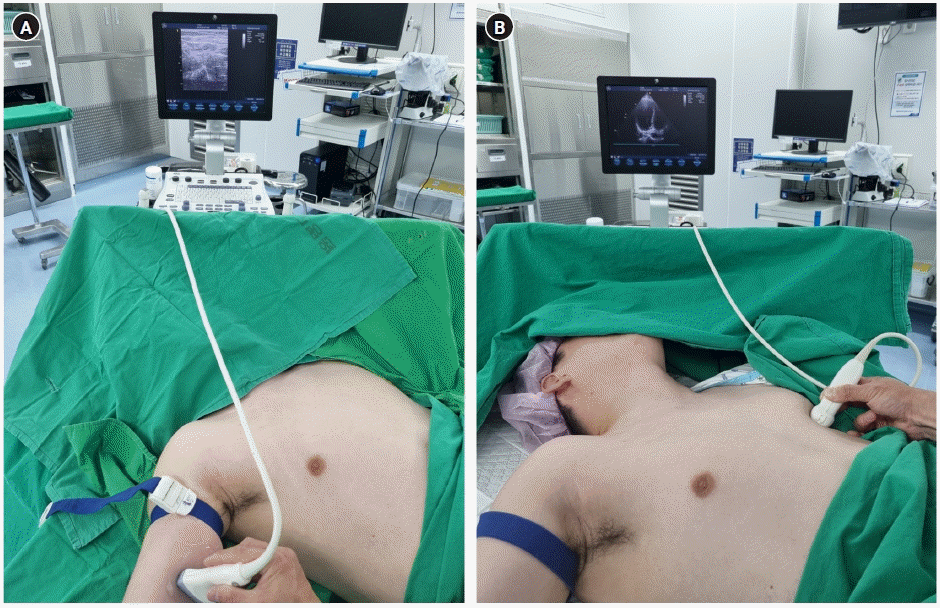
Figure 2.
Transthoracic echocardiography during peripherally inserted central catheter, apical four-chamber view: (A) hyperechogenic line (arrowhead represents the catheter heading from the RA to the RV) and (B) hyperechogenic artifact (arrow represents the catheter tip at the RV). LV: left ventricle; RV: right ventricle; RA: right atrium; LA: left atrium.
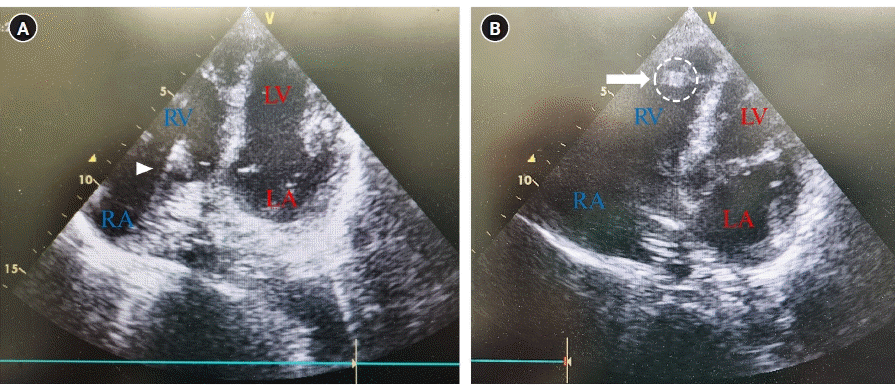
Figure 3.
Study protocol used to analyze the diagnostic test. PICC: peripherally inserted central catheter; TTE: transthoracic echocardiography; RaPeVa: rapid peripheral vein assessment; Rt: right; Lt: left; CXR: chest x-ray; SVC–RA: superior vena cava–right atrium.
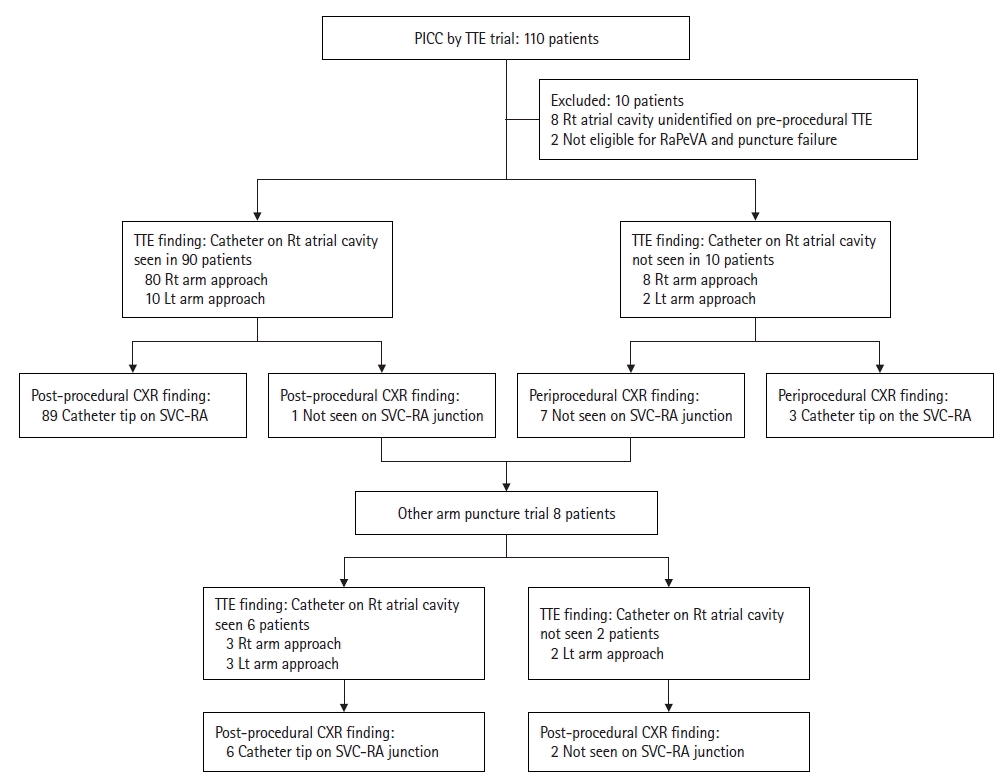
Table 1.
Enrolled patients and material characteristics
| Variable | Total (n=100) | Catheter seen in RA (n=90) | Catheter not seen in RA (n=10) |
|---|---|---|---|
| Age (yr) | 68±12 | 64±7 | 69±9 |
| Sex (male:female) | 48:52 | - | - |
| BMI (kg/m2) | 25.3±12.4 | 26.3±9.3 | 18.21±8.6 |
| Indications for PICC | |||
| After cardiac surgery | 32 | 28 | 4 |
| After orthopedic surgery/rehabilitation | 22 | 20 | 2 |
| Chronic kidney disease | 18 | 16 | 2 |
| Cancer | 11 | 10 | 1 |
| Infectious disease | 10 | 9 | 1 |
| Gastrointestinal disease | 7 | 7 | 0 |
| Arrhythmiaa) | 8 | 6 | 2 |
| Atrial fibrillation | 6 | 4 | 2 |
| Ventricular junctional rhythm | 2 | 2 | 0 |
| Patients who underwent PICC placement previously (redo PICC placement) | 7 | 4 | 3 |
| Practice unit | |||
| Intensive care unit | 61 | 59 | 2 |
| General ward and rehabilitation center | 32 | 26 | 6 |
| Operating room (for redo PICC placement) | 7 | 5 | 2 |
| PICC materialb) | |||
| 5-Fr double-lumen, arrow PICC | 69 | 65 | 4 |
| 5-Fr triple-lumen pro-PICC | 31 | 25 | 6 |
Table 2.
TTE as a diagnostic test for PICC detection
| Diagnostic test |
CXR findings |
||
|---|---|---|---|
| Positive | Negative | Total | |
| TTE detection in the right atrial cavity | |||
| Positive | 95 | 1 | 96 |
| Negative | 3 | 9 | 12 |
| Total | 98 | 10 | 108 |
Table 3.
PICC placement results and complications




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download