Abstract
Background
To investigate the prevalence, incidence, comorbidities, and management status of diabetic kidney disease (DKD) and diabetes-related end-stage kidney disease (ESKD) in South Korea.
Methods
We used the Korea National Health and Nutrition Examination Survey data (2019 to 2021, n=2,665) for the evaluation of prevalence, comorbidities, control rate of glycemia and comorbidities in DKD, and the Korean Health Insurance Service-customized database (2008 to 2019, n=3,950,857) for the evaluation of trends in the incidence and prevalence rate of diabetes-related ESKD, renin-angiotensin system (RAS) blockers and sodium glucose cotransporter 2 (SGLT2) inhibitors use for DKD, and the risk of atherosclerotic cardiovascular disease (ASCVD) and mortality according to DKD stages. DKD was defined as albuminuria or low estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 in patients with diabetes mellitus.
Results
The prevalence of DKD was 25.4% (albuminuria, 22.0%; low eGFR, 6.73%) in patients with diabetes mellitus aged ≥30 years. Patients with DKD had a higher rate of comorbidities, including hypertension, dyslipidemia, and central obesity; however, their control rates were lower than those without DKD. Prescription rate of SGLT2 inhibitors with reduced eGFR increased steadily, reaching 5.94% in 2019. Approximately 70% of DKD patients were treated with RAS blockers. The prevalence rate of diabetesrelated ESKD has been steadily increasing, with a higher rate in older adults. ASCVD and mortality were significantly associated with an in increase in DKD stage.
Conclusion
DKD is prevalent among Korean patients with diabetes and is an independent risk factor for cardiovascular morbidity and mortality, which requiring intensive management of diabetes and comorbidities. The prevalence of diabetes-related ESKD has been increasing, especially in the older adults, during past decade.
• This study explored DKD prevalence, incidence, comorbidities, and management in Korea.
• We used data from the KNHANES (2019–2021) and the Korean NHIS (2008–2021).
• New GDMTs (SGLT-2i, ns-MRAs, GLP-1 RAs) show cardiorenal benefits beyond RAS blockade.
• DKD was common in Korean patients and an independent cardiovascular risk factor.
• Diabetes-related ESKD rates have surged, especially in older adults, over the past decade.
Diabetic kidney disease (DKD), an alteration in kidney function and structure attributed to diabetes mellitus (DM) [1], is one of the major vascular complications in patients with both type 1 DM and type 2 DM [2]. A classic clinical course of DKD or diabetic nephropathy, which begin with early hyperfunction, followed by increased urinary albumin excretion and progressive decline in the kidney function, eventually progresses to end-stage kidney disease (ESKD), was described about 40 years ago [3]. Although this description has been debated with epidemiologic evidence and observations against the classic pattern, particularly in patients with type 2 DM [4,5], albuminuria and decreased kidney function are evidently strong risk factors and predictors of advanced chronic kidney disease (CKD) [6,7]. Accordingly, the diagnosis of DKD in clinical practice generally consider albuminuria or low estimated glomerular filtration rate (eGFR), regardless of the typical histopathological changes observed in diabetic nephropathy [8].
The increased prevalence of DKD is concurrent with the increased prevalence of DM worldwide, with an estimated prevalence of approximately 20% to 40% with regional and ethnic variations [2,9,10]. In South Korea, the prevalence of DKD was approximately 30% in patients with DM in multiple literature [11,12]; however, most of them were reported in the early 2010s, which needs to be updated. Understanding the basic epidemiology of DKD is essential for establishing policies for patient care, and has a direct and indirect impact on DKD management.
DKD is a leading cause of ESKD, and a significant risk factor for atherosclerotic cardiovascular diseases (ASCVD) [13,14]. Accordingly, optimal management of DKD comprises glycemic control, optimal use of renoprotective drugs including reninangiotensin system (RAS) blockers and sodium glucose cotransporter 2 (SGLT2) inhibitors, nutritional intervention, and intensive management of cardiovascular risk factors [15,16]. However, decreased kidney function hampers the proper engagement of antidiabetic drugs, requires multiple drug combinations for hypertensive management, and often leads to possible drug-drug interactions [17,18]. Hemodynamic alterations in DKD affects heart function in multiple ways [19]. Thus, optimal management of DKD is critical for the prevention of advanced stages of CKD and ASCVD.
We aimed to investigate the recent prevalence of DKD and diabetes-related ESKD, their changes, associated renal and cardiovascular risk factors, and the current status of DKD management in South Korea using representative nationwide cohorts.
This study used two different nationwide epidemiologic databases. The Korea National Health and Nutrition Examination Survey (KNHANES) is a national surveillance system conducted by the Korea Centers for Disease Control and Prevention. Briefly, the surveys collect information on medical health conditions, health-related behaviors, health care utilization, biochemical and anthropometric measures, nutritional status, and dietary behaviors through interviews and health examinations of approximately 10,000 individuals annually. For the representative sample selection of the entire population of South Korea, the surveys use a sampling plan of a multi-stage clustered probability design. More details have been described elsewhere [20]. This study used the 8th KNHANES dataset (KNHANES VIII, 2019 to 2021), comprising 22,559 individuals.
The Korean National Health Insurance Service (NHIS) cohort is a semi-dynamically constructed longitudinal cohort based on the national medical insurance system, which includes information on the epidemiologic, social, and medical information of approximately 50 million people covered by the national health insurance in South Korea. Moreover, information on participants’ demographics, medical and pharmaceutical records, medical procedures, hospitalizations, drugs, and anthropometric and biochemical measurements from the national health examination program conducted biennially is also included. The details of the cohort have been published previously [21,22].
We used the KNHANES (2019 to 2021, number of DM patients aged ≥30 years, 2,665) database for the cross-sectional evaluation of prevalence, comorbidities, optimal control rate of diabetes and comorbidities in patients with DKD, and the NHIS-customized database (2008 to 2019, number of DM patients aged ≥30 years in 2008 [n=2,019,979], and in 2019 [n=3,950,857]) for the longitudinal evaluation of the trends in the incidence and prevalence of diabetes-related ESKD, RAS blockers, and SGLT2 inhibitors use for DKD, and risk of ASCVD and mortality according to DKD stages. This study was approved by the Institutional Review Board at Soonchunhyang University Gumi Hospital, Gumi, Korea (IRB No. 2023-03). Written informed consent by the patients was waived due to a retrospective nature of our study.
DKD was defined as elevated urinary albumin excretion (albuminuria, urine albumin to creatinine ratio ≥30 mg/g) or a low eGFR, less than 60 mL/min/1.73 m2 in patients with DM. Glomerular filtration rate (GFR) was estimated using the CKD-Epidemiology Collaboration (CKD-EPI) creatinine equation [23]. Owing to the lack of information on albuminuria, the prevalence of DKD was assessed only by the KNHANES, and not the NHIS. ESKD was defined using the N18 code of the International Classification of Diseases 10th Revision (ICD-10) or ESKD-related procedures (O7020, O7021, O7061, O7062, O7071, O7072, O7073, O7074, O7075, R3280) in the NHIS database. Special exemption codes (V001 hemodialysis, V003 peritoneal dialysis, and V005 kidney transplant) for ESKD were mandatory for defining ESKD.
The comorbidities of interest in the patients with DKD were hypertension, dyslipidemia, obesity, and central obesity. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg, or diastolic BP ≥90 mm Hg, or antihypertensive use. Hypercholesterolemia was defined as low-density lipoprotein cholesterol (LDL-C) ≥160 mg/dL or use of lipid-lowering drugs. Dyslipidemia was defined as LDL-C ≥160 mg/dL or triglyceride ≥200 mg/dL or high-density lipoprotein cholesterol <40 mg/dL in men <50 mg/dL in women, or use of lipid-lowering drugs. Obesity was defined as a body mass index of ≥25 kg/m2, and central obesity was defined as a waist circumference of ≥90 cm in men ≥85 cm in women. Diabetic retinopathy was defined using funduscopic examination in the KNHNANES VIII program (n=544). During the survey period, a total of 544 participants underwent ophthalmoscopic retinal examination. Two ophthalmologists read the images and defined diabetic retinopathy when retinal findings were consistent with nonproliferative diabetic retinopathy, proliferative diabetic retinopathy and macular edema. The cardiovascular outcomes of interest included myocardial infarction (MI), stroke, and cardiovascular death. In the NHIS, MI was defined as hospitalization for MI (identified using ICD-10 codes I21–I22). Ischemic stroke was defined as hospitalization for stroke (identified using ICD-10 code I63) plus brain imaging studies. Cardiovascular death was defined as death from CVD (ICD codes I00–I99). In the KNAHNES, ASCVD (MI, angina, or stroke) was defined using questionnaires based on the diagnosis by clinicians.
The prevalence of DKD was estimated by the number of DKD patients divided by the number of DM patients, based on the cross-sectional information of the KNHANES VIII (2019 to 2021). The prevalence and control rates of comorbidities were compared between DM patients with and without DKD. Trends in the prevalence and incidence rates of diabetes-related ESKD were estimated annually between 2008 and 2019 using the NHIS-customized database. The prevalence and incidence rates were estimated as age, and sex-standardized rates per 100,000 individuals as a reference to the patients with DM in 2011. Standardized rates of prevalence and incidence were also stratified by age groups (30–59, 60–79, and ≥80 years). The prescription rate of SGLT2 inhibitors and RAS blockers between 2014 and 2019 were estimated by the number of DKD patients who had received the drugs divided by the number of all DKD patients each year based on the NHIS-customized database. In this case, DKD was only defined as low eGFR (<60 mL/min/1.73 m2) owing to the lack of information on albuminuria in the NHIS. DKD stages 3 and 4 were defined as eGFR of 30–59 and 15–29 mL/min/1.73 m2, respectively. The risks of ASCVD and death according to eGFR were estimated using Cox-proportional regression analysis. Cox-proportional hazards regression analysis was performed to evaluate the hazard ratio (HR) across the categories of eGFR such as ≥90, 60–89, 30–59, <30 mL/min/1.73 m2, and ESKD. Adjusted model was adjusted for sex, age, body mass index, smoking, alcohol consumption, exercise, hypertension, and dyslipidemia.
Data on various lifestyle habits, such as smoking status, alcohol consumption, and physical activity levels, were collected using structured questionnaires. In NHIS, we utilized data regarding smoking status and alcohol habits gathered from self-reported surveys conducted during national health screenings. The questions about smoking habits and smoking status included categories like current alcohol drinker, non-drinker and current smoker and non-smoker. Regular physical activity was identified as engaging in moderate exercise for at least 5 days per week or intense exercise for a minimum of 3 days per week.
The primary outcome was development of MI and stroke between January 1, 2012 and December 31, 2018 in each participant. In addition, the secondary outcome of this study was the presence of newly death between January 1, 2012 and December 31, 2019 in each participant.
The HR and 95% confidence intervals (CIs) for the main outcomes were estimated employing a multivariate Cox regression model. A prior diagnosis of ischemic heart disease and MI was ascertained based on the classifications I20–25 and I21–22. This was done if there were multiple diagnoses recorded either during hospital admissions or in outpatient settings. Data regarding the date and primary cause of mortality were acquired from the National Mortality Database using individual resident identification numbers. The reasons for death were classified according to the ICD-10 coding system.
A 5% level of significance was used to test statistical significance, and all statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
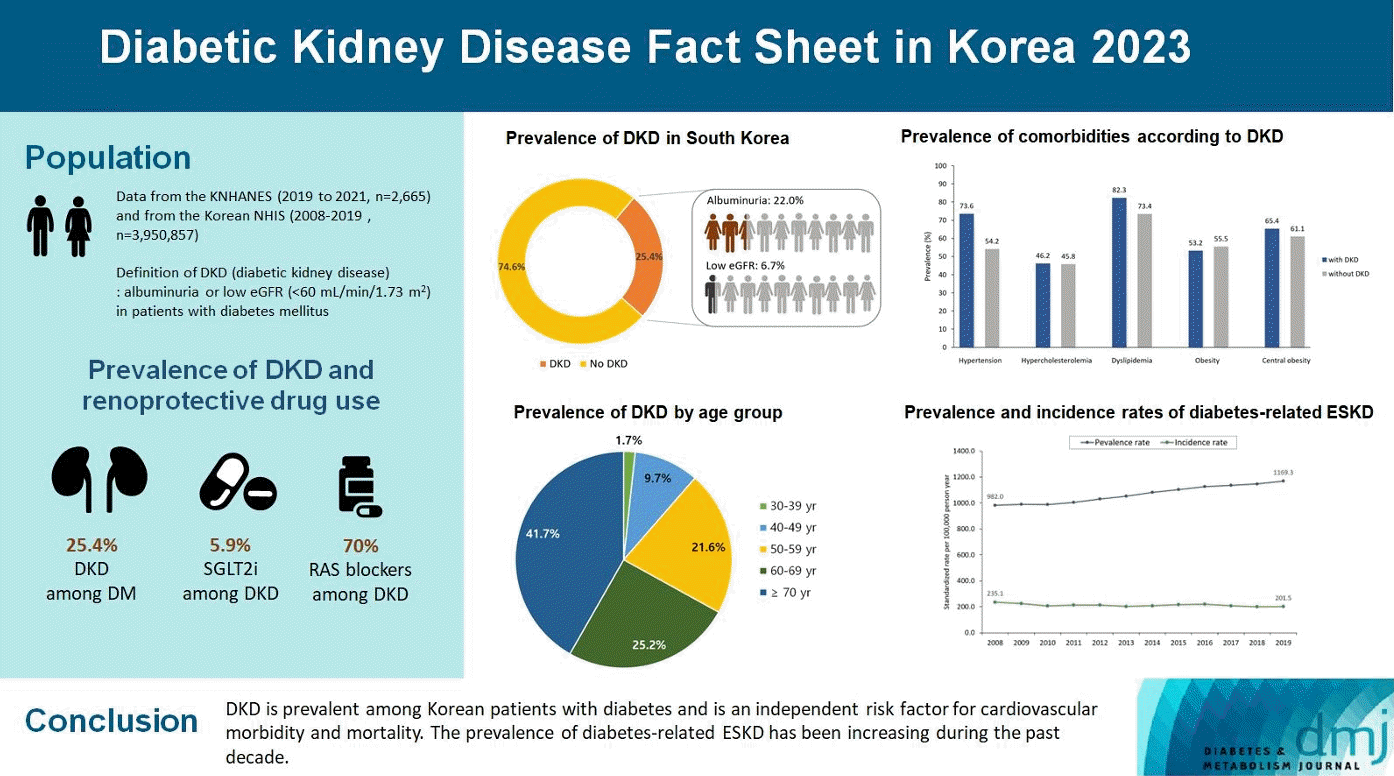
The prevalence of DKD among patients with DM aged ≥30 years was 25.4% (Fig. 1A), indicating that the estimated number of DKD patients in the entire Korean population is 1,309,900. Albuminuria and low eGFR (CKD stage 3 or higher) were reported in 22.0% (estimated number=1,133,716) and 6.7% (estimated number=362,249) of patients with DM, respectively. The estimated number of DKD without albuminuria was 176,184, which was 3.4% of the entire patients with DM, and 13.4% of patients with DKD (Fig. 1B). The estimated number of DKD patients in the entire Korean population is approximately 1.3 million. DKD is more prevalent with increasing age; 9.6%, 22.8%, and 37.3% of patients with DM are in the age groups of 30, 60, and ≥70 years, respectively (Fig. 1C). Moreover, the proportion of older adults among patients with DKD was high: 41.7% aged ≥70 years and 66.9% aged ≥60 years (Fig. 1D).
Hypertension and dyslipidemia were the most common comorbidities in patients with DKD (73.6% had hypertension and 82.3% had dyslipidemia) (Fig. 2A). The prevalence of hypercholesterolemia (defined as LDL-C ≥160 mg/dL or use of lipid-lowering drugs) was 46.2%. The obesity and abdominal obesity rates were 53.2% and 65.4%, respectively. The prevalence of these comorbidities was higher in patients with DKD than in those without DKD, except obesity. Fig. 2B illustrates the control rates of blood glucose levels and comorbidities in patients with and without DKD. The control rate of glycosylated hemoglobin (HbA1c) below 7.0% was 44.1% in patients with DKD, which was lower than 59.5% in patients without DKD. Among patients with DKD, the rate of HbA1c control below 6.5% was 24.0%, and the rates of systolic BP control below 140/90 and 130/80 mm Hg were 65.6% and 38.1%, respectively. The rates of LDL-C control at <100 mg/dL were 61.3% and below 70 mg/dL was 28.1%, respectively. Unlike other parameters, the rate of LDL-C control in patients with DKD was higher than that in patients without DKD. The rate of comprehensive control of HbA1c, BP, and LDL-C was only 20.5%, while the rate was only 3.6% when strict criteria were used (HbA1c <6.5%, BP <130/80 mm Hg, and LDL-C <70 mg/dL) in patients with DKD. Diabetic retinopathy was assessed based on a fundus examination conducted during the KNHANES VIII. The prevalence of diabetic retinopathy in patients with DKD aged 40 to 59 years was 43.4%, which was more than twice the prevalence of 19.4% in patients without DKD. The prevalence of ASCVD coexistence was 14% in DKD patients aged ≥30 years and 19% in DKD patients aged ≥65 years.
Fig. 3A and Supplementary Table 1 show the age, and sex-standardized prevalence and incidence of diabetes-related ESKD between 2008 and 2019. The incidence rate has remained constant (201.5–235.1 per 100,000 person-years) since 2008, whereas the prevalence rate has been steadily increasing. The prevalence of diabetes-related ESKD increased by 19.1% over 11 years, from 982.0 per 100,000 person-years in 2008 to 1,169.3 per 100,000 person-years in 2019. The prevalence rate of diabetes-related ESKD has increased in all age groups (Fig. 3B); however, the increase in the incidence rate was observed only in older adults aged ≥80 years (Fig. 3C).
The prescription rate of SGLT2 inhibitors in patients with reduced eGFR has been steadily increasing but remains relatively low (Fig. 4A, Supplementary Table 2). The prescription rate for SGLT2 inhibitors was 5.94% in 2019. The prescription rate of RAS blockers was steady from 2014 to 2019, used by approximately 70% of patients with DKD (Fig. 4B). This finding is similar to that of the coexistence of hypertension in patients with DKD.
Decreased kidney function was a significant risk factor for ASCVD and mortality (Table 1). The risk of MI and stroke increased linearly with increasing DKD stage. The risk of MI was 1.6-fold higher (adjusted HR, 1.60; 95% CI, 1.54 to 1.66) in stage 3 DKD patients and 3-fold higher (adjusted HR, 3.00; 95% CI, 2.77 to 3.25) in patients with stage 4 or higher DKD than in those with normal kidney function. The corresponding risks of stroke were 1.47- and 2.25-fold, respectively. Analysis of mortality risk showed similar results, particularly highlighting the close association between DKD stage and cardiovascular death. The risk of cardiovascular disease mortality was 2.05-fold higher in stage 3 patients with DKD, 4.64-fold higher in stage 4 or higher patients, and 7.27-fold higher in patients with ESKD than in those with normal kidney function. The corresponding risks of all-cause death were 1.50-, 3.90-, and 7.40-fold higher, respectively.
This study reported several key statistics related to DKD and diabetes-related ESKD in South Korea. Among patients with DM aged >30 years, 25.4% had DKD with 22.0% of albuminuria, and 6.7% of a low eGFR. Among those aged ≥70 years, 37.3% had DKD. Patients with DKD had higher rates of cardiometabolic comorbidities than those without DKD; however, the control rates were lower. The prescription rate of RAS blockers exceeded 70%, while the prescription rate of SGLT2 inhibitors remained low (only 6% in 2019) among patients with DKD. The incidence of diabetes-related ESKD has remained steady over the past 10 years, whereas its prevalence rate has been continuously increasing.
DKD prevalence was reported by analyzing the 2011 KNHAN ES data. Among patients with DM, the prevalence of albuminuria was 26.7% and low eGFR was 8.6% [12]. In the current study, the results show a small decrease in albuminuria to 22.0% and low eGFR to 6.7%, compared to the previous study. This might be, in part, due to the characteristics of the KNHANES program which uses different samplings in different periods. In addition, a total target population of each period of KNHANES is about 10,000 individuals, including only around 2,000 diabetes patients. Therefore, it is difficult to consider these results as fully representative of all diabetes patients in Korea. Therefore, it is cautious to conclude that the prevalence of DKD is decreasing. Nevertheless, compared with multiple previous reports [11,12], it appears that the prevalence of DKD in Korea over the past 10 years at least has not increased significantly. This is somewhat surprising since the prevalence of DM in Korea has been continuously increasing, and the proportion of elderly population among them has also been increasing [24]. Thus, it is likely that there have been improvements in the prevention and management of DKD. In addition, this study confirmed that age was a significant factor in the occurrence of DKD, as the prevalence of DKD increased linearly with age. DKD was reported in 9.6% and 37.3% of patients with DM in the age groups of 30 and ≥70 years, respectively. Two-thirds of all DKD patients were aged ≥60 years, necessitating more rigorous DKD prevention and management for older patients.
In this study, the prevalence of DM with reduced eGFR without albuminuria, the so-called normoalbuminuric DKD, 3.4% among DM patients and 13.4% among DKD patients, which is lower than those reported by other countries [25,26]. However, several epidemiologic studies have indicated that the prevalence of albuminuria declined while that of a reduced GFR increased [9,27]. Some possible explanations include the wide introduction of RAS blockers, specifically in older patients with multiple cardiovascular risk factors [2]. Ethnic differences or nondiabetic etiologies for the development of CKD might also be involved [1]. The increasing use of SGLT2 inhibitors, which lower albuminuria or prevent the progression of albuminuria, is expected to change the phenotype of DKD in the near future. Because we do not have sufficient secular information on the prevalence of DKD, we are unsure whether normoalbuminuric DKD has been increasingly prevalent in South Korea, which requires further investigation.
As anticipated, the comorbidity rate among the patients with DKD was high. Of these patients, 73.6% had hypertension, 82.3% had dyslipidemia, and 65.4% had central obesity, all of which were higher than in patients without DKD. The control rates of HbA1c and BP were also unsatisfactory in patients with DKD. Only 24% of patients achieved the target HbA1c level (<6.5%), and the rate of achieving the target BP (<130/80 mm Hg) was only 38.1%. Patients with DKD were evaluated to have a high risk of ASCVD, thus requiring an optimal LDL-C target of less than 70 mg/dL according to the current guidelines for dyslipidemia management [28]. However, only 28.1% of the patients reached the target LDL-C level. Interestingly, the LDL-C target achievement rate in patients with DKD was higher than that in patients without DKD. Previous studies have also observed that CKD patients have lower LDL-C levels than the general population, possibly due to the Friedewald formula underestimating LDL-C levels in CKD patients [29]. More aggressive lipid-lowering medication use may have been common in patients with DKD. Nonetheless, only 2.7% of the patients achieved all strict target levels for metabolic indicators (HbA1c <6.5%, BP <130/80 mm Hg, and LDL-C <70 mg/dL), indicating the need for more aggressive management.
Currently, SGLT2 inhibitors are the first line of treatment for patients with DKD according to current diabetes management guidelines [15,16]. The aggressive recommendation for SGLT2 inhibitors in patients with DKD was introduced in the Korean Diabetes Association clinical guidelines in 2021. Before 2021, the use of SGLT2 inhibitors was not recommended to patients with an eGFR <60 mL/min/1.73 m2 by the regulation of the Korean Ministry of Food and Drug Safety, which may explain the low prescription rate of SGLT2 inhibitors before 2021. According to the recent reports indicating more prevalent use of SGLT2 inhibitors than before in South Korea, it is expected to increase in the use of SGTL2 inhibitors in patients with DKD as well as DM.
DM is the most common cause of ESKD in many developed and developing countries, including South Korea [30,31]. In this study, the prevalence of diabetes-related ESKD continued to increase. The incidence of diabetes-related ESKD remains at approximately 200 per 100,000 person-years or may even decrease slightly. Specifically, the incidence and prevalence rates increased in the population aged ≥80 years, whereas in those aged ≤80 years, the incidence rate gradually decreased. These findings can be interpreted in several ways. The increase in the prevalence of diabetes-related ESKD is likely due to an increase in the prevalence of DM as well as an increase in the lifespan of patients with diabetes. The decrease in the incidence rate in patients aged ≤80 years could be attributed to increased health screening, leading to an earlier diagnosis of DM and delayed progression of DKD. Nevertheless, the continuous increase in the incidence rate in patients aged ≥80 years highlights the need for more proactive management of DKD risk factors over an extended period.
A decline in kidney function is an independent risk factor for ASCVD and mortality [6,13] consistent with the findings of this study. Patients with DKD stage 3 had a 1.6 times higher risk of MI, 1.47 times higher risk of stroke, 2.05 times higher risk of cardiovascular death, and 1.5 times higher overall mortality risk than those with normal kidney function. As DKD progresses to ESKD, these risks further increase. The low control rates of comorbidities in patients with DKD might have contributed to increased morbidity and mortality risks.
This study has some limitations. First, the definition of DKD simply relies on the presence of albuminuria or low eGFR in patients with DM; therefore, the exact terminology of DKD is CKD in DM or diabetes-related CKD. However, most epidemiological studies generally accept this somewhat imprecise definition because of difficulties in the precise discrimination between DKD and CKD in patients with DM with other etiologies in routine clinical practice. Second, information on albuminuria was not included in the NHIS database, and trends in the prevalence and incidence of DKD were not determined. Third, the number of patients with DM was approximately 2,000 in the KNHANES VIII, which may not guarantee sufficient power to detect precise statistics on DKD.
This study reported recent statistics on DKD and diabetes-related ESKD in South Korea. This would serve as fundamental data for the future treatment and management of patients with DKD.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0310.
Supplementary Table 1.
Trends in the prevalence and incidence of diabetes-related ESKD-NHIS database (2008 to 2019)
Supplementary Table 2.
Prescription rates of SGLT2 inhibitors and RAS blockers in DKD patients (2012 to 2019)
Notes
REFERENCES
1. Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015; 1:15018.

2. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018; 25:121–32.

3. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease: with emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983; 32 Suppl 2:64–78.

4. Thomas MC, Macisaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care. 2009; 32:1497–502.

5. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006; 55:1832–9.
6. Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009; 20:1813–21.

7. Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med. 2022; 386:2120–8.

8. Persson F, Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl (2011). 2018; 8:2–7.

9. Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016; 316:602–10.

10. Guo K, Zhang L, Zhao F, Lu J, Pan P, Yu H, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: cross-sectional study. J Diabetes Complications. 2016; 30:803–10.

11. Yang CW, Park JT, Kim YS, Kim YL, Lee YS, Oh YS, et al. Prevalence of diabetic nephropathy in primary care type 2 diabetic patients with hypertension: data from the Korean Epidemiology Study on Hypertension III (KEY III study). Nephrol Dial Transplant. 2011; 26:3249–55.

12. Ahn JH, Yu JH, Ko SH, Kwon HS, Kim DJ, Kim JH, et al. Prevalence and determinants of diabetic nephropathy in Korea: Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2014; 38:109–19.

13. Palsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014; 21:273–80.

14. Sasso FC, De Nicola L, Carbonara O, Nasti R, Minutolo R, Salvatore T, et al. Cardiovascular risk factors and disease management in type 2 diabetic patients with diabetic nephropathy. Diabetes Care. 2006; 29:498–503.

15. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021; 45:461–81.

16. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 11. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S191–202.
17. Neumiller JJ, Alicic RZ, Tuttle KR. Overcoming barriers to implementing new therapies for diabetic kidney disease: lessons learned. Adv Chronic Kidney Dis. 2021; 28:318–27.

18. Yildirim T, Arici M, Piskinpasa S, Aybal-Kutlugun A, Yilmaz R, Altun B, et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Ren Fail. 2012; 34:1095–9.

19. Garcia-Donaire JA, Ruilope LM. Cardiovascular and renal links along the cardiorenal continuum. Int J Nephrol. 2011; 2011:975782.
20. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77.

21. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.

22. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. 2022; 11:103–10.

23. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010; 5:1003–9.

24. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J. 2018; 42:93–100.

25. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013; 24:302–8.

26. Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2013; 36:2301–10.
27. Kume S, Araki SI, Ugi S, Morino K, Koya D, Nishio Y, et al. Secular changes in clinical manifestations of kidney disease among Japanese adults with type 2 diabetes from 1996 to 2014. J Diabetes Investig. 2019; 10:1032–40.
28. Yang YS, Kim HL, Kim SH, Moon MK; Committee of Clinical Practice Guideline; Korean Diabetes Association and Clinical Practice Guideline Committee; Korean Society of Lipid and Atherosclerosis. Lipid management in Korean people with type 2 diabetes mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis consensus statement. J Lipid Atheroscler. 2023; 12:12–22.

29. Bauer F, Seibert FS, Rohn B, Babel N, Westhoff TH. Estimation of LDL cholesterol in chronic kidney disease. Eur J Prev Cardiol. 2021; 28:1402–8.

Fig. 1.
Prevalence of diabetic kidney disease (DKD) in South Korea-Korea National Health and Nutrition Examination Survey database 2019 to 2021. (A) Prevalence of DKD (aged ≥30 years). (B) Proportion of DKD with and without albuminuria. (C) Prevalence of DKD by age groups. (D) Distribution of age in DKD.
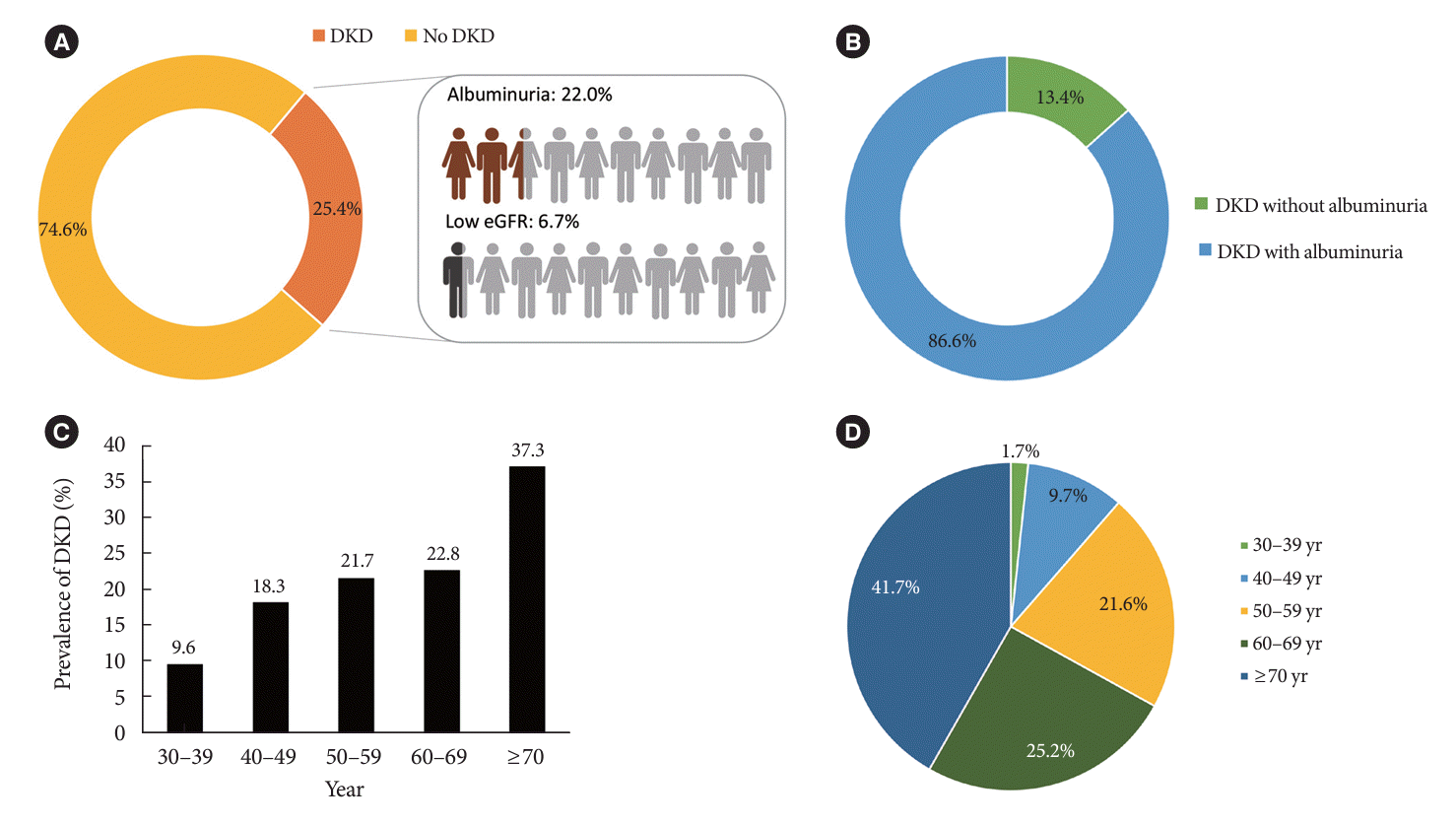
Fig. 2.
Prevalence and control rate of comorbidities in patients with and without diabetic kidney disease (DKD)-Korea National Health and Nutrition Examination Survey database 2019 to 2021. (A) Prevalence of comorbidities in patients with and without DKD. (B) Optimal control rate of hyperglycemia and comorbidities in patients with and without DKD. Comprehensive control rate (1) indicates the proportion of patients whose glycosylated hemoglobin (HbA1c) <6.5%, blood pressure (BP) <130/80 mm Hg, and low-density lipoprotein-cholesterol (LDL-C) <70 mg/dL. Comprehensive control rate (2) indicates the proportion of patients whose HbA1c <7.0%, BP <140/90 mm Hg, and LDL-C <100 mg/dL.
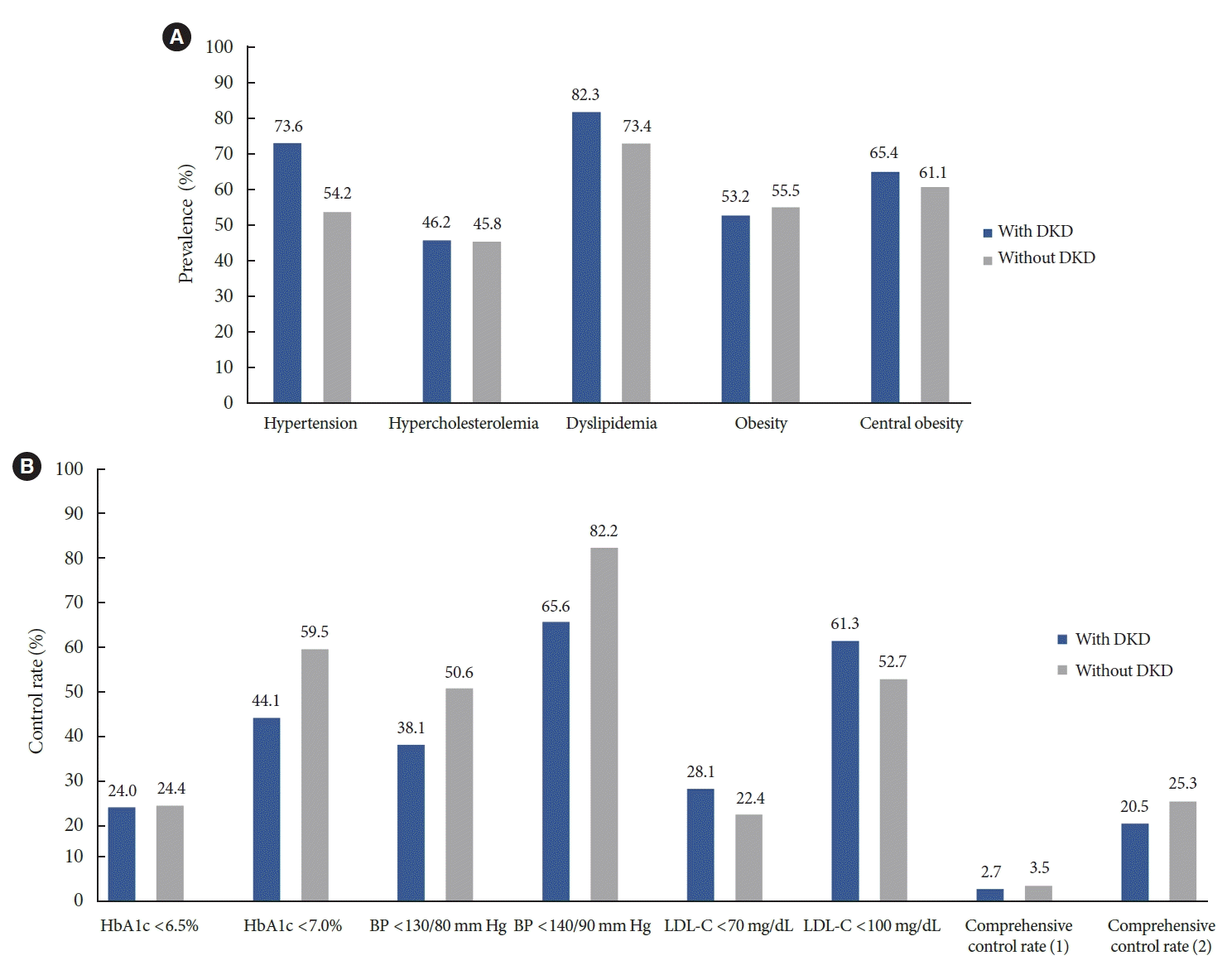
Fig. 3.
Trends in the prevalence and incidence rate of diabetes-related end-stage kidney disease (ESKD)-National Health Insurance Service (NHIS) database 2008 to 2019. (A) Age, and sex-standardized prevalence and incidence rate of diabetes-related ESKD. (B) Prevalence rate of diabetes-related ESKD by age group. (C) Incidence rate of diabetes-related ESKD by age group.
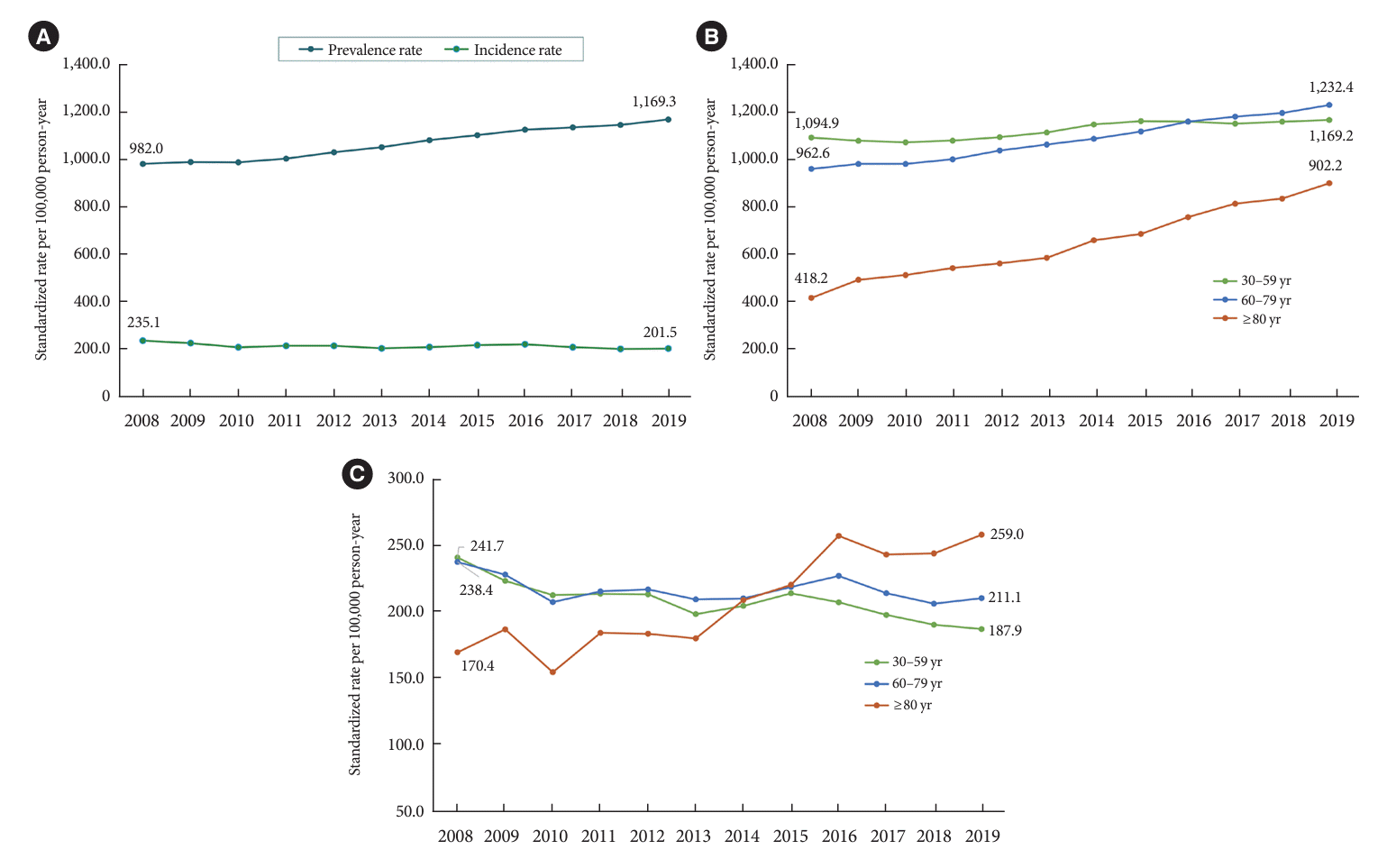
Fig. 4.
Prescription rates of (A) sodium glucose cotransporter 2 inhibitors and (B) renin-angiotensin system blockers in diabetic kidney disease National Health Insurance Service database 2014 to 2019. DKD, diabetic kidney disease.
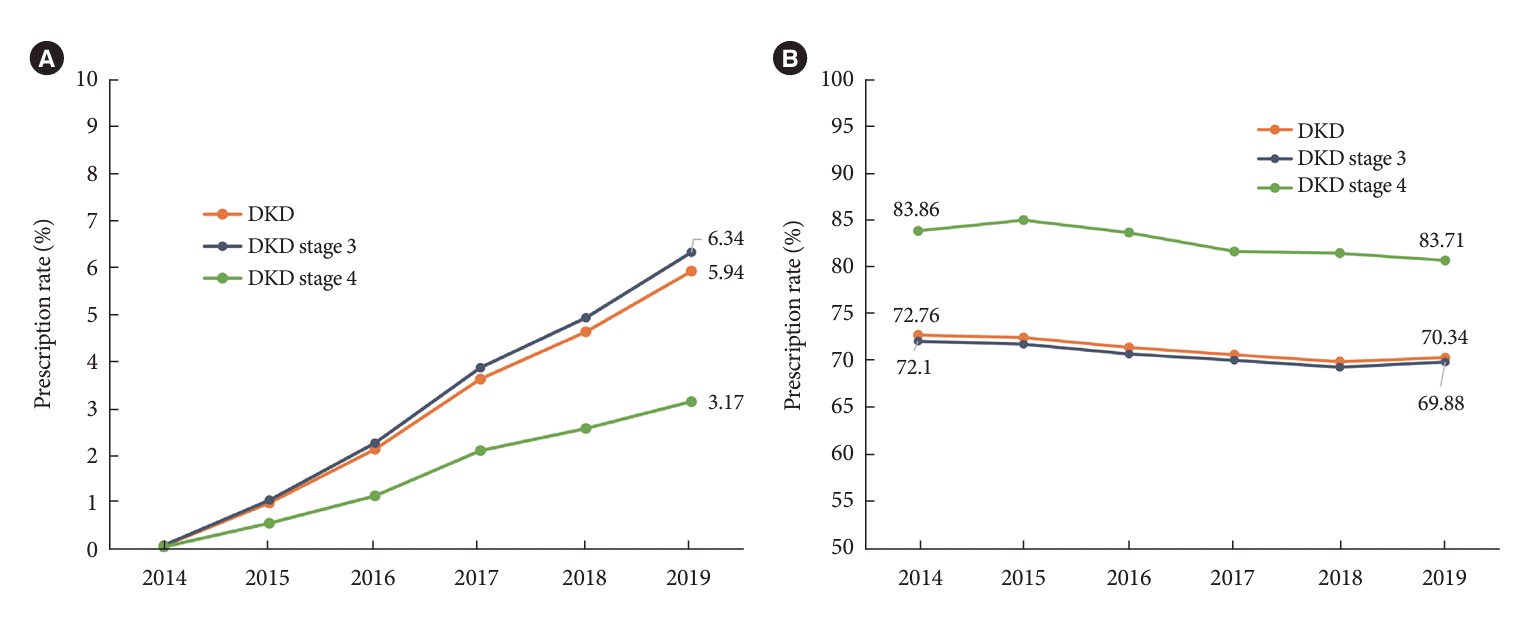
Table 1.
Risk of atherosclerotic cardiovascular disease and mortality according to eGFR
| Variable |
eGFR, mL/min/1.73 m2 |
||||
|---|---|---|---|---|---|
| ≥90 | 60–89 | 30–59 | <30 | ESKD | |
| Myocardial infarction | |||||
| Incidence ratea | 3.0 | 4.5 | 8.1 | 15.7 | 19.9 |
| Unadjusted HR | 1.00 (ref) | 1.52 (1.48–1.57) | 2.74 (2.64–2.84) | 5.31 (4.91–5.74) | 6.67 (5.96–7.46) |
| Adjustedb HR | 1.00 (ref) | 1.15 (1.11–1.19) | 1.60 (1.54–1.66) | 3.00 (2.77–3.25) | 4.87 (4.35–5.45) |
| Stroke | |||||
| Incidence ratea | 4.0 | 6.9 | 12.7 | 19.7 | 22.5 |
| Unadjusted HR | 1.00 (ref) | 1.72 (1.68–1.76) | 3.17 (3.08–3.27) | 4.91 (4.58–5.27) | 5.56 (5.00–6.19) |
| Adjustedb HR | 1.00 (ref) | 1.13 (1.10–1.16) | 1.47 (1.42–1.52) | 2.25 (2.09–2.42) | 3.85 (3.46–4.28) |
| Cardiovascular death | |||||
| Incidence ratea | 1.2 | 2.7 | 7.6 | 16.2 | 13.7 |
| Unadjusted HR | 1.00 (ref) | 2.44 (2.33–2.55) | 6.86 (6.53–7.20) | 15.03 (13.73–16.44) | 12.83 (11.03–14.92) |
| Adjustedb HR | 1.00 (ref) | 1.20 (1.14–1.26) | 2.05(1.94–2.17) | 4.64 (4.23–5.10) | 7.27 (6.24–8.46) |
| All-cause death | |||||
| Incidence ratea | 7.6 | 14.2 | 32.3 | 76.5 | 84.7 |
| Unadjusted HR | 1.00 (ref) | 1.87 (1.83–1.90) | 4.28 (4.20–4.37) | 10.47 (10.06–10.90) | 11.69 (11.00–12.42) |
| Adjustedb HR | 1.00 (ref) | 0.99 (0.97–1.01) | 1.50 (1.47–1.54) | 3.90 (3.74–4.07) | 7.40 (6.96–7.87) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download