Abstract
Background
The decision to discontinue intensive care unit (ICU) treatment during the end-of-life stage has recently become a significant concern in Korea, with an observed increase in life-sustaining treatment (LST) withdrawal. There is a growing demand for evidence-based support for patients, families, and clinicians in making LST decisions. This study aimed to identify factors influencing LST decisions in ICU inpatients and to analyze their impact on healthcare utilization.
Methods
We retrospectively reviewed medical records of ICU patients with neurological disorders, infectious disorders, or cancer who were treated at a single university hospital between January 1, 2019 and July 7, 2021. Factors influencing the decision to withdraw LST were compared between those who withdrew LST and those who did not.
Results
Among 54,699 hospital admissions, LST was withdrawn in 550 cases (1%). Cancer was the most common diagnosis, followed by pneumonia and cerebral infarction. Among ICU inpatients, LST was withdrawn from 215 (withdrawal group). The withdrawal group was older (78 vs. 75 years, P=0.002), had longer total hospital stays (16 vs. 11 days, P<0.001), and higher ICU readmission rates than the control group. There were no significant differences in the healthcare costs of ICU stay between the two groups. Most LST decisions (86%) were made by family.
Since the Life-Sustaining Treatment (LST) Decision Act [1] was implemented in Korea in February 2018, the withdrawal or withholding of LST has become a form of death for patients in the intensive care unit (ICU). The LST Decision Act consists of two parts. The first part stipulates the requirements and procedures for terminating LST in patients at the end of life. The second part deals with hospice and palliative care. Our aim in this paper was to determine the effects of the first part of the law, including those on healthcare costs. Due to the enormous medical expenses possible at the end-of-life (EOL) stage [2], one of the aims of the LST Decision Act was to reduce futile treatments and decrease medical expenses. Since the law stipulates that EOL is a condition for which terminating LST is permissible, the ICU is a likely location of such termination. Furthermore, as seven intensive care criteria listed in the law are mostly performed in the ICU, it is valid to examine the pattern of medical usage in the ICU to determine the effect of enforcement of the law.
Increasingly, LST decisions are being made in ICUs, not only for patients with terminal cancer, but also for patients in catastrophic condition with various diseases [3,4]. However, the rates of advance preference for or refusal of LST by the patient and LST planning are low, and withdrawal of LST by decision of the family is common in critically ill patients nearing EOL [5-7]. This can deprive the patient of the opportunity to choose a dignified death, and family members may make a decision that is different from the patient's own intentions and disease condition [8,9]. Furthermore, relying on family members to make EOL decisions can be psychologically and ethically burdensome [10] and may delay the timing of withdrawal of LST for patients who are unlikely to recover despite treatment [11].
Healthcare providers involved in decisions regarding LST are hampered by a lack of evidence [5,8,11]. As the LST Act becomes more widely implemented, there is a need for guidelines to help clinicians make EOL decisions for critically ill patients under ICU care and to help the patient and their family with EOL planning, especially in acute and critical conditions like neurological and infectious disorders.
From the beginning of critical care medicine, the main treatment goals in the ICU have been to reduce mortality and prolong life [12]. Consequently, a large proportion of healthcare spending tends to be concentrated on elderly, EOL, and terminally ill patients [13-15]. The increasing challenges posed by limited healthcare resources due to an aging population or pandemics have hindered the efficient management and distribution of medical resources, particularly in the ICU [14,16,17]. Therefore, it is essential to determine whether there are differences in healthcare utilization behaviors and final medical expenses between critically ill patients who receive LST and those who do not.
This study aimed to identify factors that influence the decision to withdraw LST for patients admitted to the ICU, including age, disease, and identity of the decision maker. Furthermore, we investigated how the decision to withdraw LST affects the actual use of medical services by evaluating healthcare costs.
This study evaluated the effect of the LST act on ICU inpatients and identified determinants of LST withdrawal. We retrospectively reviewed the electronic medical records of patients who registered a determination form for LST withdrawal and the EOL process in a single tertiary hospital from January 1, 2019, to July 7, 2021. Factors influencing the LST withdrawal decision such as age, sex, disease, and identity of the decision maker were collected for comparison. Patients who withdrew LST according to the LST Decision Act were assigned to the withdrawal group and patients who did not were assigned to the control group. The control group comprised ICU inpatients who exhibited unstable vital signs and symptoms upon admission consistent with entering the EOL stage within the same study period as the withdrawal group. In the withdrawal group, the most prevalent disease categories were neurological disorders, infectious disorders, and cancer. For disease-matched comparison, the control group comprised ICU inpatients diagnosed with one or a combination of these three categories of disorders using International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes. Detailed diagnoses for each category are provided in Supplementary Table 1.
The Glasgow coma scale to assess mental state and the Acute Physiology and Chronic Health Evaluation (APACHE) II score were collected on admission to the ICU to gauge patient severity. Mortality was the primary outcome. Total hospital and ICU stays, readmission rate, and types of intensive care (cardiopulmonary resuscitation, hemodialysis, chemotherapy, mechanical ventilation, vasopressors, extracorporeal life support, and blood transfusion) were compared between the LST withdrawal and control groups. In the withdrawal group, days from admission to LST withdrawal decision and days from LST withdrawal decision to death were calculated. Furthermore, it was noted whether the patient or family made the decision on LST. Healthcare cost data of the groups were compared separately for total length of hospital stay and ICU stay.
According to the LST Decision Act, withdrawal of LST can be implemented only during the EOL stage. EOL implies imminent death without the possibility of recovery or revitalization despite proper treatment. EOL was determined by the physician in charge and one medical specialist in the relevant field. LST was defined as medical treatment that merely extended the duration of the EOL process without curative effect. When this research was conducted, the legal definition of LST included mechanical ventilation, hemodialysis, vasopressors, and blood transfusion, common in the ICU setting. The difference between LST and usual ICU care is based on whether the patient is in the EOL stage or not. The decision to terminate LST was made by self or family (spouse and linear ascendants or descendants) in all analyzed patients. In the ICU, patients are often incapable of self-determining withdrawal of LST due to lack of consciousness. In this case, two or more identical statements from the family members on the patient's intention or unanimous consent of all family members were required.
Categorical data are presented as proportions, normally distributed continuous data as means with standard deviations, and non-normally distributed continuous variables or ordinal variables as medians with interquartile ranges. Characteristics of subjects were summarized using descriptive statistics. Factors regarding the decision to withdraw LST first were subjected to univariate analysis, and then multivariate analysis was performed using a logistic regression model for variables with P-values less than 0.1. Student t-test and Fisher’s exact test were used to assess the significance of differences in continuous and categorical variables between groups, respectively. To compare the three disease categories, the Kruskal-Wallis test was applied. Two-tailed P-values less than 0.05 were considered significant, and all statistical analyses were performed using R version 4.1.1 (R Foundation for Statistical Computing).
The study was approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (No. SEUMC 2021-08-002-002, August 24, 2021), and the requirement for informed consent was waived due to the retrospective design of the study. The procedures in this study were performed in accordance with the ethical standards of the responsible committee on the privacy of individuals and secure data.
A total of 54,699 patients was admitted to the hospital during the study period, and 550 (1%) of these patients registered to withdraw from LST (Figure 1). In the total LST population, the median age was 78 years (interquartile range [IQR], 69–85 years), and 318 patients were male (58%). Cancer was the most common causative disease in patients who withdrew from LST, followed by pneumonia and cerebral infarction (Table 1). Among cancers, lung, biliary, and stomach cancers were the most common in descending order (Supplementary Figure 1A). Among patients who registered for an LST plan, 238 (238/550, 43%) had a history of ICU admission, of whom 90% (215/238) had a diagnosis of neurological disorder, infectious disorder, or cancer. These 215 patients were labeled as the withdrawal group. After matching the disease categories, the number of control group patients was 513 (Figure 1).
Median age of all ICU inpatients was 77 years (IQR, 64–84 years), and withdrawal group patients were significantly older than control group patients (78 vs. 75 years, P=0.002) (Table 2). There was no significant difference in the number of men or women in the withdrawal versus control groups. The most common disease in the ICU LST withdrawal group was cancer, as it was in the total population of LST withdrawal patients. Unlike the total population of LST withdrawal patients, the most common type of cancer in ICU inpatients was brain cancer (Supplementary Figure 1B). Furthermore, neurological disorders such as cerebral infarction and intracranial hemorrhage were more common in patients who withdrew from LST in the ICU than those that withdrew from LST outside the ICU (Table 2). APACHE II scores (25 [IQR, 20–34] vs. 27 [IQR, 20–34], P=0.82) and GCS (7 [IQR, 4–11] vs. 8 [IQR, 4–13], P=0.10) were not significantly different between patients in the withdrawal and control groups (Table 2).
When the three disease categories were compared, the proportion of cancer patients was significantly higher in the withdrawal group than the control group (P<0.001) (Table 2). Conversely, the numbers of patients with neurological or infectious disorder were higher in the control group than the withdrawal group (P=0.80, P<0.001, respectively). Overall, more ICU treatments were performed in the control group, while the withdrawal group received significantly fewer vasopressor treatments, hemodialysis procedures, and cardiopulmonary resuscitations than the control group, as shown in Table 2 and Figure 2.
Total hospital stay was significantly longer in the withdrawal group than the control group; however, there was no difference in ICU stay between these two groups (total hospital stay: 16 days [IQR, 8–27] vs. 11 days [IQR, 4–24], P<0.001; ICU stay: 7 days [IQR, 2–16] vs. 7 days [IQR, 2–18], P=0.59) (Table 3). Among patients who died during hospitalization (439/728, 60%), the time from admission to death was significantly shorter in the control group (8 days [IQR, 2–19]) than in the withdrawal group (14 days [IQR, 6–28]). A longer hospital stay but a shorter time from LST withdrawal to death were observed in the withdrawal group (16 days [IQR, 8–27] and 2 days [IQR, 0–7], respectively). Readmission rates during the study period were significantly higher in the withdrawal group than the control group (24% vs. 13%, P<0.001).
To determine the factors that affected the decision to withdraw LST, age, readmission, hospital stay, diagnosis of cancer, and infectious disorders were analyzed by multivariate logistic regression (Table 4). After adjusting for relevant factors, older age (odds ratio, 1.019; 95% confidence interval, 1.007–1.032; P=0.002) and higher readmission rate (odds ratio, 1.760; 95% confidence interval, 1.128–2.733; P=0.01) significantly affected the decision to withdraw from LST in the ICU. Regarding disease category, cancer patients were 3.3-fold more likely to withdraw from LST than control patients. In contrast, patients with infectious disorders tended to be twice as likely not to withdraw from LST as cancer patients and those with neurological disorders.
To assess if the decision to withdraw LST affected healthcare costs, we compared overall hospitalization costs and ICU costs for the withdrawal and control groups (Table 3). Overall hospitalization costs were higher in the withdrawal group than the control group, but there was no significant difference in ICU costs between these two groups. The higher overall cost of hospitalization in the withdrawal group was related to a longer length of stay. Narrowing of the cost gap between the two groups during the ICU stay indicates that withdrawal of LST reduces the number of medical procedures and treatments, which impacts healthcare costs. The median cost from LST withdrawal day to discharge day was 1% of the median total ICU cost.
To identify the primary decision maker regarding withdrawal from LST in EOL patients in the ICU, we compared the proportion of patients who self-determined withdrawal from LST versus family determination. In most cases, family members made the decision (184/215, 86%), and there was significant difference by disease category, occurring in 89% and 95% of respective families of patients with neurological disorders and infectious disorders (Figure 3). In contrast, 30% of patients with cancer made their own decisions, a higher rate than for other disease categories (P<0.001).
This study focused on withdrawal of LST in patients admitted to the ICU after implementation of the LST Decision Act in Korea in 2018. Patients for whom LST was withdrawn were older, had longer hospital stays, more ICU readmissions, and higher overall healthcare costs than those who did not. However, there was no difference in the cost of the ICU stay between patients in the withdrawal and control groups. This might be explained by death within 2 days after withdrawal of LST. Furthermore, the withdrawal group received significantly less intensive care, including the use of vasopressors, hemodialysis, and cardiopulmonary resuscitation, than the control group. There were different patterns in the decision to withdraw LST among cancer, infectious disease, and neurological disorder patients.
Communication with patient families needs to be improved as they are making most LST decisions. According to a previous study [18], participating in proactive family conferences resulted in less exposure of patients to non-beneficial interventions than participation in standard doctor-family conferences. The percentage of bereaved families experiencing negative emotions such as anxiety, depression, and symptoms of post-traumatic stress also decreased significantly more after proactive family conferences than standard conferences. Depending on the nature of disease, a patient may be unable to make LST decisions. Therefore, it is essential to have a process in place to reduce the ethical and psychological burden of family members. Caregivers or families of patients may request information about withdrawal of LST in the early phase of ICU care, but unexpected such decisions can be challenging. Given the uncertainty in determining the optimal time for LST withdrawal, early decisions regarding LST may compromise the commitment to appropriate intensive treatment rather than respecting the patient's will. Therefore, identified factors that influence the decision to withdraw from LST can provide useful information for decision-makers.
In our study, 30% of patients (215/728) admitted to the ICU for neurological disorders, infectious disorders, or cancer decided to terminate LST. The percentage of patients who withdrew from LST was remarkably higher in these critically ill patients compared to all inpatients (1%). This suggests that research is needed on an LST withdrawal process more suitable for critically ill patients.
Age was a significant factor in the decision to withdraw LST regardless of severity. Disease was also a significant factor in the LST withdrawal decision, with cancer in particular being a notable predictor. Infectious disorders were associated with a lower rate of LST withdrawal than cancer and neurological disorders. This discrepancy may be due to the shorter hospital stay of patients with infectious diseases and septic shock than that of those with cancer or neurological conditions. Loss of consciousness due to neurological disorders or abrupt aggravation of infectious disorders might be a barrier to self-determination of LST. This is because of the limited time available for deciding on LST withdrawal in patients with rapidly deteriorating infectious disorders, as well as the inclination of cancer patients to autonomously choose to withdraw LST. Consequently, patients at high risk of impending death in the ICU, especially elderly patients, require a wide range of advance care planning methods, including LST planning.
Hospitalization duration, overall hospitalization costs, and readmission rates were significantly higher in the withdrawal group than the control group. This can be attributed to prolonged disease aggravation or a delay in the decision to withdraw LST. The higher rate of ICU readmissions in the withdrawal group indicates that patients in this group may have gradually deteriorated, received repeated ICU care, and eventually decided to discontinue ongoing treatment. This may also have contributed to the longer length of hospital stay in the withdrawal group than the control group. The lack of difference in ICU costs between the two groups is likely because costs rapidly decrease after the decision to withdraw LST. In a similar vein, the notably low ICU costs and days from LST withdrawal to discharge provide support for the effectiveness of withdrawing or withholding LST in reducing both ICU costs and utilization. For these reasons, patients admitted to the ICU need information about prognosis and optimal timing of EOL care preparation, and the patient’s family should be involved from admission to the ICU. An appropriate approach to optimal timing and prediction of prognosis is important not only because it would help patients make their own decisions, but also because it would have economic efficacy.
Intensive care was provided more frequently in the control group than the withdrawal group, particularly cardiopulmonary resuscitation, hemodialysis, and vasopressors. This difference suggests that the decision to withdraw LST is made when the patient requires an additional invasive procedure. This may be because the decision to withdraw LST is often made by family members who are afraid of invasive intensive care that may cause patient suffering. Physicians need to consider that the nature of intensive care, especially invasive procedures, may affect a family's decision. Moreover, non-invasive alternative treatments available in the general ward may help reduce the length of stay in the ICU while ensuring that patients receive adequate supportive care. The factors that determine when to discontinue types of intensive care were not addressed in this study and require further research.
The LST Decision Act is increasingly being applied in patients at EOL, but the decision to apply the law is often left to the individual doctors' judgment. It is necessary to find ways to better time LST decisions given the critical nature of patients entering the ICU. These measures include identifying prognostic indicators according to type of disease, providing prognostic information that can guide the LST decision, and explaining the option of withdrawing LST to patients and their families from the point of ICU admission.
This study has several limitations. First, since this was a single-center study with a retrospective research design, its generalizability to other cohorts is limited. However, it is meaningful to determine how the LST Decision Act has been applied in actual clinical settings since it became law. Second, despite efforts to classify patients into disease groups based on the primary cause of admission to the ICU, such patients may have overlapping diseases. Third, we did not distinguish between withdrawal and withholding of LST in this study, as these are treated the same in the LST Decision Act [8,19]. Fourth, the comprehensive criteria used to define the control group may have influenced the study results. The definition of EOL stage varies, and there is no clear single standard for predicting the exact EOL period. Upon admission to the ICU, unstable vital signs and symptoms can signal impending death and the EOL stage. As the main objective of this study was to examine factors influencing the decision to withdraw LST based on diagnosis upon ICU admission, patients admitted to the ICU due to unstable vital signs were assumed to be nearing the EOL stage and included in the control group. Additionally, in cases where caregivers refused to proceed with LST, the absence of an EOL assessment may have resulted in inclusion of such patients in the control group. Overcoming these limitations necessitates the establishment of realistic criteria for EOL assessment based on prospective future studies. Finally, by comparing medical expenses, we attempted to determine whether futile medical interventions were reduced by implementing the LST Decision Act. Though the healthcare costs in the ICU were similar in the withdrawal and control groups, the total healthcare cost was higher in the withdrawal group. This is probably because of delayed decision-making regarding withdrawal of LST after ICU admission and the longer hospital stay of the withdrawal group. Additionally, other factors affecting healthcare utilization such as religion, geographic region, and socioeconomic background were not considered in our analyses due to the retrospective nature of the study. Supporting our findings, a recent study reported a decrease in healthcare utilization among patients who chose withdrawal or withholding of LST compared to those who did not [20]. Further comprehensive and prospective research is warranted to delve into these factors.
We identified advanced age, frequent ICU readmissions, and cancer as significant factors in the ICU in the decision to withdraw LST. Given the nature of critically ill patients who are often incapable of making self-decisions due to rapid disease deterioration or a decline in mental status, there is a growing need for more targeted research on LST decisions in the ICU. Additionally, proper processes should be established to provide timely information after ICU admission to patients and their families to support them in their decision-making process. The findings of this study underscore the complex nature of LST decisions in the ICU, emphasizing the need for enhanced coordination in patient decision-making processes and allocation of healthcare resources. Further investigation is imperative to tailor LST decision-making in the ICU to support patients, caregivers, and ICU clinicians.
▪ Older age, frequent intensive care unit (ICU) readmissions, cancer, and infectious disorders significantly influence the decision to withdraw life-sustaining treatment (LST) in ICU patients.
▪ Although total healthcare costs were higher for the LST withdrawal group, ICU costs remained similar between patients who withdrew LST and those who did not, possibly due to reduced intensive care following withdrawal.
▪ Decision-making regarding LST withdrawal was mostly determined by family members and varied depending on the disease, highlighting the need for personalized LST decision plans for critically ill patients.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2023.01130.
Supplementary Table 1.
Disease categories of diagnoses in patients admitted to the intensive care unit by ICD-10 code
acc-2023-01130-Supplementary-Table-1.pdf
Supplementary Figure 1.
Differences in cancer distribution for life-sustaining treatment withdrawal patients between total in-hospital population and ICU inpatients.
acc-2023-01130-Supplementary-Fig-1.pdf
REFERENCES
1. Welfare Ministry of Health and Welfare. Act on decisions on life-sustaining treatment for patients in hospice and palliative care or at the end of life. No. 17218. Welfare Ministry of Health and Welfare;2020.
2. Kim HA, Cho M, Son DS. Temporal change in the use of laboratory and imaging tests in one week before death, 2006-2015. J Korean Med Sci. 2023; 38:e98.

3. Council on Ethical and Judician Affairs, American Medical Association. Medical futility in end-of-life care: report of the council on ethical and judicial affairs. JAMA. 1999; 281:937–41.
4. Curtis JR, Vincent JL. Ethics and end-of-life care for adults in the intensive care unit. Lancet. 2010; 376:1347–53.

5. Lee SI, Hong KS, Park J, Lee YJ. Decision-making regarding withdrawal of life-sustaining treatment and the role of intensivists in the intensive care unit: a single-center study. Acute Crit Care. 2020; 35:179–88.

6. Park SY, Lee B, Seon JY, Oh IH. A national study of life-sustaining treatments in South Korea: what factors affect decision-making? Cancer Res Treat. 2021; 53:593–600.

7. Kim JS, Yoo SH, Choi W, Kim Y, Hong J, Kim MS, et al. Implication of the life-sustaining treatment decisions act on end-of-life care for Korean terminal patients. Cancer Res Treat. 2020; 52:917–24.

8. Phua J, Joynt GM, Nishimura M, Deng Y, Myatra SN, Chan YH, et al. Withholding and withdrawal of life-sustaining treatments in intensive care units in Asia. JAMA Intern Med. 2015; 175:363–71.

9. Yun YH, Lee CG, Kim SY, Lee SW, Heo DS, Kim JS, Lee KS, Hong YS, et al. The attitudes of cancer patients and their families toward the disclosure of terminal illness. J Clin Oncol. 2004; 22:307–14.

10. Manalo MF. End-of-life decisions about withholding or withdrawing therapy: medical, ethical, and religio-cultural considerations. Palliat Care. 2013; 7:1–5.

11. Kim H, Im HS, Lee KO, Min YJ, Jo JC, Choi Y, et al. Changes in decision-making process for life-sustaining treatment in patients with advanced cancer after the life-sustaining treatment decisions-making act. BMC Palliat Care. 2021; 20:63.

12. Luce JM. End-of-life decision making in the intensive care unit. Am J Respir Crit Care Med. 2010; 182:6–11.

13. Yildirim S, Durmaz Y, Şan Y, Taşkıran İ, Cinleti BA, Kirakli C. Cost of chronic critically ill patients to the healthcare system: a single-center experience from a developing country. Indian J Crit Care Med. 2021; 25:519–23.

14. Khandelwal N, Benkeser D, Coe NB, Engelberg RA, Teno JM, Curtis JR. Patterns of cost for patients dying in the intensive care unit and implications for cost savings of palliative care interventions. J Palliat Med. 2016; 19:1171–8.

15. Slatyer MA, James OF, Moore PG, Leeder SR. Costs, severity of illness and outcome in intensive care. Anaesth Intensive Care. 1986; 14:381–9.

16. Kim D, Yoo SH, Seo S, Lee HJ, Kim MS, Shin SJ, et al. Analysis of cancer patient decision-making and health service utilization after enforcement of the life-sustaining treatment decision-making act in Korea. Cancer Res Treat. 2022; 54:20–9.

17. Arabi YM, Myatra SN, Lobo SM. Surging ICU during COVID-19 pandemic: an overview. Curr Opin Crit Care. 2022; 28:638–44.

18. Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007; 356:469–78.

Figure 1.
Distribution of diseases between patients for whom life-sustaining treatment was withdrawn and the control group. Patients in the life-sustaining treatment (LST) group and control group were assigned to one of three common critical disease categories of neurological disorders, infectious disorders, and cancers. The proportion of cancer patients was significantly higher in the withdrawal group than the control group. Conversely, the numbers of patients with neurological disorders and infectious disorders were higher in the control group than the withdrawal group. ICU: intensive care unit.
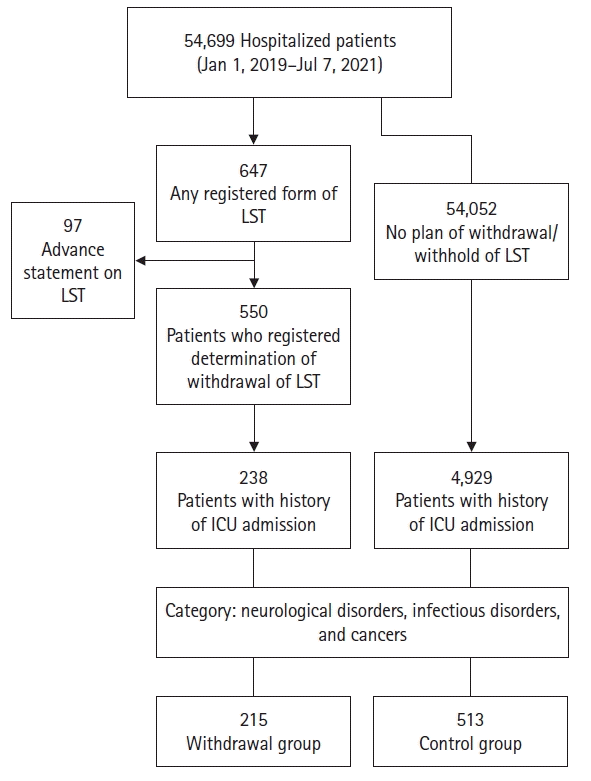
Figure 2.
Types of intensive care performed in the life-sustaining treatment (LST) withdrawal group (A) and control group (B) during their intensive care unit stay. The legal definition of LST includes not only cardiopulmonary resuscitation (CPR), but also mechanical ventilation, hemodialysis, vasopressors, and blood transfusion, which are commonly performed in intensive care units. The most common intensive care received during the intensive care unit stay among those who withdrew LST was, in order of frequency, mechanical ventilation, vasopressor usage, and blood transfusions. ECLS: extracorporeal life support.
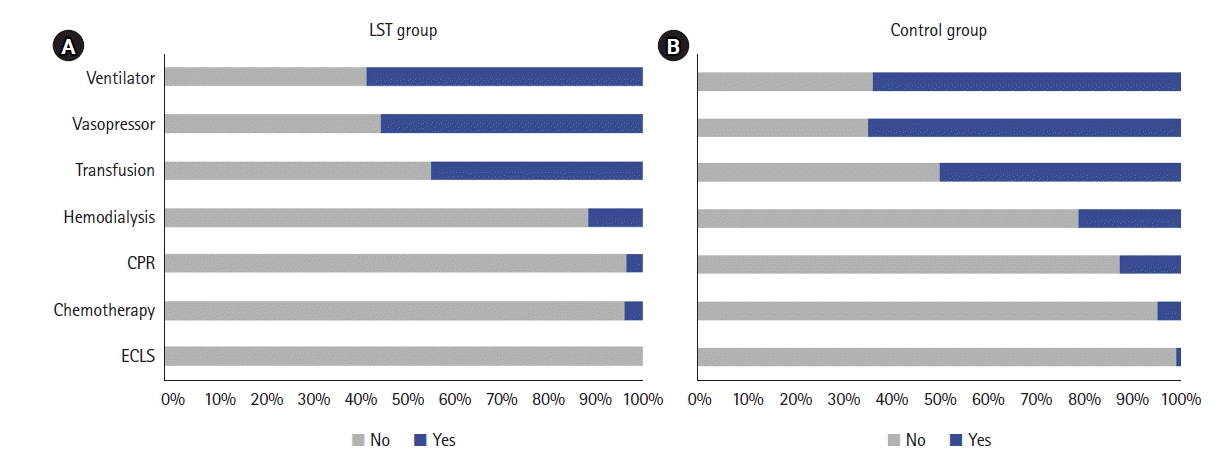
Figure 3.
Identity of decision-makers to withdraw life-sustaining treatment among intensive care unit inpatients. Overall, withdrawal of life-sustaining treatment was predominantly by family determination, but it varied by the nature of the disease: self-determination was higher for cancer patients compared to other diseases and lower for neurological disorders with altered consciousness and sepsis with the possibility of rapid deterioration.
Table 1.
Diseases of the patients who withdraw LST between the total population and ICU inpatients
Table 2.
Demographics of withdrawal of life-sustaining treatment group and control group in ICU patients with three disease categories
Table 3.
Outcomes and healthcare costs between the withdrawal of LST group and control group
| Variable | Total (n=728) | Withdrawal (n=215) | Control (n=513) | P-value |
|---|---|---|---|---|
| Hospital stay (day) | 12 (5–24) | 16 (8–27) | 11 (4–24) | <0.001 |
| ICU stay (day) | 7 (2–18) | 7 (2–16) | 7 (2–18) | 0.59 |
| Admission to death (day) | 10 (3–22) | 14 (6–28) | 8 (2–19) | <0.001 |
| Admission to LST (day) | 8 (3–20) | |||
| LST to death (day) | 2 (0–7) | |||
| Readmission | 118 (16) | 52 (24) | 66 (13) | <0.001 |
| Mortality | 439 (60) | 141 (66) | 298 (58) | 0.07 |
| Healthcare cost (KRW) | ||||
| Total admission | 25,399,018 (11,389,713–49,619,676) | 29,853,224 (13,697,848–53,778,748) | 20,855,966 (9,247,913–45,047,298) | 0.001 |
| ICU | 14,672,692 (7,308,024–32,439,968) | 15,781,577 (8,138,538–32,571,558) | 14,349,285 (6,908,488–32,438,511) | 0.35 |
| From ICU to LST withdrawala) | 12,949,487 (6,437,354–27,491,472) | |||
| From LST withdrawal to dischargeb) | 282,659 (0–4,586,083) |
Table 4.
Multivariate analysis of factors affect to decision of withdrawing life-sustaining treatment in patients admitted ICU




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download