Highlights
INTRODUCTION
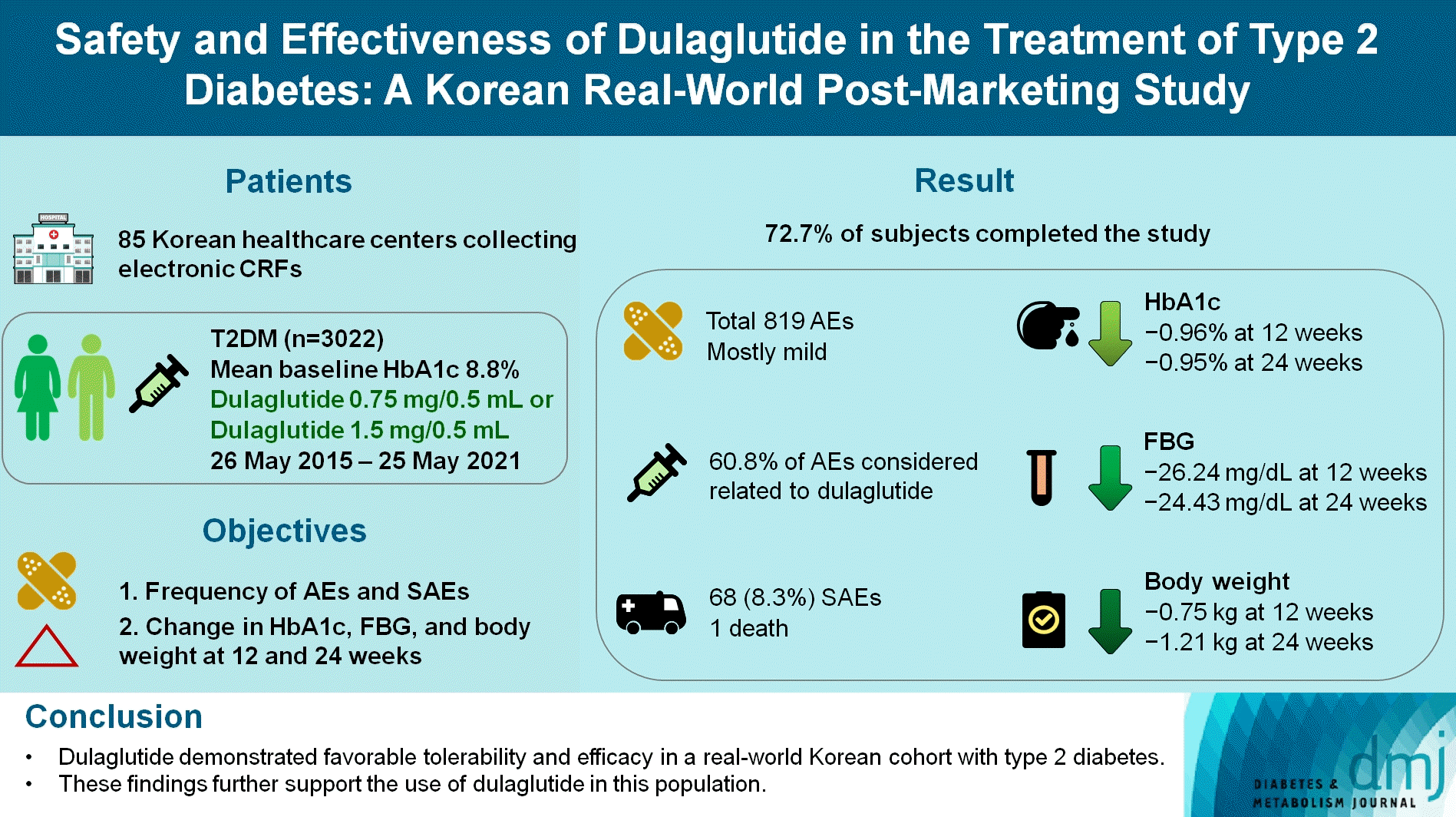
METHODS
RESULTS
Table 1.
| Category | Number | Statistic |
|---|---|---|
| Female sex | 3,022 | 1,600 (52.95) |
| Age, yr | 3,022 | 56.22±12.56 |
| <30 | 97 (3.21) | |
| ≥30 and <40 | 209 (6.92) | |
| ≥40 and <50 | 533 (17.64) | |
| ≥50 and <60 | 907 (30.01) | |
| ≥60 and <70 | 854 (28.26) | |
| ≥70 and <80 | 372 (12.31) | |
| ≥80 | 50 (1.65) | |
| Patients stratified by age 65 years, yr | ||
| ≥65 | 785 (25.98) | |
| <65 | 2,237 (74.02) | |
| Height, cm | 2,341 | 163.25±9.34 |
| Waist circumference, cm | 1,088 | 91.23±14.19 |
| Baseline weight, kg | 2,335 | 75.45±15.22 |
| Baseline BMI, kg/m2 | 2,148 | 28.31±4.64 |
| Underweight (BMI <18.5) | 9 (0.42) | |
| Normal (18.5≤ BMI <23.0) | 195 (9.08) | |
| Overweight (23.0≤ BMI <25.0) | 294 (13.69) | |
| Obese I (25.0≤ BMI <30.0) | 985 (45.86) | |
| Obese II (BMI ≥30.0) | 665 (30.96) | |
| HbA1c, % | 2,731 | 8.84±1.47 |
| FBG, mg/dL | 2,174 | 172.61±63.24 |
| Duration of T2DM, yrb | 3,022 | 11.19±7.83 |
| Previously received T2DM treatment | 3,022 | 2,449 (81.04) |
| Pre-existing conditions | 3,022 | 2,659 (87.99) |
| Hypertension | 3,022 | 1,599 (52.91) |
| Dyslipidemiac | 3,022 | 1,067 (35.31) |
| Hyperlipidemiad | 3,022 | 838 (27.73) |
| Renal impairmente | 3,022 | 258 (8.54) |
| Hepatic impairmente | 3,022 | 325 (10.75) |
| Allergiese | 3,022 | 84 (2.78) |
| Coronary artery disease | 3,022 | 2 (0.07) |
| Concomitant medication for T2DM | 3,022 | 2,884 (95.43) |
| Other concomitant medication | 3,022 | 2,491 (82.43) |
Values are presented as number (%) or mean±standard deviation.
BMI, body mass index; HbA1c, glycosylated hemoglobin; FBG, fasting blood glucose; T2DM, type 2 diabetes mellitus.
a The safety analysis set (n=3,022) includes subjects from the effectiveness analysis set (n=2,368). The effectiveness set includes subjects whose HbA1c, FBG, or body weight data were collected at baseline,
c Dyslipidaemia refers to the imbalance of lipids in the blood (low-density lipoprotein cholesterol, triglycerides, and high-density lipoprotein),
Safety data
Table 2.
| Event type | No. of events | No. of subjects (%) | Most common symptom (%)a |
|---|---|---|---|
| Adverse event | 819 | 589 (19.49) | Nausea (5.03) |
| Diarrhea (2.08) | |||
| Decreased appetite (1.29) | |||
| Adverse drug reaction | 498 | 403 (13.34) | Nausea (4.93) |
| Diarrhea (1.75) | |||
| Drug in-effectivity (1.26) | |||
| Unexpected adverse event | 325 | 255 (8.44) | Increased blood glucose (0.86) |
| Dizziness (0.53) | |||
| Hyperglycemia (0.5) | |||
| Unexpected adverse drug reaction | 62 | 60 (1.99) | Increased blood glucose (0.26) |
| Dizziness (0.26) | |||
| Headache (0.20) | |||
| Pruritis (0.20) | |||
| Serious adverse eventb | 68 | 51 (1.69) | Increased blood glucose (0.30) |
| Inadequate DM control (0.1) | |||
| Cardiac failure (0.07) | |||
| Serious adverse drug reaction | 15 | 14 (0.46) | Increased blood glucose (0.26) |
| Inadequate control of DM, diabetic gastropathy, diabetic nephropathy, and urinary disorders (0.03%, respectively) | |||
| Unexpected serious adverse event | 59 | 50 (1.65) | Increased blood glucose (0.3) |
| Inadequate DM control (0.1) | |||
| Cardiac failure (0.07) | |||
| Unexpected serious adverse drug reaction | 13 | 13 (0.43) | Increased blood glucose (0.26) |
| Inadequate DM control, diabetic gastropathy, dizziness, diabetic nephropathy, urinary disorders (0.03, respectively) | |||
| Deathc | 1 | 1 |
a Preferred term as defined by MedDRA 24.0 (MedDRA-K 24.0) for system organ class and investigations,
b Serious adverse events included adverse events that resulted in death, required either inpatient hospitalization or the prolongation of hospitalization, were life-threatening (immediate risk of death), resulted in a persistent or significant disability/incapacity or resulted in a congenital anomaly/birth defect, or any adverse event that did not result in death or require inpatient hospitalization but was considered significant based on the investigator’s judgment,
Table 3.
| Factor | Subject | AE incidence | 95% CI | P valuea |
|---|---|---|---|---|
| Sex | ||||
| Male | 1,422 | 232 (16.32) | 14.43–18.34 | <0.0001b |
| Female | 1,600 | 357 (22.31) | 20.29–24.43 | |
| Total | 3,022 | 589 (19.49) | ||
| Age, yr | ||||
| <30 | 97 | 16 (16.49) | 9.73–25.40 | 0.7101 |
| ≥30–<40 | 209 | 39 (18.66) | 13.62–24.61 | |
| ≥40–<50 | 533 | 93 (17.45) | 14.32–20.94 | |
| ≥50–<60 | 907 | 177 (19.51) | 16.98–22.25 | |
| ≥60–<70 | 854 | 181 (21.19) | 18.50–24.09 | |
| ≥70–<80 | 372 | 74 (19.89) | 15.96–24.32 | |
| ≥80 | 50 | 9 (18.00) | 8.58–31.44 | |
| Total | 3,022 | 589 (19.49) | ||
| Patients stratified by age 65 years, yr | ||||
| ≥65 | 785 | 158 (20.13) | 17.38–23.11 | 0.6005 |
| <65 | 2,237 | 431 (19.27) | 17.65–20.96 | |
| Total | 3,022 | 589 (19.49) | ||
| BMI, kg/m2 | ||||
| Underweight (BMI <18.5) | 9 | 2 (22.22) | 2.81–60.01 | 0.0182b |
| Normal (18.5≤ BMI <23.0) | 195 | 55 (28.21) | 22.01–35.08 | |
| Overweight (23.0≤ BMI <25.0) | 294 | 52 (17.69) | 13.50–22.54 | |
| Obese I (25.0≤ BMI <30.0) | 985 | 179 (18.17) | 15.81–20.73 | |
| Obese II (BMI ≥30.0) | 665 | 141 (21.20) | 18.15–24.51 | |
| Total | 2,148 | 429 (19.97) | ||
| Previous history of T2DM treatment | ||||
| Yes | 2,449 | 518 (21.15) | 19.55–22.82 | <0.0001b |
| No | 573 | 71 (12.39) | 9.81–15.37 | |
| Total | 3,022 | 589 (19.49) | ||
| Medical historyc | ||||
| Yes | 270 | 76 (28.15) | 22.87–33.92 | 0.0002b |
| No | 2,752 | 513 (18.64) | 17.20–20.15 | |
| Total | 3,022 | 589 (19.49) | ||
| Pre-existing conditions | ||||
| Yes | 2,659 | 557 (20.95) | 19.41–22.54 | <0.0001b |
| No | 363 | 32 (8.82) | 6.11–12.22 | |
| Total | 3,022 | 589 (19.49) | ||
| Renal impairment | ||||
| Yes | 258 | 56 (21.71) | 16.83–27.24 | 0.3477 |
| No | 2,764 | 533 (19.28) | 17.83–20.80 | |
| Total | 3,022 | 589 (19.49) | ||
| Hepatic impairment | ||||
| Yes | 325 | 70 (21.54) | 17.19–26.41 | 0.3238 |
| No | 2,697 | 519 (19.24) | 17.77–20.78 | |
| Total | 3,022 | 589 (19.49) | ||
| Allergies | 0.1159 | |||
| Yes | 84 | 22 (26.19) | 17.20–36.93 | |
| No | 2,938 | 567 (19.30) | 17.89–20.77 | |
| Total | 3,022 | 589 (19.49) | ||
| Concomitant medication for T2DM | ||||
| Yes | 2,884 | 571 (19.80) | 18.36–21.30 | 0.0503 |
| No | 138 | 18 (13.04) | 7.92–19.83 | |
| Total | 3,022 | 589 (19.49) | ||
| Other concomitant medication | <0.0001b | |||
| Yes | 2,491 | 544 (21.84) | 20.23–23.51 | |
| No | 531 | 45 (8.47) | 6.25–11.18 | |
| Total | 3,022 | 589 (19.49) | ||
| Dulaglutide administration duration, wk | ||||
| <10 | 332 | 106 (31.93) | 26.94–37.24 | <0.0001b |
| 10–14 | 253 | 71 (28.06) | 22.62–34.03 | |
| >14–<22 | 310 | 74 (23.87) | 19.23–29.01 | |
| 22–26 | 905 | 145 (16.02) | 13.69–18.58 | |
| >26 | 1,222 | 193 (15.79) | 13.79–17.96 | |
| Total | 3,022 | 589 (19.49) | ||
| Average weekly dose of dulaglutide, mg/wk | ||||
| ≤0.75 | 1,548 | 320 (20.67) | 18.68–22.78 | 0.0075b |
| >0.75–<1.5 | 1,398 | 264 (18.88) | 16.86–21.04 | |
| ≥1.5 | 76 | 5 (6.58) | 2.17–14.69 | |
| Total | 3,022 | 589 (19.49) | ||
| Follow-up period, wk | ||||
| <22 | 761 | 190 (24.97) | 21.93–28.20 | <0.0001b |
| ≥22 | 2,261 | 399 (17.65) | 16.10–19.28 | |
| Total | 3,022 | 589 (19.49) |
Table 4.
| Factor | Odds ratio (95% CI) | P valuea |
|---|---|---|
| Sex | ||
| Male | 0.61 (0.49–0.77) | <0.0001b |
| Female | Reference | |
| BMI, 1 kg/m2 | 0.99 (0.96–1.01) | 0.2865 |
| Previous history of T2DM treatment with medicine | ||
| Yes | 1.70 (1.19–2.42) | 0.0034b |
| No | Reference | |
| Medical history | ||
| Yes | 1.34 (0.96–1.87) | 0.0894 |
| No | Reference | |
| Pre-existing conditions | ||
| Yes | 1.64 (0.80–3.37) | 0.1762 |
| No | Reference | |
| Other concomitant medication | ||
| Yes | 2.52 (1.43–4.44) | 0.0014b |
| No | Reference | |
| Administration duration of dulaglutide, 1 week | 0.94 (0.93–0.95) | <0.0001b |
Effectiveness data
Table 5.
| Factor | LS mean difference (mean±SE) | 95% CI | P valuea |
|---|---|---|---|
| HbA1c change from baseline, %b | |||
| 12±4 weeks | –0.96±0.03 | –1.01 to –0.91 | <0.0001c |
| 24±4 weeks | –0.95±0.03 | –1.01 to –0.89 | <0.0001c |
| FBG change from baseline, mg/dLd | |||
| 12±4 weeks | –26.24±1.19 | –28.56 to –23.91 | <0.0001c |
| 24±4 weeks | –24.43±1.37 | –27.12 to –21.73 | <0.0001c |
| Body weight change from baseline, kge | |||
| 12±4 weeks | –0.75±0.07 | –0.89 to –0.62 | <0.0001c |
| 24±4 weeks | –1.21±0.08 | –1.36 to –1.06 | <0.0001c |
Table 6.
| Factor | Odds ratio (95% CI) | P valuea |
|---|---|---|
| Age, 1 year | 0.97 (0.96–0.98) | <0.0001b |
| BMI, 1 kg/m2 | 1.03 (1.00–1.06) | 0.0542 |
| Hepatic impairment | ||
| Yes | 1.42 (0.96–2.10) | 0.0781 |
| No | Reference | |
| Other concomitant medication | ||
| Yes | 0.62 (0.45–0.87) | 0.0053b |
| No | Reference |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download