INTRODUCTION
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an accurate and safe procedure for diagnosing unknown mediastinal or abdominal masses and lymphadenopathy.
1-
4 To diagnose lymphadenopathy, large tissue samples are required for a pathological assessment, which includes immunohistochemistry, flow cytometry, and a cytogenetic assessment. The use of a large-gauge (19-gauge, 19-G) needle has enabled the sampling of large amounts of tissue from lymphadenopathy.
5 Recently, Franseen needles have been reported to be useful for tissue sampling of pancreatic and subepithelial lesions
6-
9; additionally, 19-G Franseen needles seem to be suitable for obtaining large tissue samples. However, the efficacy of diagnosing lymphadenopathy using the 19-G Franseen needle remains unclear. This study aimed to compare the effectiveness of 19-G conventional and Franseen needles in the diagnosis of lymphadenopathy and classification of malignant lymphoma (ML).
METHODS
Patients
This retrospective study was conducted at the Gifu Municipal Hospital, where more than 100 EUS-FNA procedures are performed annually. The patient database, including clinical data of EUS-FNA performed between January 2012 and February 2022, was searched. The inclusion criteria were as follows: (1) age >20 years, (2) lymphadenopathy, and (3) use of a 19-G needle. The exclusion criteria were (1) use of needles other than the 19-G needle, (2) inability to provide informed consent, and (3) an individual considered ineligible by the investigators.
EUS-FNA procedure
EUS-FNA was performed using an oblique forward-viewing electronic linear scanning video echoendoscope (GF-UC240AL-5, or GF-UCT 260; Olympus Optical). The echoendoscope was inserted into the patient while in the left lateral decubitus position. After visualizing the lymph node (LN), Doppler mode was used to confirm blood flow to avoid puncturing the vessel with the aspiration line. Following the EUS evaluation, a puncture was performed under EUS guidance via the esophageal, gastric, or duodenal wall (
Fig. 1A). Our institution is a core hematology hospital; therefore, many patients with lymphadenopathy suspected of having ML visit our hospital. Therefore, EUS-FNA is typically performed using a 19-G needle to obtain sufficient tissue samples, considering the wide range of pathological examinations. The needle was advanced to the LN and the stylet was removed. The needle was passed back and forth 10 times in the LN with a 10 mL syringe suction. The puncture session continued until sufficient whitish material was obtained for pathological examination. One or two additional punctures were made to obtain a sufficient amount of material. These samples were divided into two tubes containing RPMI 1640 medium, one for flow cytometry and the other for cytogenetic assessment.
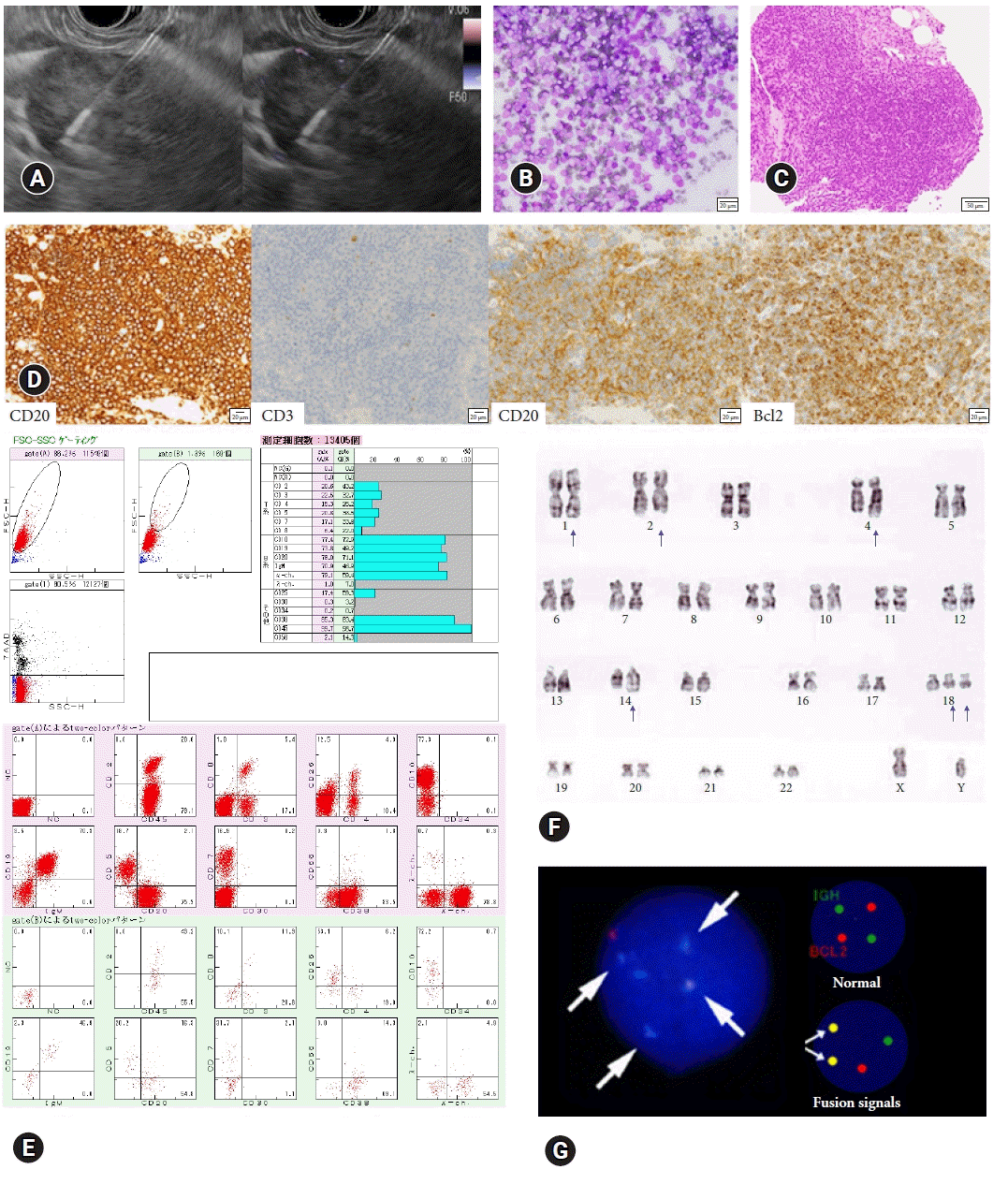
Fig. 1.
The process of lymphadenopathy and malignant lymphoma diagnosis using a 19-gauge conventional or Franseen needle. (A) Endoscopic ultrasound (EUS) fine needle aspiration performed under EUS guidance in Doppler mode. (B) Cytology stained with Giemsa shows small, atypical lymphocytes. Scale bar=20 μm. (C) Histopathology shows a lymphoid follicle consisting of small, atypical lymphocytes. Scale bar=50 μm. (D) Immunohistochemical staining is positive in CD20, CD10, Bcl-2, and negative in CD3. (E) The flow cytometry analysis of this case shows the expression of B-cell lineage antigens (CD10, CD19, and CD20) and immunoglobulin light chains (κ). Scale bar=20 μm. (F) A G-banded karyotyping analysis finds t(14;18)(q32;q21) chromosomal translocation (arrows). (G) The fluorescence in situ hybridization assay of this case indicates a green signal for IGH, a red signal for BCL-2, and a yellow signal (arrows) for a fusion of IGH and BCL-2.

Rapid on-site evaluations
Rapid on-site evaluation (ROSE) was performed by a cytopathologist/cytotechnologist in the procedural room for all EUS-FNA procedures. The EUS-FNA specimens were placed on glass slides and smeared for on-site preparation. Before staining, the obtained sample was gently placed on the side to avoid crushing the artifacts. Subsequently, each slide was air-dried for Diff-Quik staining. Finally, a cytopathologist or cytotechnologist determined whether the samples were positive (definitive cytopathological evidence of malignancy) or negative (no malignant cells), after which the samples were processed for further examination. EUS-FNA was performed until adequate tissue samples that could be analyzed using ROSE were obtained.
Pathological diagnosis, flow cytometry, cytogenetic assessment, and final diagnosis
The aspirated material was expelled onto a glass slide. A whitish portion of the material was cut and removed from the clot using tweezers. The collected whitish tissue was fixed in 10% neutral buffered formalin solution for pathological and immunohistochemical examinations. The remaining whitish samples were smeared onto glass slides for cytological examination (
Fig. 1B). Finally, the material was embedded in paraffin wax and processed to prepare 3 to 4 mm-thick serial sections for hematoxylin-eosin staining and immunohistochemistry (
Fig. 1C,
D). Several monoclonal and polyclonal antibodies, including those against ML, have been used in immunohistochemistry to obtain an accurate pathological diagnosis of lymphadenopathy. Pathologists (YK, NW, and TT) independently made cytological and histopathological diagnoses. For ML, flow cytometry was performed using the following antibodies: B-cell lineage antigens CD10, CD19, CD20, and Bcl2; T-cell lineage antigens CD2, CD3, CD4, CD5, and CD7; additional antigens CD25, CD30, CD34, CD38, CD45, and CD56; and IgM, immunoglobulin light chains (κ and λ) (
Fig. 1E). Cytogenetic abnormalities were assessed using conventional G-banded karyotyping (
Fig. 1F). A fluorescence
in situ hybridization (FISH) assay was performed to determine the diagnosis in cases requiring a detailed clinical evaluation (
Fig. 1G). The final diagnoses of ML, determined according to the World Health Organization (WHO) classification
10 or metastasis of malignant lesions, were determined based on the pathological findings and clinical course. Benign diagnoses were confirmed when lymphadenopathy progression did not occur on follow-up imaging for ≥6 months.
Study endpoints and needle type
The study endpoint was to compare 19-G conventional and Franseen needles for diagnosing lymphadenopathy. Conventional (end-cut) 19-G needles (Echo Tip, Wilson-Cook; EZ shot3, Olympus; SonoTip Pro Control, Medi-Globe; Expect Slimline, Boston Scientific) were used frequently until December 2017 (conventional group), and Franseen needles (Acquire, Boston Scientific; SonoTip TopGain, Medi-Globe) were frequently used after January 2018 (Franseen group). The procedures and specimen handling methods were the same for both groups. The outcomes of both groups were compared to evaluate sensitivity (malignant), specificity (benign), positive predictive value (PPV), negative predictive value (NPV), and lymphadenopathy accuracy based on histological and cytological findings. As patients were diagnosed with ML, the sensitivity of the ML classification diagnostic examinations, including immunohistochemical evaluation of histological specimens, flow cytometry, and cytogenetic assessment, was compared between the two groups. Adverse events (AEs) after EUS-FNA were also compared.
Statistical analyses
All analyses were conducted using R ver. 4.0.2 (The R Foundation for Statistical Computing). Values are expressed as number of patients or median (range). Fisher exact test and the Mann-Whitney U-test were used for categorical and continuous variable analyses, respectively. A p-value <0.05 was considered statistically significant. Subgroup analyses were performed to evaluate the diagnostic yield of conventional and Franseen needle sampling for each lymphadenopathy puncture route. To identify the factors of accurate lymphadenopathy diagnostic performance, a logistic analysis was performed with an adjustment for clinically significant findings, including age, sex, lesion size, number of needle passes, needle type, and puncture route, on cytological or histological accuracy. Factors related to accurate lymphadenopathy diagnosis (p<0.20) in the univariate analysis were further assessed using multivariate analysis. The regression analysis results are expressed as odds ratios (ORs). Median values were used to determine the cut-off values for age, lesion size, and number of needle passes.
Ethics approval
This study was approved by the Institutional Review Board of Gifu Municipal Hospital (no. 749) and adhered to the Declaration of Helsinki. The study protocol was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000046873).
RESULTS
Patient selection and characteristics
We enrolled 172 patients with lymphadenopathy who underwent EUS-FNA between January 2012 and February 2022. Out of this group, 26 were excluded based on eligibility criteria: a 22-G needle was used in 25 patients and a 25-G needle was used in one (
Supplementary Tables 1,
2). A total of 146 patients met the inclusion criteria (conventional group, 70 patients; Franseen group, 76 patients) (
Fig. 2). Among them, 110 were diagnosed with ML. Of the remaining participants, five had metastatic diseases (lung cancer [four patients] and gastric cancer [one patient]) and 31 benign cases (sarcoidosis [13 patients] and nonspecific lymphadenopathy [18 patients]).
Table 1 summarizes the baseline patient characteristics according to the WHO classification of ML cases. The median lesion size was 29 mm (range, 10–83 mm) in the conventional group and 34 mm (range, 12–110 mm) in the Franseen group, without statistical significance. Additionally, there were no significant differences between groups in terms of age, sex, lesion location, or final diagnosis.
Fig. 2.
Flowchart of patient selection.
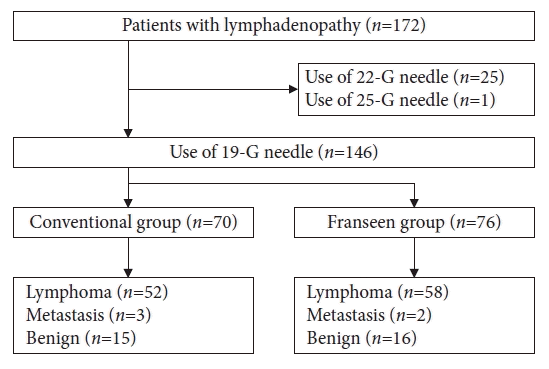

Table 1.
Characteristics of patients with lymphadenopathy according to the World Health Organization classifications of malignant lymphoma
|
Characteristic |
Conventional group (n=70) |
Franseen group (n=76) |
p-value |
|
Age (yr) |
71 (26–87) |
73 (24–93) |
0.25 |
|
Female |
35 (50.0) |
29 (38.2) |
0.18 |
|
Size of the lesion |
29 (10–83) |
34 (12–110) |
0.14 |
|
Location of the lesion |
|
|
|
|
Mediastinum |
14 (20.0) |
21 (27.6) |
0.33 |
|
Paraarotic |
54 (77.1) |
54 (71.1) |
0.45 |
|
Hepatic hilum |
2 (2.9) |
1 (1.3) |
0.61 |
|
Final diagnosis |
|
|
|
|
Malignant |
|
|
|
|
Lymphoma |
52 (74.3) |
58 (76.3) |
0.85 |
|
Hodgkin lymphoma |
0 |
3 |
0.25 |
|
Mixed cellularity classical |
0 |
3 |
0.25 |
|
Mature B-cell neoplasms |
48 |
48 |
0.60 |
|
DLBCL, not otherwise specified |
25 |
26 |
0.85 |
|
Follicular lymphoma |
16 |
15 |
0.69 |
|
Mantle cell lymphoma |
1 |
3 |
0.62 |
|
Burkitt lymphoma |
2 |
0 |
0.23 |
|
Splenic marginal zone lymphoma |
0 |
2 |
0.50 |
|
MALT lymphoma |
1 |
0 |
0.48 |
|
T cell/histiocyte-rich large B-cell lymphoma |
0 |
1 |
1.0 |
|
Nodal marginal zone lymphoma |
0 |
1 |
1.0 |
|
Unclassified |
3 |
0 |
0.10 |
|
Matura T-cell neoplasms |
4 |
7 |
0.54 |
|
PTCL, not otherwise specified |
1 |
3 |
0.62 |
|
AILT |
1 |
1 |
1.0 |
|
ALCL, ALK-negative |
0 |
1 |
1.0 |
|
ATLL |
1 |
0 |
1.0 |
|
Follicular T-cell lymphoma |
0 |
1 |
1.0 |
|
Unclassified |
1 |
1 |
1.0 |
|
Metastasis |
3 (4.3) |
2 (2.6) |
0.67 |
|
Lung cancer |
2 |
2 |
1.0 |
|
Gastric cancer |
1 |
0 |
0.48 |
|
Benign |
15 (21.4) |
16 (21.1) |
1.0 |
|
Sarcoidosis |
4 |
9 |
0.25 |
|
Nonspecific |
11 |
7 |
0.31 |

Outcomes of EUS-FNA
The outcomes in both groups are shown in
Table 2. There were no significant differences in the puncture routes. Tissue samples were obtained from both groups and evaluated using cytology and histology. The median number of needle passes was significantly higher in the Franseen group than in the conventional group (median [range]; 4 [1–6] vs. 3 [1–6] times,
p=0.023). The occurrence of AEs was not significant; however, three cases of bleeding were observed in the Franseen group. There was one case of severe bleeding that required embolization following angiography after transesophageal puncture of the mediastinal LN (
Fig. 3). There were no significant differences between the two groups in sensitivity, specificity, PPV, NPV, or accuracy for malignant diseases (ML and metastasis). To evaluate the factors related to diagnostic accuracy, the continuous variable factor was divided by the median values of 72 years of age, 32 mm lesion size, and four needle passes for logistic analysis. Based on the results of the univariate logistic analyses, the cytological factors of the female and Franseen groups were included in the multivariate analysis. However, no significant predictors for improved cytological accuracy were identified. No significant histological changes were identified either (
Table 3).
Fig. 3.
A bleeding case in the Franseen needle group. (A) A 20-mm mediastinum lymphadenopathy (arrow) was detected by computed tomography. (B) The lymphadenopathy was punctured via a transesophageal route using a 19-gauge Franseen needle. (C) Mediastinal enlargement with chest pain was observed one day after puncture test. The vessel (arrowheads) was observed in the hematoma around the lymphadenopathy using contrast computed tomography. (D) Embolization of the vessel was required for hemostasis.
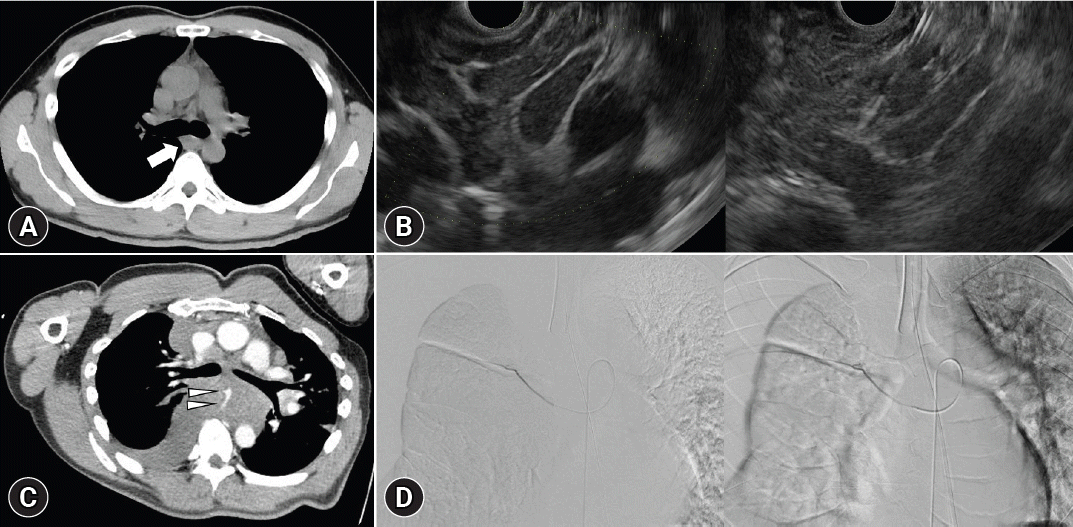

Table 2.
Comparison of the lymphadenopathy diagnosis for malignant disease
|
|
Conventional group (n=70) |
Franseen group (n=76) |
p-value |
|
Puncture route |
|
|
|
|
Transesophageal |
14 (20.0) |
21 (27.6) |
0.33 |
|
Transgastric |
52 (74.3) |
48 (63.2) |
0.16 |
|
Transduodenal |
4 (5.7) |
7 (9.2) |
0.54 |
|
No. of passes |
3 (1–6) |
4 (1–6) |
0.023 |
|
Cytology |
|
|
|
|
Sensitivity |
53/55 (96.4) |
53/60 88.3) |
0.16 |
|
Specificity |
14/15 (93.3) |
14/16 87.5) |
1.0 |
|
PPV |
53/54 (98.1) |
53/55 (96.4) |
1.0 |
|
NPV |
14/16 (87.5) |
14/21 (66.7) |
0.25 |
|
Accuracy |
67/70 (95.7) |
67/76 (88.2) |
0.13 |
|
Histology |
|
|
|
|
Sensitivity |
52/55 (94.5) |
58/60 (96.7) |
0.67 |
|
Specificity |
14/15 (93.3) |
16/16 (100.0) |
0.48 |
|
PPV |
52/53 (98.1) |
58/58 (100.0) |
0.48 |
|
NPV |
14/17 (82.4) |
16/18 (88.9) |
0.66 |
|
Accuracy |
66/70 (94.3) |
74/76 (97.4) |
0.43 |
|
Adverse events |
|
|
|
|
Bleeding |
0 (0) |
3 (3.9) |
0.25 |

Table 3.
A logistic analysis of the relating factors in the accurate diagnosis of lymphadenopathy through cytology or histology
|
Factor |
Univariate analysis (cytology) |
Multivariate analysis (cytology) |
Univariate analysis (histology) |
|
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
OR (95% CI) |
p-value |
|
Franseen group |
0.33 (0.09–1.29) |
0.11 |
0.37 (0.10–1.46) |
0.16 |
2.24 (0.40–12.60) |
0.36 |
|
Age, ≥72 yr |
0.69 (0.21–2.29) |
0.55 |
|
|
1.00 (0.20–5.13) |
1.0 |
|
Sex, Female |
0.23 (0.05–1.10) |
0.07 |
0.26 (0.05–1.22) |
0.09 |
2.67 (0.47–15.00) |
0.27 |
|
Tumor size, ≥32 mm |
0.71 (0.22–2.36) |
0.58 |
|
|
2.12 (0.38–11.90) |
0.40 |
|
Number of passes, ≥4 times |
1.23 (0.38–4.00) |
0.73 |
|
|
1.22 (0.24–6.27) |
0.81 |
|
Puncture route (gastric) |
1.10 (0.31–3.84) |
0.89 |
|
|
1.09 (0.19–6.18) |
0.92 |

Subgroup analysis of the lymphadenopathy diagnosis according to puncture route
The cytology and histology sensitivity, specificity, PPV, NPV, and accuracy were compared among the different puncture routes (esophageal, gastric, and duodenal) for lymphadenopathy (
Table 4). No significant differences were observed between the two groups in any category for each puncture route.
Table 4.
Subgroup analyses of the lymphadenopathy diagnosis by the puncture route
|
Conventional group |
Franseen group |
p-value |
|
Esophageal |
|
|
|
|
Cytology |
|
|
|
|
Sensitivity |
8/8 (100.0) |
9/10 (90.0) |
1.0 |
|
Specificity |
6/6 (100.0) |
10/11 (90.9) |
1.0 |
|
PPV |
8/8 (100.0) |
9/10 (90.0) |
1.0 |
|
NPV |
6/6 (100.0) |
10/11 (90.9) |
1.0 |
|
Accuracy |
14/14 (100.0) |
19/21 (90.5) |
0.51 |
|
Histology |
|
|
|
|
Sensitivity |
8/8 (100.0) |
9/10 (90.0) |
1.0 |
|
Specificity |
6/6 (100.0) |
11/11 (100.0) |
1.0 |
|
PPV |
8/8 (100.0) |
9/9 (100.0) |
1.0 |
|
NPV |
6/6 (100.0) |
11/12 (91.7) |
1.0 |
|
Accuracy |
14/14 (100.0) |
20/21 (95.2) |
1.0 |
|
Gastric |
|
|
|
|
Cytology |
|
|
|
|
Sensitivity |
43/45 (95.6) |
39/45 (86.7) |
0.27 |
|
Specificity |
7/7 (100.0) |
3/3 (100.0) |
1.0 |
|
PPV |
43/43 (100.0) |
39/39 (100.0) |
1.0 |
|
NPV |
7/9 (77.8) |
3/9 (33.3) |
0.15 |
|
Accuracy |
50/52 (96.2) |
42/48 (87.5) |
0.15 |
|
Histology |
|
|
|
|
Sensitivity |
42/45 (93.3) |
44/45 (97.8) |
0.62 |
|
Specificity |
7/7 (100.0) |
3/3 (100.0) |
1.0 |
|
PPV |
42/42 (100.0) |
44/44 (100.0) |
1.0 |
|
NPV |
7/10 (70.0) |
3/4 (75.0) |
1.0 |
|
Accuracy |
49/52 (94.2) |
47/48 (97.9) |
0.62 |
|
Duodenum |
|
|
|
|
Cytology |
|
|
|
|
Sensitivity |
2/2 (100.0) |
5/5 (100.0) |
1.0 |
|
Specificity |
1/2 (50.0) |
2/2 (100.0) |
1.0 |
|
PPV |
2/3 (66.7) |
5/5 (100.0) |
0.38 |
|
NPV |
1/1 (100.0) |
2/2 (100.0) |
1.0 |
|
Accuracy |
3/4 (75.0) |
7/7 (100.0) |
0.36 |
|
Histology |
|
|
|
|
Sensitivity |
2/2 (100.0) |
5/5 (100.0) |
1.0 |
|
Specificity |
1/2 (50.0) |
2/2 (100.0) |
1.0 |
|
PPV |
2/3 (66.7) |
5/5 (100.0) |
0.38 |
|
NPV |
1/1 (100.0) |
2/2 (100.0) |
1.0 |
|
Accuracy |
3/4 (75.0) |
7/7 (100.0) |
0.36 |

Comparison of the needle type of diagnostic sensitivity for ML and WHO classifications
Overall, 110 (75.3%) of the 146 patients were diagnosed with ML.
Table 5 summarizes the comparison of ML cases in 52 patients in the conventional group and 58 patients in the Franseen group. There was no significant difference between the two groups in terms of the puncture route or number of needle passes for tissue sampling. Both groups showed high sensitivities for cytology (96% vs. 88%,
p=0.17) and histology (94% vs. 97%,
p=0.67), without significance. Immunohistochemistry of histological specimens was performed in 46 of 52 (88.5%) patients in the conventional group and in 56 of 58 (96.6%) in the Franseen group (
p=0.14).
Table 5.
Comparison of the malignant lymphoma diagnoses in the conventional and Franseen groups
|
Conventional group (n=52) |
Franseen group (n=58) |
p-value |
|
Puncture route |
|
|
|
|
Transesophageal |
5 (9.6) |
8 (13.8) |
0.56 |
|
Transgastric |
45 (86.5) |
45 (77.6) |
0.32 |
|
Transduodenal |
2 (3.8) |
5 (8.6) |
0.44 |
|
No. of passes |
3 (1–6) |
4 (1–6) |
0.22 |
|
Sensitivity |
|
|
|
|
Cytology |
50 (96.2) |
51 (87.9) |
0.17 |
|
Histology |
49 (94.2) |
56 (96.6) |
0.67 |
|
IHC evaluation rate in histological specimen |
46 (88.5) |
56 (96.6) |
0.14 |
|
Flow cytometry |
47 (90.4) |
49 (84.5) |
0.40 |
|
Cytogenetic assessment |
33 (63.5) |
45 (77.6) |
0.14 |
|
Diagnostic rate of WHO classifications |
48 (92.3) |
57 (98.3) |
0.19 |

Flow cytometry was performed in all ML cases. A concordant result between ML classification and flow cytometry was obtained in 47 out of 52 patients (90.4%) in the conventional group and 49 out of 58 patients (84.5%) in the Franseen group (p=0.40). The remaining five cases in the conventional group and nine cases in the Franseen group were B-cell ML cases (11 diffuse large B-cell lymphoma [DLBCL], two follicular lymphoma [FL], and one mucosa-associated lymphoid tissue lymphoma); however, flow cytometry showed T-cell-like expression in these cases.
Cytogenetic assessment using G-banded karyotyping was performed for all ML cases. Specific translocations were found in 35 patients: t(14;18)(q32;q21) in 10 FL and three DLBCL cases in the conventional group, and nine FL and five DLBCL cases in the Franseen group; t(3;14)(q27;q32) in two DLBCL cases in the conventional group and three DLBCL cases in the Franseen group; and t(11;14)(q13:q32) in one mantle case in the Franseen group. Nonspecific and/or complex abnormalities were found in 14 and nine patients in the conventional and Franseen groups, respectively. A normal karyotype was observed in 11 and 13 patients in the conventional and Franseen groups, respectively. Of the remaining 30 patients, cell proliferation was insufficient during cell culture in 13 patients in the conventional group and 17 patients in the Franseen group. Hence, the rate of specific cytogenetic abnormalities was 63.5% (33/52) in the conventional group and 77.6% (45/58) in the Franseen group (not significant, p=0.14). The FISH assay was performed in nine patients (eight conventional and one Franseen case). In the conventional group, fusion signals were detected in the FISH assay (BCL-1/IGH probe fusion in one mantle lymphoma case, BCL-2/IGH probe fusion in one FL case, MYC/IGH probe fusion in one DLBCL case, and one Burkitt lymphoma case). ML WHO classifications were possible for 92.3% (48/52) of patients in the conventional group and 98.3% (57/58) of patients in the Franseen group through a combination of the immunohistochemistry evaluation, flow cytometry, and cytogenetic assessment (p=0.19).
DISCUSSION
There have been three comparative studies of 22-G or 25-G conventional and Franseen needles for the diagnosis of lymphadenopathy, with the diagnostic accuracy of 22-G or 25-G needles reportedly at 65% to 88%.
11-
13 However, no previous studies have compared 19-G conventional and Franseen needles in cases of lymphadenopathy. The use of a 19-G needle ensured that large amounts of tissue samples were obtained, and the diagnostic accuracy of our study with both 19-G needle types was 94–97%. Therefore, using a 19-G needle ensures that sufficient tissue samples were obtained for the accurate diagnosis of lymphadenopathy. Franseen needles have been reported to be useful for tissue sampling
6-
9,
11; however, there was no significant difference in the diagnostic accuracy of lymphadenopathy between the two 19-G needle types. The 19-G conventional needle can obtain sufficient tissue samples because of the structure of lymphadenopathy. The pathological findings showed that the lymphadenopathy had high cell density, low fibrous tissue, and weak tissue connection (
Fig. 1C). These structures differ from those in pancreatic cancer, suggesting that even conventional needles can be used to collect sufficient tissue. In this study, the median number of needle passes was significantly higher in the Franseen group (four) than in the conventional group (three). This difference was mainly due to the revision of our strategy for obtaining cytogenetic assessment samples. We typically performed two needle passes to obtain pathological specimens and added another needle pass to obtain samples for cytogenetic assessment in the early half-period when the conventional needle was frequently used. However, due to the low sensitivity (14.3%) of the cytogenetic assessment performed in a previous report,
2 we increased the number of needle passes required to collect samples for cytogenetic assessment from one to two in the late half-period; at this time, the Franseen needle was frequently used. Therefore, the increased number of needle passes in the Franseen group may have only affected the sensitivity of the cytogenetic assessment and not pathological sample acquisition.
ML classification is important for determining treatment plans and is based on the results of immunohistochemistry, flow cytometry, and/or cytogenetic assessments. Immunohistochemistry was performed in 92.7% of patients (102/110) using both 19-G needle types. Sufficient histological ML tissue samples could not be obtained in five of the remaining cases, and the other three cases were difficult to evaluate because the samples included a high concentration of necrotic tissues. As both the conventional and Franseen groups showed a high evaluation rate, there was no significant difference in the immunohistochemical evaluation rate (88% vs. 97%, p=0.14). Therefore, the 19-G needle can be used to obtain sufficient tissue samples for immunohistochemistry, regardless of the needle type. Flow cytometry requires smaller tissue sample sizes and can obtain results faster than immunohistochemistry. Notably, this rapid provision of information is particularly important for commencing treatment. The flow cytometry sensitivity was also high in both groups (90% in the conventional group versus 85% in the Franseen group). Previous reports showed high sensitivity (81.0%) of flow cytometry using a 19-G conventional needle2; therefore, flow cytometry can be evaluated using a 19-G needle, regardless of the needle type.
Cytogenetic assessment using G-banded karyotyping and/or FISH is also helpful for evaluating ML classifications with genetic abnormalities, such as with t(14;18)(q32;q21) and t(11;14)(q13:q32) translocation.
14-
17 However, this method requires a large number of cells to enable the examination of cells while in metaphase, and the sensitivity of G-banded karyotyping was reported to be only 14.3% using 19-G conventional needle-obtained tissue.
2,
10 In this study, G-band sensitivity in the Franseen group (78%) was higher than in the conventional group (63%). This suggests that the use of the 19-G Franseen needle can improve the sensitivity of cytogenetic assessment; however, the median pass number was larger in the Franseen group (four times) than in the conventional group (three times). Therefore, the results should be evaluated by subtracting the number of needle passes. Finally, 105 (95%) of the 110 patients with ML were diagnosed using a combination of immunohistochemistry, flow cytometry, and/or cytogenetic assessment. There was no significant difference in the rate of ML diagnosis between the conventional (92%) and Franseen groups (98%,
p=0.19). This result suggests that 19-G needles have high diagnostic ability for ML classification.
Three cases of bleeding occurred in the Franseen group. Although blood vessels were observed in all cases using the Doppler mode before EUS-FNA, undetectable minor vessels were injured. Benign lymphadenopathies, such as sarcoidosis, involve several minor vessels that can only be detected by contrast-enhanced EUS,
18 and two of the bleeding cases were sarcoidosis. A large-gauge needle can increase the risk of vessel injury; however, the 19-G conventional needle group did not experience any AEs. Although it is difficult to determine the relationship between the Franseen needle and bleeding, the shape of the three needle tips may increase the risk of a minor vessel injury. Considering that both needle types can achieve an accurate diagnosis of lymphadenopathy and ML classification, the 19-G conventional needle seems to be a safe option for tissue sampling.
This study had some limitations. First, because this was a long-term, single-center, retrospective study, improvements in endoscopy and ultrasound processors could potentially favor the use of EUS-FNA in late-stage cases. However, although most Franseen needle cases were included in the late half-period of the study, there were no significant differences in the pathological findings. This suggests that the use of a conventional needle is sufficient for diagnosing lymphadenopathy and classifying ML. Second, there was no sampling protocol for the two tubes containing the RPMI 1640 medium. Tissue samples obtained from one needle pass were divided into two tubes during the early half-period. However, in the late half-period, the tissue sample obtained after two needle passes was divided into two tubes to improve the tissue volume and sensitivity of the cytogenetic assessment, which also affected the number of needle passes in each group.
In conclusion, both 19-G conventional and Franseen needles showed high accuracy for lymphadenopathy and ML classification. Both needle types ensured sufficient tissue collection and avoidance of AEs; therefore, a 19-G conventional needle can be used for lymphadenopathy diagnosis.






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download