Abstract
Heart failure (HF) management guidelines recommend individualized assessments based on HF phenotypes. Adiposity is a known risk factor for HF. Recently, there has been an increased interest in organ-specific adiposity, specifically the role of the epicardial adipose tissue (EAT), in HF risk. EAT is easily assessable through various imaging modalities and is anatomically and functionally connected to the myocardium. In pathological conditions, EAT secretes inflammatory cytokines, releases excessive fatty acids, and increases mechanical load on the myocardium, resulting in myocardial remodeling. EAT plays a pathophysiological role in characterizing both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). In HFrEF, EAT volume is reduced, reflecting an impaired metabolic reservoir, whereas in HFpEF, the amount of EAT is associated with worse biomarker and hemodynamic profiles, indicating increased EAT activity. Studies have examined the possibility of therapeutically targeting EAT, and recent studies using sodium glucose cotransporter 2 inhibitors have shown potential in reducing EAT volume. However, further research is required to determine the clinical implications of reducing EAT activity in patients with HF.
· EAT, easily assessed via imaging, is anatomically and functionally connected to the myocardium.
· Cytokines and fatty acids from EAT worsen myocardial remodeling, causing HF.
· In HFrEF, reduced EAT volume indicates metabolic dysfunction.
· Conversely, in HFpEF, higher EAT levels correlate with adverse hemodynamic profiles.
· EAT may serve as a target for HF therapies.
The epicardial adipose tissue (EAT) is a visceral adipose tissue (VAT) located in the heart [1]. With an increased interest in organ-specific adiposity, a close association between EAT and heart failure (HF) has been suggested because of its anatomical proximity to the heart. EAT is located in the interventricular or atrioventricular groove and surrounds nearly all coronary arteries, constituting 15% to 20% of the heart mass [2]. Fig. 1 shows the gross anatomy of EAT in an 81-year-old woman with threevessel coronary artery disease (CAD) who underwent a coronary artery bypass graft. EAT has unique characteristics compared to those of the pericardial adipose tissue (PAT), as EAT is located between the myocardium and visceral pericardium, has an embryonic origin from the splanchnopleuric mesoderm, and is supplied by branches of the coronary artery. In contrast, PAT originates from the primitive thoracic mesenchyme and is vascularized by branches of the internal mammary artery [3]. EAT and the myocardium share the same blood supply, and there is no fascia separating them histologically, making them anatomically and functionally contiguous [4]. Some studies do not differentiate between EAT and PAT, describing PAT as a broader concept that includes EAT. However, this study specifically concentrates on EAT as a true VAT of heart [5].
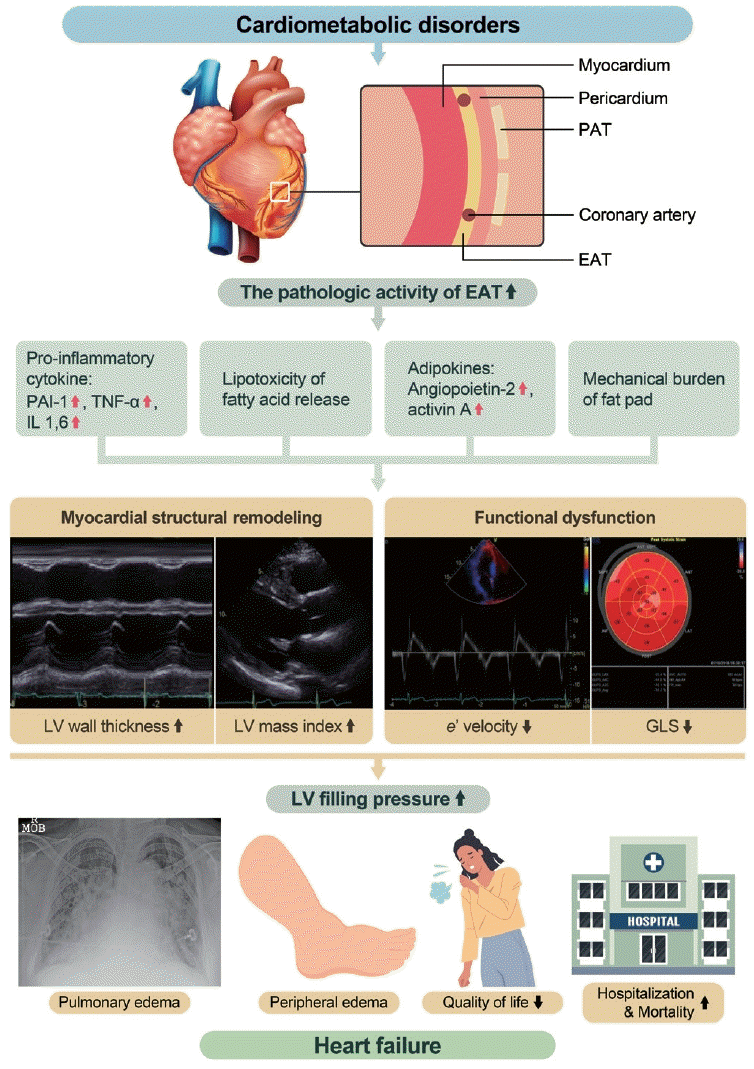
EAT is a bioactive organ that secretes several adipokines, including pro-inflammatory and pro-fibrotic cytokines, which can induce myocardial remodeling and dysfunction [6]. In pathological conditions, such as obesity, EAT releases excessive free fatty acids (FFAs) from its lipid store, which can induce lipotoxic effects on the myocardium [7]. The close proximity of EAT to the myocardium and its secretion of pathological cytokines and FFAs have led to investigations into the role of EAT in the development and progression of HF [8].
Despite the dramatic improvement in short- and long-term outcomes of HF with established guideline-directed medical therapy, HF remains a significant health burden worldwide with increasing prevalence [9-13]. Current HF guidelines emphasize the importance of individualized assessment and management according to HF phenotypes [14-16]. Obesity is a wellknown risk factor for HF, as it is associated with a high-volume load, impaired insulin resistance, and metabolic disturbances [17]. Several surrogate markers of adiposity reflect the risk of HF. In this review, we discuss the pathological role of EAT on the myocardium and its association with HF, as well as the possibility of EAT as a therapeutic target in HF.
In the current era of multi-modal imaging, various tools are available to assess the amount and location of EAT. Echocardiography is a non-invasive and relatively inexpensive technique that can easily evaluate the amount of EAT [4,18]. The presence of EAT between the myocardium and the visceral layer of the pericardium can be identified by echocardiography. Representative echocardiographic images of EAT are shown in Fig. 2. Echocardiographic EAT thickness is typically defined as the maximal thickness during end-systole [2,19,20] and is measured perpendicular to the right ventricle (RV) free wall of the aortic annulus in the parasternal long-axis view. Echocardiographic EAT thickness is a well-validated and reproducible marker in the general population and in patients with various cardiovascular diseases (CVDs), with EAT thicknesses ranging from 1 to 23 mm [1,21,22]. In a study of a large population, mean EAT thickness values were found to be 7 mm in men and 6.5 mm in women [4]. However, echocardiography only provides a linear measurement of EAT; thus, the amount of EAT evaluated by this method may be less accurate than that measured by computed tomography (CT) or magnetic resonance imaging (MRI) [8]. Although CT and MRI may be more expensive and invasive techniques, they provide a more accurate evaluation of the amount of EAT by volumetric or area assessment [23,24]. Additionally, using CT and MRI, characterization of EAT at any location with higher resolution can be achieved, thus providing more location-specific implications of EAT. For example, EAT around the left atrium (LA) is closely associated with atrial fibrillation and the LA volume index [25]. The characteristics of each imaging modality for evaluating EAT are summarized in Table 1.
Metabolic syndrome is a constellation of various cardiovascular risk factors, including hypertension, abdominal obesity, dyslipidemia, and insulin resistance [26]. Although body mass index (BMI) is commonly used as a marker of general obesity, it cannot accurately reflect the burden of visceral obesity because it does not differentiate between fat content and muscle mass [27,28]. Abdominal obesity, as assessed by measuring waist circumference (WC), is a well-established risk factor for myocardial dysfunction and development of CVDs [29,30]. For a more detailed evaluation, the amount of VAT can be quantified using bioelectrical impedance analysis. We have previously reported that VAT, as evaluated by bioelectrical impedance analysis, but not abdominal obesity evaluated by measuring WC, is correlated with myocardial structural and functional remodeling in individuals with early dysmetabolic states [17]. VAT secretes pro-inflammatory and pro-atherogenic cytokines that have adverse systemic effects on the cardiovascular system [31]. Moreover, VAT is an active source of several adipokines. In pathological conditions, dysregulated secretion of adiponectin and leptin contributes to changes in myocardial fatty acid metabolism, resulting in myocardial remodeling and dysfunction [32].
EAT, a component of VAT located in the heart, has metabolic, mechanical, and thermogenic roles. The heart is a vital organ with a high energy demand and no energy reserves in the myocardium. FFA oxidation through β-oxidation is a major source of energy, accounting for approximately 40% to 60% [33]. EAT has the ability to release and uptake FFAs, providing energy to the heart. Additionally, EAT acts as a buffer to protect the myocardium from exposure to toxic levels of FFA [34]. EAT also surrounds the epicardial coronary arteries, acting as a cushion, protecting against mechanical torsion [35]. EAT has a brown adipose tissue-like function that supplies heat to the myocardium [36].
In pathological conditions, EAT has unique effects on the myocardium compared to other VATs. Studies have investigated the inflammatory role of EAT and showed that CD8-positive T-cells and macrophage infiltration in EAT are highly increased in patients with CAD compared with those without CAD [37]. Furthermore, the level of serine proteinase inhibitor A3, which regulates the inflammatory status, was significantly increased in the EAT of patients with HF compared to those without HF [38]. In a study comparing EAT and subcutaneous adipose tissue (SAT) samples in patients with HF, p53 mRNA levels were highly up-regulated in EAT compared to those in SAT [39]. Chronic activation of p53 leads to the production of reactive oxygen species, adipose tissue apoptosis, and tissue inflammation [39]. In another study using EAT and SAT samples, p53 mRNA, which causes inflammation, was found to accelerate coronary atherosclerosis and myocardial remodeling. Notably, the inflammatory activity of EAT is not limited to local effects but also has systemic effects. EAT was found to be linearly related to high-sensitivity C-reactive protein (hs-CRP) levels in asymptomatic individuals who underwent a cardiovascular health survey [40]. Additionally, EAT thickness was reported to be linearly associated with hs-CRP levels in middle-aged men with suspected metabolic syndrome [41].
In addition to its inflammatory role, EAT is also involved in the regulation of myocardial energy metabolism. EAT stores FFAs and directly supplies them to the myocardium as an energy source. However, in pathological conditions, excessive release of fatty acids from EAT can lead to lipid infiltration into the myocardium, because EAT and the myocardium share the same coronary microcirculation and have no fascia separating them [42]. Several studies have reported an association between EAT volume and myocardial fat content [43,44]. For instance, a cardiac MRI study of healthy participants showed that increased EAT volume was independently associated with higher myocardial triglyceride content and worse left ventricular contractile function, even after controlling for visceral adiposity and other covariates [45]. This study also found an association between EAT volume and myocardial interstitial fibrosis, as assessed by T1 mapping. Another study showed that increased myocardial fat content was correlated with left ventricular diastolic dysfunction, particularly in patients with HF with preserved ejection fraction (HFpEF), indicating the potential role of EAT in the pathogenesis of HFpEF [46]. In addition to direct lipid infiltration, EAT has been implicated in the development of myocardial dysfunction through the dysregulated secretion of adipokines. Experimental studies using EAT samples have shown that EAT secretes adipokines, such as angiopoietin-2 and activin A, which can induce cardiomyocyte contractile dysfunction and altered cytosolic calcium fluxes in a dose-dependent manner [47].
EAT is a large fat pad that surrounds the myocardium. This protects the coronary arteries within EAT against torsion. However, excessive amounts of EAT can cause a mechanical burden on the myocardium, leading to myocardial dysfunction. This negative relationship between EAT and myocardial function has been observed in elderly women, with a stronger association in the lateral e´ and s´ than in the septal e´ and s´ [48]. This supports the hypothesis that the mechanical burden of EAT induces myocardial dysfunction.
In addition to its mechanical effects, EAT also plays an important role in the pathogenesis of coronary atherosclerosis. EAT surrounds the epicardial coronary artery and shares microcirculation. Inflammatory cytokines, adipokines, and metabolic substrates secreted from EAT affect the pathogenesis of coronary atherosclerosis [49]. EAT is not equally distributed throughout the heart and tends to accumulate excessively at the focal site. Thus, EAT appears to be a transducer of metabolic disturbances and systemic inflammation in the underlying coronary artery [42]. In patients with suspected CAD, EAT thickness is independently associated with the presence of obstructive CAD and vasospasm, as confirmed by invasive angiography [50]. Moreover, studies using CT have demonstrated that an increased EAT volume is related to the vulnerable type of plaque, suggesting that EAT may contribute to the progression and vulnerability of coronary plaque [51]. Interestingly, EAT has also been implicated in coronary microvascular dysfunction, which plays an important role in the development of HF without obstructive CAD [52]. In patients without obstructive CAD, increased EAT volume is related to microvascular dysfunction, as evaluated using Rb-82 positron emission tomography [53]. These findings suggest that EAT is involved not only in the pathogenesis of epicardial CAD, but also in coronary microcirculation dysfunction. Fig. 3 summarizes the pathophysiologic mechanism of EAT.
The association between the amount of EAT and myocardial dysfunction has been investigated in various clinical situations, including the general population, patients with metabolic disease without CVD, and patients with established CVD. We previously reported that a greater EAT thickness was correlated with a higher left ventricle (LV) mass index, worse LV systolic dysfunction represented by LV global longitudinal strain, and LV diastolic dysfunction in patients with suspected metabolic syndrome and no overt CVD [1]. In elderly women without established CVD, an increase in the EAT amount was related to worse LV systolic and diastolic dysfunction [48]. In patients with acute myocardial infarction, EAT amount progressively increased according to the grade of LV diastolic dysfunction [54].
Excessive epicardial fat pad has an adverse effect on the myocardium. The amount of EAT varies according to the HF subtype: HF with reduced ejection fraction (HFrEF) or HFpEF. Fig. 4 shows the EAT amount in the HFrEF, HFpEF, and control groups. When patients with HFrEF and controls were compared, both cardiac MRI and echocardiography showed that the EAT amount was consistently reduced in patients with HFrEF than in controls [55-57]. However, the EAT amount in the HFpEF group was reported to increase in two studies and decrease in one study, when compared with that in the control group [58-60]. As the HFpEF study population was heterogeneous, this might have led to conflicting results. Few studies have compared patients with HFrEF and HFpEF with controls. In the study by Tromp et al. [61], which utilized both cardiac magnetic resonance (CMR) and echocardiography, it was observed that EAT thickness, as measured by echocardiography, was lower in HFrEF compared to the control group. However, EAT mass, as determined by CMR, was higher in the HFrEF group. It’s worth noting that the method of measuring EAT can significantly influence the study’s findings. Echocardiography primarily assesses EAT thickness at the RV free wall, whereas CMR provides a more comprehensive evaluation of EAT mass across the entire heart. Given that EAT covers a larger portion of the heart in cases of larger heart mass, Tromp et al. [61] indexed EAT mass to heart mass, revealing that the indexed EAT mass was lower in the HFrEF group when compared to the control. Thus, for CMR studies, it is necessary to measure EAT mass and adjust for body surface area.
Although the exact explanation for different status of EAT according to HF classification is not clear, we can propose some possible explanations. Studies with HFrEF mainly enrolled patients with ischemic cardiomyopathy and dilated cardiomyopathy. These advanced HF conditions not only leads to LV dysfunction but also RV dysfunction, followed by intestinal congestion. Intestinal congestion in HF can lead to anorexia and poor nutrition absorption, resulting in catabolic conditions such as sarcopenia [62]. This might be related to the decreased volume of EAT in HFrEF. Conversely, HFpEF is frequently accompanied by obesity, and this induces an increase in intravascular volume, which imposes hemodynamic stress by increasing myocardial workload [30]. Given the higher proportion of obese patients in HFpEF, EAT volume may increase in HFpEF. However, this issue requires further precise research focused on the classification of HFrEF and HFpEF. Pugliese et al. [63] conducted a more advanced study analyzing EAT thickness in patients with HFrEF, HFpEF, and controls and investigated the association between EAT thickness and multiple biomarkers and cardiorespiratory fitness evaluated by peak oxygen consumption. Increased EAT thickness was associated with worse cardiorespiratory fitness, biomarker profile, RV-pulmonary arterial uncoupling, and mortality in patients with HFpEF. Conversely, reduced EAT thickness was associated with worse LV dysfunction and mortality in patients with HFrEF. This study showed that EAT had a pathophysiological role in the characterization of HFrEF and HFpEF. Because EAT has a brown adipose tissue-like function, it serves as an energy reservoir and reflects the catabolic status of HFrEF. In HFpEF, EAT is associated with a worse biomarker profile, suggesting enhanced EAT activity. In HFpEF, EAT maybe associated with hemodynamic stress and poor cardiorespiratory fitness, leading to increased adverse events. In the study with HF with midly reduced ejection fraction and HFpEF, EAT accumulation was associated with the adverse prognosis after adjusting for the conventional risk factors and the severity of HF [64].
Contrary to the findings of various studies on EAT and prevalent HF, the implications of EAT burden on the development of HF have rarely been investigated. The Multi-Ethnic Study of Atherosclerosis group explored whether the baseline pericardial fat volume, including epicardial and pericardial fat, was linked to the occurrence of HF [65]. In this study, pericardial fat volume was evaluated in 6,785 community-based individuals by CT. Pericardial fat volume was linearly associated with an increased risk of HF after adjustment for baseline characteristics, including anthropometric parameters (1-standard deviation increase in pericardial fat volume: hazard ratio, 1.22; 95% confidence interval, 1.12 to 1.31; P<0.001). In the Jackson Heart study with 2,882 participants without prevalent HF, a higher volume of PAT and VAT was associated with an increased risk of HFpEF. Although the replication of these findings is warranted in other community-based studies, this study suggested that increasing the amount of EAT in a community-based population might be a novel risk factor for newly diagnosed HF.
EAT can be visualized and quantified using two-dimensional echocardiography or other imaging modalities. EAT volume changes more quickly than other anthropometric parameters of body fat [66]. The role of EAT as a therapeutic target has been elucidated using several emerging cardiovascular drugs. A randomized controlled trial (RCT) evaluated the effect of an intensive dose of atorvastatin and a moderate dose of pravastatin on the progression of the coronary calcium score evaluated by CT [67]. The investigators also evaluated the effect of statins on changes in the EAT volume. Only the intensive-intensity statin group showed significantly decreased EAT volume but not the moderate-intensity group. The degree of lipid reduction did not correlate with EAT regression. This finding suggested that the effect of statins on EAT volume might be related to pleiotropic effects, such as anti-inflammatory effects, and not lipid-lowering effects. In patients with aortic stenosis, statin treatment was associated with lower EAT thickness and an in vitro statin-modulated inflammatory profile of human EAT [68]. In patients with coronary artery stenosis, the use of 20-mg atorvastatin was associated with lower EAT thickness than that with the use of 10-mg simvastatin and ezetimibe [69]. However, the effects of statins on EAT thickness in patients with HF require further investigation.
The lipolytic effect of sodium glucose cotransporter 2 (SGLT2) inhibitors, known as the statins of the 21st century [70], has been investigated with a focus on VAT, including EAT [71]. SGLT2 inhibitors increase glucose excretion in the urine, leading to reduced blood glucose levels. Consequently, insulin secretion decreases, and the release of glucagon, which has counter-regulatory effects, increases. Unlike insulin, which promotes lipogenesis and lowers blood glucose, glucagon stimulates lipolysis, elevating blood glucose levels. In other words, SGLT2 inhibitors promote the lipolysis through increased glucagon secretion, as evidenced by several studies showing that the use of SGLT2 inhibitors can reduce visceral obesity [72,73]. Lipolysis results in an increase in blood ketone levels, which can explain one of the notable side effects of SGLT2 inhibitors, known as ketoacidosis. Interestingly, blood ketones serve as an efficient metabolic energy source for the heart. Given the heart’s continuous need for hemodynamic energy, it relies on various substances as energy sources, making it a ‘metabolic omnivore.’ Since ketone produce highest adenosine triphosphate via ketone oxidation, ketone is often referred to as ‘the superfuel of the heart.’ This rise in blood ketone levels enhances the energy efficiency of the heart, leading to an improved prognosis for HF. In a non-diabetic pig model, use of SGLT2 inhibitor improved myocardial energetics and increased the myocardial ketone uptake [74]. This may explain the outstanding findings of improved HF-related outcomes with SGLT2 inhibitors (Fig. 5) [75]. Based on these findings, the effect of SGLT2 inhibitors on lowering EAT volume and activity has been studied in several studies, including RCTs [72,76-81]. Table 2 summarizes the major RCTs that have investigated the effects of SGLT2 inhibitors on EAT volume. The Iacobellis group studied the effect of dapagliflozin compared to placebo in patients with type 2 diabetes mellitus with a BMI ≥27 kg/m2 [79]. Dapagliflozin reduced EAT thickness by 20% from baseline in 6 months. The reduction in EAT thickness was higher in the dapagliflozin group than in the metformin group. In this study, the reduction in EAT thickness did not correlate with weight loss, suggesting that the EAT reduction effect of SGLT2 inhibitors might be mediated beyond weight loss. Requena-Ibanez et al. [81] performed an RCT comparing empagliflozin and placebo in patients with HFrEF without diabetes. Empagliflozin reduced the EAT volume compared to the placebo, as evaluated by cardiac MRI after 6 months. EAT reduction was associated with improvements in inflammatory biomarkers, myocardial fibrosis, and aortic stiffness. The lipolytic effect of SGLT2 inhibitors on EAT volume varies according to the study population, drugs, study duration, and imaging modalities. A few studies have failed to demonstrate the effects of SGLT2 inhibitors on EAT volume [77,78]. These studies enrolled a small-sized population with a low cardiovascular risk, and the study period was short. A meta-analysis of RCTs concluded that SGLT2 inhibitors significantly reduced EAT volume in patients with type 2 diabetes mellitus [80]. The effect of SGLT2 inhibitors on the amount of EAT and whether the intervention-induced reduction of EAT volume or activity leads to the improvement in clinical outcomes of HF remains unclear. Therefore, further studies are required to address this issue.
A recent study demonstrated that glucagon-like peptide-1 receptor agonists (GLP-1RA) improved quality of life in patients with HFpEF and obesity [82]. GLP-1RA also exhibit pleiotropic effects, such as weight loss and cardiovascular protection beyond glucose control. They reduce appetite, delay gastric emptying, and can even alter body fat distribution [72]. EAT expresses receptors for GLP-1, and GLP-1RA have been found to be associated with increased gene expression related to the differentiation of white to brown adipose tissue and decreased expression of pro-adipogenic genes [83]. In light of this background, researchers have explored the effects of GLP-1RA injection on EAT. In patients with type 2 diabetes mellitus and obesity, a 24-week liraglutide treatment successfully reduced echocardiographic EAT thickness from 9.6±2.0 to 6.2±1.5 mm, marking a 36% reduction [84]. Long-acting GLP-1RA, such as semaglutide, also decreased EAT thickness in a dose-dependent manner [85]. In a study involving liraglutide, the reduction in EAT thickness was independently correlated with LV mass reduction [84]. This suggests that the reduction of EAT thickness by GLP-1RA could serve as a surrogate marker for myocardial reverse remodeling. Further studies are needed to investigate the association between EAT reduction and cardiovascular protection.
EAT is a promising biomarker for identifying the HF phenotype and guiding treatment strategies because of its close anatomical and functional connection with the myocardium. It is easily measurable and modifiable, which makes it an attractive therapeutic target. As contemporary medicine for HF emphasizes individualized approaches based on HF phenotypes, further studies are required to fully explore the therapeutic potential of EAT.
REFERENCES
1. Cho DH, Joo HJ, Kim MN, Lim DS, Shim WJ, Park SM. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc Diabetol. 2018; 17:95.

2. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005; 2:536–43.

4. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009; 22:1311–9.

5. Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring). 2009; 17:625.

6. Fei J, Cook C, Blough E, Santanam N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis. 2010; 212:488–94.

7. Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022; 19:593–606.

8. Song Y, Song F, Wu C, Hong YX, Li G. The roles of epicardial adipose tissue in heart failure. Heart Fail Rev. 2022; 27:369–77.

9. Park JJ, Lee CJ, Park SJ, Choi JO, Choi S, Park SM, et al. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021; 3:224–36.

10. Lee HY, Oh BH. Paradigm shifts of heart failure therapy: do we need another paradigm? Int J Heart Fail. 2020; 2:145–56.

11. Lee KS, Noh J, Park SM, Choi KM, Kang SM, Won KC, et al. Evaluation and management of patients with diabetes and heart failure: a Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement. Diabetes Metab J. 2023; 47:10–26.

12. Cho DH, Yoo BS. Current prevalence, incidence, and outcomes of heart failure with preserved ejection fraction. Heart Fail Clin. 2021; 17:315–26.

13. Cho JY, Cho DH, Youn JC, Kim D, Park SM, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: definition and diagnosis. Int J Heart Fail. 2023; 5:51–65.

14. Kim KJ, Cho HJ, Kim MS, Kang J, Kim KH, Kim D, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019; 1:4–24.

15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–726.

16. Youn JC, Kim D, Cho JY, Cho DH, Park SM, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: treatment. Int J Heart Fail. 2023; 5:66–81.

17. Cho DH, Kim MN, Joo HJ, Shim WJ, Lim DS, Park SM. Visceral obesity, but not central obesity, is associated with cardiac remodeling in subjects with suspected metabolic syndrome. Nutr Metab Cardiovasc Dis. 2019; 29:360–6.

18. Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003; 88:5163–8.

19. Iacobellis G, Barbaro G, Gerstein HC. Relationship of epicardial fat thickness and fasting glucose. Int J Cardiol. 2008; 128:424–6.

20. Malavazos AE, Ermetici F, Cereda E, Coman C, Locati M, Morricone L, et al. Epicardial fat thickness: relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr Metab Cardiovasc Dis. 2008; 18:523–30.

21. Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013; 111:73–8.

22. Fernandes-Cardoso A, Santos-Furtado M, Grindler J, Ferreira LA, Andrade JL, Santo MA. Epicardial fat thickness correlates with P-wave duration, left atrial size and decreased left ventricular systolic function in morbid obesity. Nutr Metab Cardiovasc Dis. 2017; 27:731–8.

23. Cetin M, Kocaman SA, Durakoglugil ME, Erdogan T, Ergul E, Dogan S, et al. Effect of epicardial adipose tissue on diastolic functions and left atrial dimension in untreated hypertensive patients with normal systolic function. J Cardiol. 2013; 61:359–64.

24. Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012; 220:223–30.

25. Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011; 22:647–55.

26. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005; 28:385–90.

27. Hwang IC, Choi HM, Yoon YE, Park JJ, Park JB, Park JH, et al. Body mass index, muscle mass, and all-cause mortality in patients with acute heart failure: the obesity paradox revisited. Int J Heart Fail. 2022; 4:95–109.

28. Kim SE, Lee CJ. The paradox in defining obesity in patients with heart failure. Int J Heart Fail. 2022; 4:91–4.

29. Ho JE, McCabe EL, Wang TJ, Larson MG, Levy D, Tsao C, et al. Cardiometabolic traits and systolic mechanics in the community. Circ Heart Fail. 2017; 10:e003536.

30. Shen Q, Hiebert JB, Rahman FK, Krueger KJ, Gupta B, Pierce JD. Understanding obesity-related high output heart failure and its implications. Int J Heart Fail. 2021; 3:160–71.

31. Lemieux I, Pascot A, Prud’homme D, Almeras N, Bogaty P, Nadeau A, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001; 21:961–7.
32. Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013; 18:631–44.

33. Lopaschuk GD. Metabolic modulators in heart disease: past, present, and future. Can J Cardiol. 2017; 33:838–49.

34. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015; 11:363–71.

35. Prati F, Arbustini E, Labellarte A, Sommariva L, Pawlowski T, Manzoli A, et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat?: a three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J. 2003; 24:329–36.

36. Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009; 94:3611–5.
37. Hirata Y, Kurobe H, Akaike M, Chikugo F, Hori T, Bando Y, et al. Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011; 52:139–42.

38. Zhao L, Guo Z, Wang P, Zheng M, Yang X, Liu Y, et al. Proteomics of epicardial adipose tissue in patients with heart failure. J Cell Mol Med. 2020; 24:511–20.

39. Agra RM, Teijeira-Fernandez E, Pascual-Figal D, Sanchez-Mas J, Fernandez-Trasancos A, Gonzalez-Juanatey JR, et al. Adiponectin and p53 mRNA in epicardial and subcutaneous fat from heart failure patients. Eur J Clin Invest. 2014; 44:29–37.

40. Lai YH, Yun CH, Yang FS, Liu CC, Wu YJ, Kuo JY, et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: a study validated with computed tomography. J Am Soc Echocardiogr. 2012; 25:234–41.

41. Cho DH, Joo HJ, Kim MN, Kim HD, Lim DS, Park SM. Longitudinal change in myocardial function and clinical parameters in middle-aged subjects: a 3-year follow-up study. Diabetes Metab J. 2021; 45:719–29.

42. Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017; 22:889–902.

43. Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006; 91:4689–95.

44. Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010; 105:1831–5.

45. Ng AC, Strudwick M, van der Geest RJ, Ng AC, Gillinder L, Goo SY, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imaging. 2018; 11:e007372.

46. Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, et al. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020; 22:445–54.

47. Greulich S, de Wiza DH, Preilowski S, Ding Z, Mueller H, Langin D, et al. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J Cell Mol Med. 2011; 15:2399–410.

48. Kim SA, Kim MN, Shim WJ, Park SM. Epicardial adipose tissue is related to cardiac function in elderly women, but not in men. Nutr Metab Cardiovasc Dis. 2017; 27:41–7.

49. Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005; 29:251–5.

50. Kim MN, Kim HL, Park SM, Shin MS, Yu CW, Kim MA, et al. Association of epicardial adipose tissue with coronary spasm and coronary atherosclerosis in patients with chest pain: analysis of data collated by the KoRean wOmen’S chest pain rEgistry (koROSE). Heart Vessels. 2018; 33:17–24.

51. Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010; 210:150–4.

52. Kim SR, Cho DH, Kim MN, Park SM. Rationale and study design of differences in cardiopulmonary exercise capacity according to coronary microvascular dysfunction and body composition in patients with suspected heart failure with preserved ejection fraction. Int J Heart Fail. 2021; 3:237–43.

53. Alam MS, Green R, de Kemp R, Beanlands RS, Chow BJ. Epicardial adipose tissue thickness as a predictor of impaired microvascular function in patients with non-obstructive coronary artery disease. J Nucl Cardiol. 2013; 20:804–12.

54. Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, et al. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol. 2014; 114:1663–9.

55. Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010; 12:40.

56. Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, et al. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring). 2013; 21:E253–61.

57. Tabakci MM, Durmus HI, Avci A, Toprak C, Demir S, Arslantas U, et al. Relation of epicardial fat thickness to the severity of heart failure in patients with nonischemic dilated cardiomyopathy. Echocardiography. 2015; 32:740–8.

58. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017; 136:6–19.

59. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail. 2018; 6:640–9.

60. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018; 20:1559–66.

61. Tromp J, Bryant JA, Jin X, van Woerden G, Asali S, Yiying H, et al. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail. 2021; 23:835–8.

62. Valentova M, von Haehling S, Krause C, Ebner N, Steinbeck L, Cramer L, et al. Cardiac cachexia is associated with right ventricular failure and liver dysfunction. Int J Cardiol. 2013; 169:219–24.

63. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021; 23:1858–71.

64. van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VP, Willems TP, de Boer RA, et al. Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ Heart Fail. 2022; 15:e009238.

65. Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021; 77:2638–52.
66. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011; 22:450–7.

67. Alexopoulos N, Melek BH, Arepalli CD, Hartlage GR, Chen Z, Kim S, et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic postmenopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol. 2013; 61:1956–61.
68. Parisi V, Petraglia L, D’Esposito V, Cabaro S, Rengo G, Caruso A, et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int J Cardiol. 2019; 274:326–30.

69. Park JH, Park YS, Kim YJ, Lee IS, Kim JH, Lee JH, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010; 18:121–6.
70. Braunwald E. SGLT2 inhibitors: the statins of the 21st century. Eur Heart J. 2022; 43:1029–30.
71. Kato ET, Kimura T. Sodium-glucose co-transporters-2 inhibitors and heart failure: state of the art review and future potentials. Int J Heart Fail. 2020; 2:12–22.
72. Iacobellis G, Baroni MG. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J Endocrinol Invest. 2022; 45:489–95.
74. Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020; 11:2127.

75. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.
76. Fukuda T, Bouchi R, Terashima M, Sasahara Y, Asakawa M, Takeuchi T, et al. Ipragliflozin reduces epicardial fat accumulation in non-obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther. 2017; 8:851–61.

77. Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol. 2021; 20:57.

78. Hiruma S, Shigiyama F, Hisatake S, Mizumura S, Shiraga N, Hori M, et al. A prospective randomized study comparing effects of empagliflozin to sitagliptin on cardiac fat accumulation, cardiac function, and cardiac metabolism in patients with early-stage type 2 diabetes: the ASSET study. Cardiovasc Diabetol. 2021; 20:32.

79. Iacobellis G, Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity (Silver Spring). 2020; 28:1068–74.

80. Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2-inhibitors on epicardial adipose tissue: a meta-analysis. Cells. 2021; 10:2150.

81. Requena-Ibanez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021; 9:578–89.
82. Kosiborod MN, Abildstrom SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023; 389:1069–84.
83. Romanelli MM, Vianello E, Malavazos AE, Tacchini L, Schmitz G, Iacobellis G, et al. GLP‐1 receptor is associated with genes involved in fatty acids oxidation and white‐to‐brown fat differentiation in epicardial adipose tissue (EAT). FASEB J. 2019; 33(S1):662.

Fig. 1.
The gross anatomy of epicardial adipose tissue (EAT) in an 81-year-old woman with three-vessel coronary artery disease who underwent a coronary artery bypass graft.
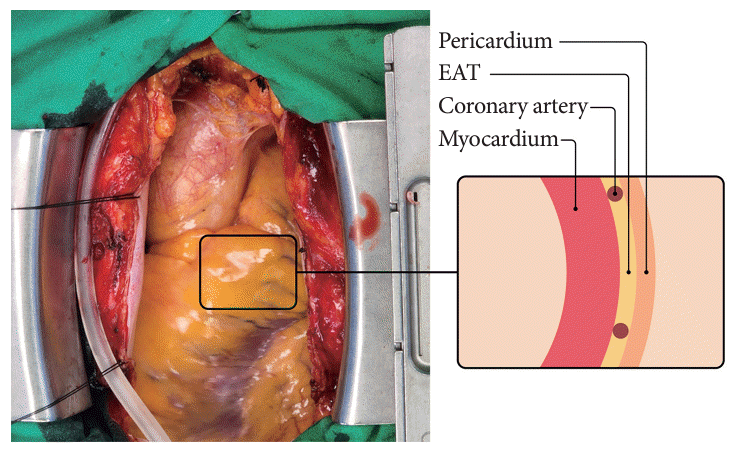
Fig. 2.
Representative images of epicardial adipose tissue (EAT). (A) Echocardiographic images of EAT. (B) Cardiac magnetic resonance images of EAT. Asterisk indicates EAT and white arrow indicates pericardium. (C, D) Computed tomography images of EAT. Green colored area indicates EAT. PAT, pericardial adipose tissue; RV, right ventricle; LV, left ventricle; LA, left atrium.
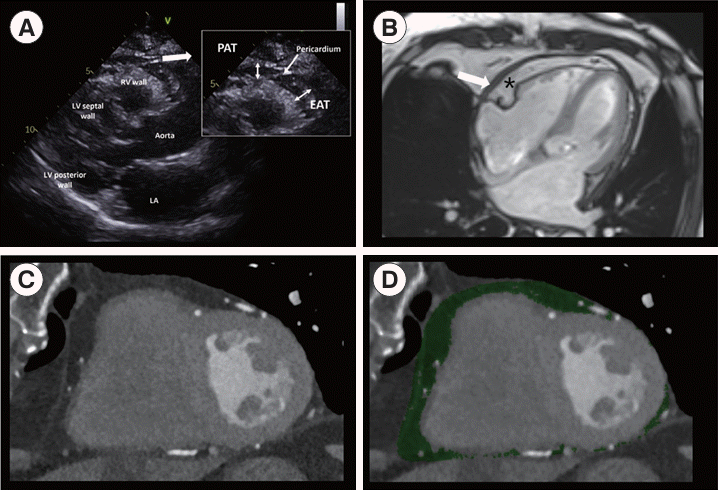
Fig. 3.
The pathophysiologic mechanism of epicardial adipose tissue (EAT). PAT, pericardial adipose tissue; PAI-1, plasminogen activator inhibitor 1; TNF-α, tumor necrosis factor-alpha; IL, interleukin; LV, left ventricle; e` velocity, early diastolic velocity of the mitral annulus; GLS, global longitudinal strain.
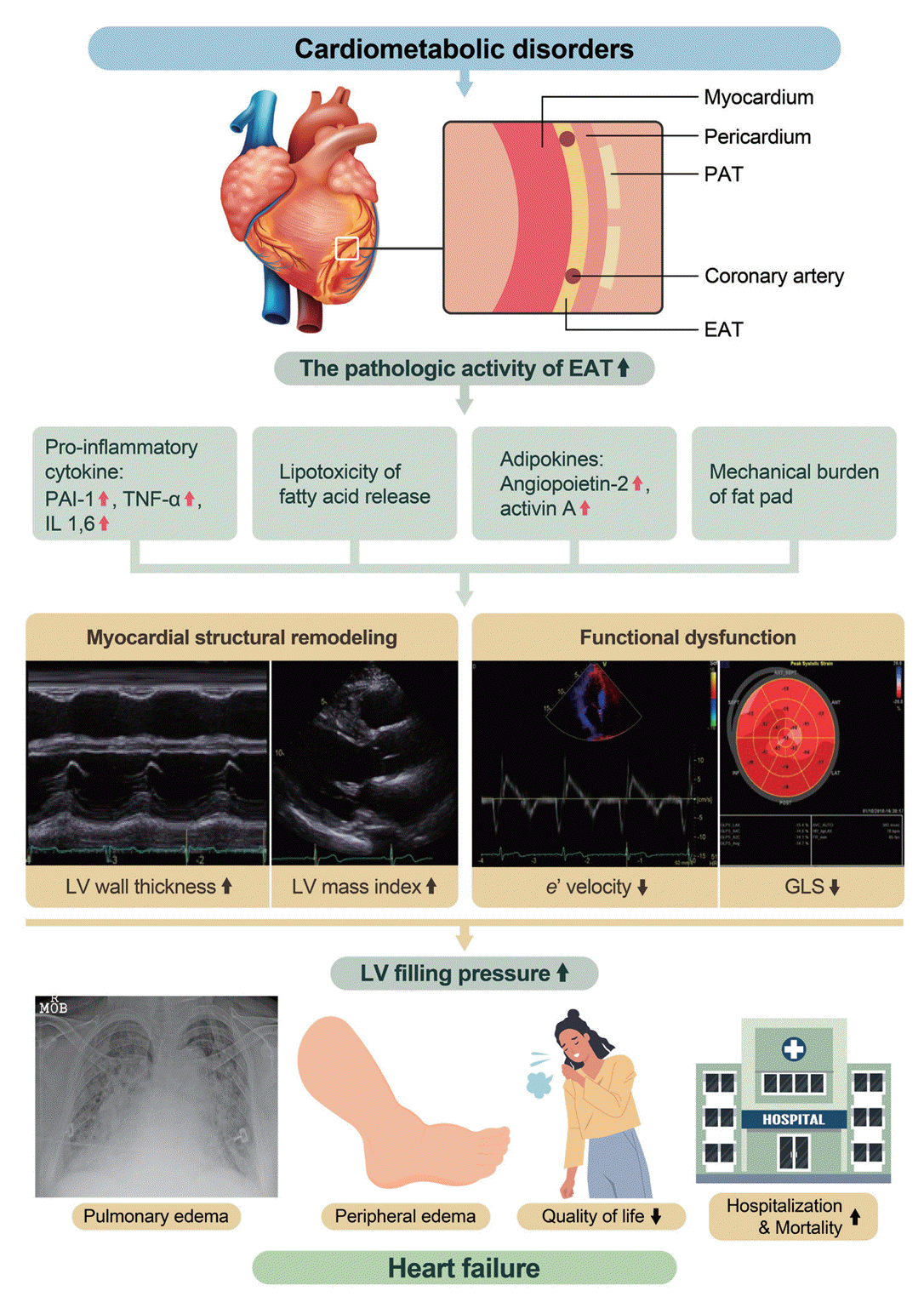
Fig. 4.
The epicardial adipose tissue (EAT) amount in the heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), and control groups. (A) EAT amount in HFrEF vs. control. (B) EAT amount in HFpEF vs. control. (C) EAT amount in HFrEF, HFpEF, and control. CMR, cardiac magnetic resonance. aThis study used cm3 as the measurement unit of EAT.
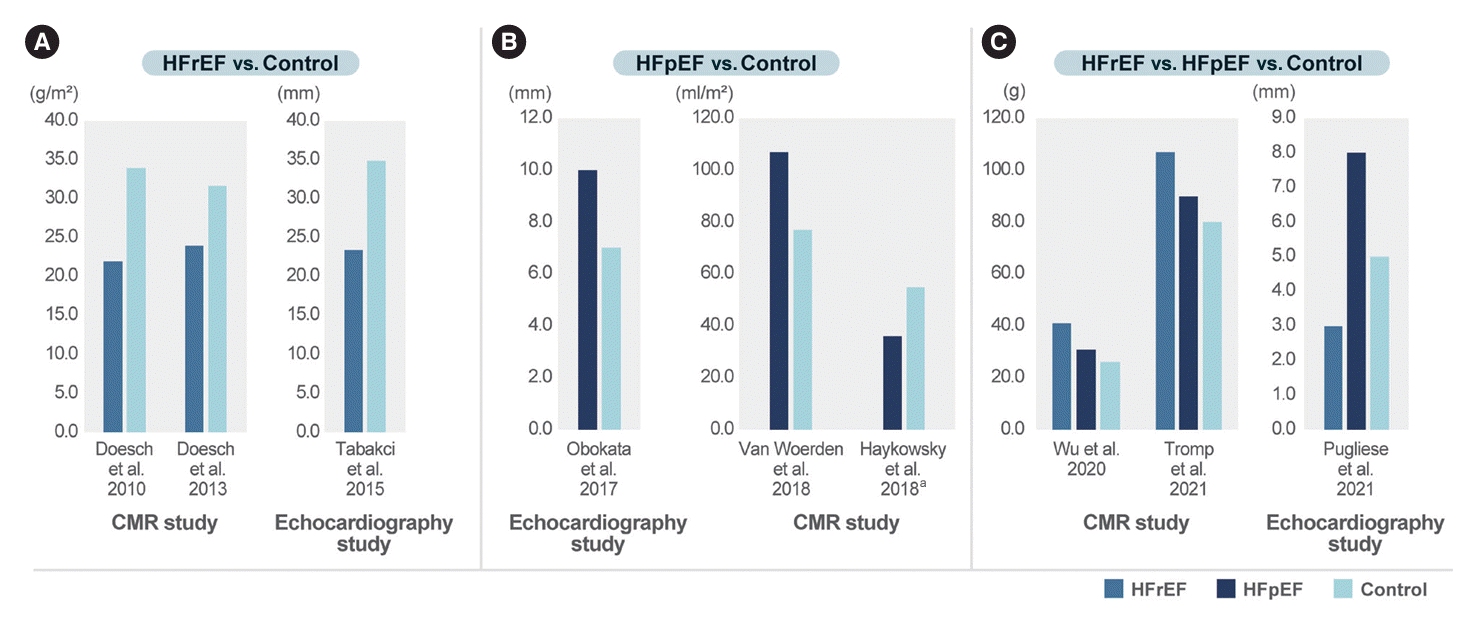
Fig. 5.
Mechanism of sodium glucose cotransporter 2 (SGLT2) inhibitor reducing epicardial adipose tissue (EAT). VAT, visceral adipose tissue; OHA, oral hypoglycemic agent.
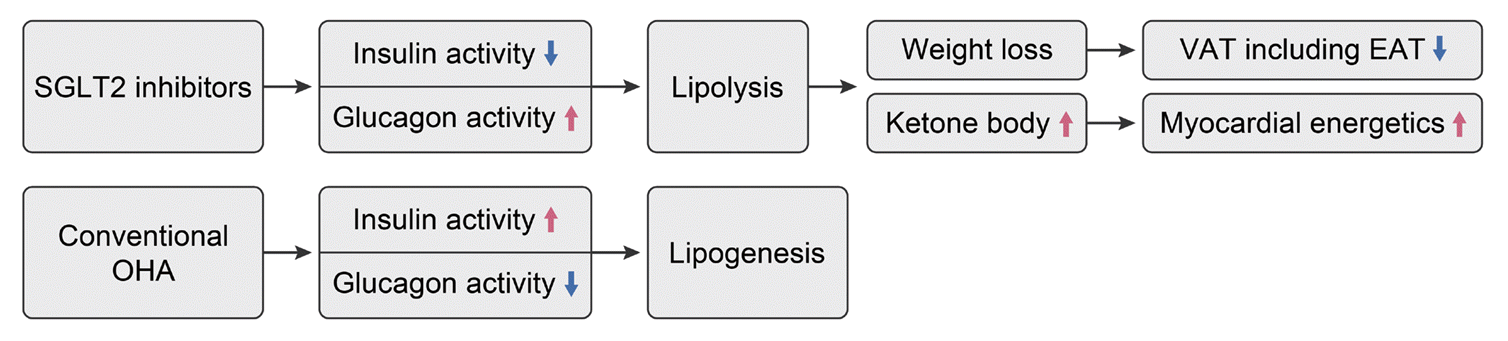
Table 1.
Imaging modalities used to access EAT
| Variable | Echocardiography [1,4,18] | CT [23-25] | Cardiac MRI [56,60,61] |
|---|---|---|---|
| Measurement unit | Thickness (mm) | Area (cm2) or volume (mL) | Area (cm2) or volume (mL) |
| Linear two-dimensional measurement | High spatial resolution for volume measurement | Highest image resolution for volume measurement | |
| Location | Single location (only RV free wall) | Various locations | Various locations |
| Accessibility | Easily available | Low | Very low |
| Accuracy | Less accurate | More accurate | Reproducible |
| Reproducibility | Reproducible | Reproducible | Reproducible |
| Cost | Relatively cheap | Expensive | Expensive |
Table 2.
Major clinical studies investigating the role of SGLT2 inhibitors on EAT volume
| Study | Population | Imaging modality | Study type | Drug | Control | Duration, wk | Major findings |
|---|---|---|---|---|---|---|---|
| Requena-Ibanez et al. (2021) [81] | HFrEF (n=84) | CMR | RCT | Empagliflozin | Placebo | 24 | Empagliflozin significantly reduced EAT volume, myocardial fibrosis, and inflammatory markers compared with placebo |
| Gaborit et al. (2021) [77] | T2DM (n=56) | CMR | RCT | Empagliflozin | Placebo | 12 | No effect on myocardial or epicardial fat volume |
| Hiruma et al. (2021) [78] | T2DM without CVD (n=44) | CMR | RCT | Empagliflozin | Sitagliptin | 12 | No effect on myocardial or epicardial fat volume |
| Iacobellis et al. (2020) [79] | T2DM & obesity (n=100) | Echocardiography | RCT | Dapagliflozin | Placebo | 24 | Dapagliflozin reduced EAT thickness by 20% from baseline |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download