Abstract
Purpose
One of the novel cell sources of cell-based liver regenerative medicine is human chemically-derived hepatic progenitors (hCdHs). We previously established this cell by direct hepatocyte reprogramming with a combination of small molecules (hepatocyte growth factor, A83-01, CHIR99021). However, there have been several issues concerning the cell’s stability and maintenance, namely the occurrences of epithelial-mesenchymal transition (EMT) that develop fibrotic phenotypes, resulting in the loss of hepatic progenitor characteristics. These hepatic progenitor attributes are thought to be regulated by SOX9, a transcription factor essential for hepatic progenitor cells and cholangiocytes.
Methods
To suppress the fibrotic phenotype and improve our long-term hCdHs culture technology, we utilized the epigenetic modulating drugs DNA methyltransferase inhibitor (5-azacytidine) and histone deacetylase inhibitor (sodium butyrate) that have been reported to suppress and revert hepatic fibrosis. To confirm the essential role of SOX9 to our cell, we used clustered regularly interspaced short palindromic repeats-interference (CRISPRi) to repress the SOX9 expression.
Results
The treatment of only 5-azacytidine significantly reduces the fibrosis/mesenchymal marker and EMT-related transcription factor expression level in the early passages. Interestingly, this treatment also increased the hepatic progenitor markers expression, even during the reprogramming phase. Then, we confirmed the essential role of SOX9 by repressing the SOX9 expression with CRISPRi which resulted in the downregulation of several essential hepatic progenitor cell markers.
The liver is one of the most vital organs in our body with a special capability to regenerate on its own, enabling it to recover from injury or disease, and therefore maintain its essential physiological and biological functions [1]. This unique regenerative capability is known to be attributed, in part, to hepatic progenitor cells, which can be derived from both hepatocytes and cholangiocytes [12]. This specific type of cell possesses the potential to be differentiated into various hepatic cells and contributes to liver tissue regeneration after its subsequent damage [3]. However, this regeneration by the hepatic progenitor cells is often hindered by many factors, such as epithelial-mesenchymal transition (EMT)-driven fibrogenesis [4]. This process could lead to excessive accumulation of fibrous tissue, impaired liver function, and ultimately, various end-stage liver diseases such as liver fibrosis, cirrhosis, and cancer [5]. Therefore, novel strategies to inhibit EMT are essential to enhance the regenerative potential of hepatic progenitor cells for effective regenerative medicine options and improved therapeutic outcomes [6].
Epigenetic modulation has recently emerged as a promising therapeutic option that could manipulate cell function and behavior [7]. This specific class of drugs, which target and modify various epigenetic states such as DNA methylation and histone modification [8], can change the hepatic cell state by cellular reprogramming due to alteration of gene expression profile and signaling pathways [9]. In addition, it is thought that EMT is driven by a number of epigenetic factors that lead to cellular plasticity change [10], particularly in cancer metastasis that enables polarized immotile epithelial cells to gain fibroblast-like mesenchymal abilities, such as enhanced motility [11].
Liver transplantation remains the gold standard treatment for many end-stage liver diseases [12]. However, there have been many research efforts that aim to replace this such as hepatocyte transplantation [1314]. It is known that this method has similar drawbacks to previous organ transplantations, for which many research efforts have been attempting to develop various alternatives for hepatocytes as the cell source for transplantation [215]. Previously, we have reported the generation of human chemically-derived hepatic progenitor cells (hCdHs) that could be robustly expanded from human primary hepatocytes (hPHs) [2]. This cell has demonstrated significant potential as a regenerative medicine [16], novel ex vivo gene therapy method [17], disease modeling, and drug screening platform [18]. However, there have been some drawbacks to the hCdHs, namely the incidence of cellular morphological change to fibroblast-like cells and a lower efficiency in generating hCdHs from elderly patients.
In this study, we utilized 2 specific classes of epigenetic modulating drugs (epidrugs) that have been reported to rejuvenate elderly primary hepatocytes [19] and increase the regenerative potential of these cells, DNA methyltransferase inhibitor 5-azacytidine (5-Aza), and histone deacetylase inhibitor sodium butyrate (NaB) [20]. These epidrugs, especially 5-Aza, have been reported and used clinically to alleviate fibrosis in various organs, as well as various diseases. We hypothesize that this drug could also have potential to inhibit EMT-driven fibrogenesis in hCdHs cells as well as rejuvenating its hepatic stem cell properties. In addition, we suggest that SOX9, a transcription factor that is highly related to hepatic progenitor cells [21] and cholangiocytes [22], is essential to hCdHs generation and maintenance.
The study was approved by the Institutional Review Board of Hanyang University (No. HYUH201711012020-HE001) and written informed consent were obtained from all research participants.
Human liver tissue fragments were obtained and isolated from 3 hepatectomy patients who underwent surgical treatment for various reasons at Hanyang University Medical Center in Seoul, Korea (Supplementary Table 1). The isolation of mouse primary hepatocytes or hPHs followed a previously published method [21723]. For mouse samples, the livers were perfused in vivo through a portal vein. For human samples, hPHs were isolated with a perfusion pump (BT100-1F, Dongbang Hitech) using 2-step perfusion as follows; first, perfuse the sample with warmed liver perfusion solution supplemented with tris-EDTA (Sigma-Aldrich); second, continue with enzymatic digestion by perfusion of collagenase/elastase mixture (Worthington Biochemical) and calcium chloride solution (Sigma-Aldrich). Next, the digested tissue was minced with a surgical blade and filtered with a 100-µm cell strainer (Corning) to isolate the single-cell hPH suspension. Lastly, viable and live hPHs were sorted by 25% Percoll (GE Healthcare) isodensity centrifugation and seeded on a 10-cm2 dish collagen-coated plate (Advanced BioMatrix) in William’s E Media Gibco with Primary Hepatocyte Maintenance Supplement (Gibco) supplementation.
Following the primary hepatocytes isolation from the clinical sample or C57BL/6 mouse, the seeded primary hepatocytes were incubated with HGF, A83-01, and CHIR99021 (HAC) reprogramming medium consisting of DMEM/F12 + GlutaMax (Gibco) supplemented with: 1% fetal bovine serum (Gibco), 10-mM nicotinamide (Sigma-Aldrich), 1% insulin–transferrin–selenium (Gibco), 1% penicillin/streptomycin (Gibco), 0.1-µM dexamethasone (Sigma-Aldrich), 20-ng/mL human recombinant epidermal growth factor (Peprotech), 20-ng/mL human recombinant hepatocyte growth factor (HGF) (Peprotech), 3-µM CHIR-99021 (Sigma-Aldrich), and 4-µM A83-01 (Sigma-Aldrich), according to the protocol reported by Kim et al. [2]. After cell confluency reached 80%–90%, the cells were passaged by TrypLE Select enzyme (Gibco) and 5 × 105 cells were reseeded into a new 10-cm2 collagen-coated dish.
For treatment with epidrugs during the hCdHs generation, William’s E Media was supplemented with 0.5-µM 5-Aza (Sigma-Aldrich) for 16–24 hours. During the reprogramming period, cells were treated with single or combination epidrugs of 0.5 µM 5-Aza and 0-100 µM NaB (Sigma-Aldrich) for the first 48 hours. After passage, the cells were re-treated with only epidrugs NaB for the first 48 hours.
Total RNA was isolated from cell pellets using TRIZOL Reagent (Invitrogen). Complimentary DNA (cDNA) was reverse transcripted with Transcriptor First Strand cDNA Synthesis Kit (Roche) from 1-µg isolated total RNA. Real-time (RT) PCR was performed with gene-specific primers (Supplementary Table 2) using PCR PreMix (Dyne Bio) using CFX Connect RT-PCR Detection System (BioRad) according to the manufacturer’s recommendation. PCR conditions were 40 cycles of 95 ℃ for 20 seconds and 60 ℃ for 40 seconds. Expression levels were normalized to GAPDH housekeeping gene expression level. Fold differences were calculated by the ΔΔCt method. All quantitative data are presented with triplicate (n = 3) as the mean ± standard error of mean with P-values. Statistical significance was evaluated by 2-tailed t-test with the significance at P < 0.05, P < 0.01, and P < 0.001.
To construct single guide RNA (sgRNA)-expressing plasmids, complementary oligos representing the target sequences were annealed and cloned sgRNA plasmid. The oligos are listed in Supplementary Table 3.
Electroporation was performed using a Neon Transfection System (Thermo Fisher). Using the Neon Transfection System, mouse CdHs (mCdHs; 1 × 105 cells) were transfected with 2.5 µg of dCAS9-KRAB (clustered regularly interspaced short palindromic repeats-interference, CRISPRi) plasmid (Addgene #110820) and 2.5 µg of corresponding SOX9 targeting sgRNA plasmid with the following parameters: voltage, 1,200; width, 50 ms; and number, 1. Then, the transfected cells were cultured in a reprogramming medium for cell expansion and, after several days, cells could be harvested for subsequent transcriptome analysis.
In this study, we utilized a 2-step perfusion method to isolate hPHs from human liver tissue. These hPHs were subsequently cultured in a reprogramming medium, supplemented with a specific small molecule cocktail comprising HGF, A83-01, and CHIR99021 (referred to as HAC), as previously described by Kim et al. [2]. To further enhance the reprogramming process, we introduced epidrugs, specifically 5-Aza and NaB, in 5 distinct concentrations into the HAC media (referred to as HAC+) (Fig. 1A).
Intriguingly, our efforts yielded the successful generation of proliferative hCdHs from hPHs, starting as early as day 2 after reprogramming with the HAC+ reprogramming media. We did not observe any deleterious or side effects of epidrugs treatment on the hCdHs cells, as they exhibited similar epithelial-like typical phenotype to the hCdHs cultured in the HAC media (Fig. 1B).
Subsequently, since we could not observe any obvious impact of the epidrugs on the cell phenotype, our investigation delved into the transcriptome profile of these reprogrammed cells, with a particular focus on the expression of hepatic progenitor markers (epithelial cell adhesion molecule [EpCAM] and SOX9), EMT transcription factors (twist family bHLH transcription factor 1, TWIST1), and mesenchymal markers (collagen type i alpha 1 chain, COL1A1). Notably, treatment with 5-Aza resulted in a significant upregulation of EpCAM and SOX9 expression levels, while the dual treatment with NaB specifically increased the expression of SOX9 but not EpCAM (Fig. 1C). Moreover, 5-Aza treatment led to a significant reduction in TWIST1 transcription factor expression (Fig. 1D), consequently downregulating COL1A1 expression (Fig. 1E). In contrast, treatment with NaB resulted in the comparable expression level of TWIST1 and significant upregulation of COL1A1 compared to the baseline HAC media and treatment with 5-Aza (Fig. 1D, 1E).
Taken together, our findings suggest the potent impact of 5-Aza treatment in enhancing hepatic progenitor cell markers and simultaneously inhibiting EMT by downregulating TWIST1 transcription factor, shedding new light on the potential of reprogramming strategies in the context of regenerative medicine.
Subsequently, we determined the effects of epidrugs on the in vitro maintenance of hCdHs over an extended duration. It came to our attention that in the prolonged cultivation of hCdHs, a concerning phenomenon emerged—the development of fibrogenic features that transformed these cells into fibrotic hCdHs, thus undermining their differentiation capabilities (Fig. 2A). This problem led us to hypothesize that EMT may be the driving force behind this fibrogenesis. In light of this, the hCdHs were treated with 5-Aza or NaB for 48 hours after each passage (‘a’ in Fig. 2B) and then continued to be cultured in HAC culture media without epidrugs until the confluence reached ~90% (‘b’ in Fig. 2B).
Following the long-term passage with epidrug treatment, those cells treated only with 5-Aza retained their typical morphology of epithelial and hepatic progenitor cell features and exhibited minimal evidence of early fibrogenesis (Fig. 2C). In stark contrast, cells exposed to NaB displayed a more distinct fibrotic phenotype when compared to their untreated counterparts and cells treated solely with 5-Aza. Moreover, the administration of high concentrations of NaB resulted in the premature senescence of the hCdHs.
Further corroborating the pivotal role of 5-Aza in sustaining the hCdHs, our RT-quantitative PCR transcriptome analysis unequivocally demonstrated a significant increase in the expression of EpCAM in the treated hCdHs cells. Conversely, high-dose NaB treatment (NaB >50 µM) exerted a suppressive effect on the expression of EpCAM and SOX9. In contrast, 50-µM NaB increases EpCAM expression level (Fig. 2D), but significantly increases the expression level of both TWIST1 and SNAI1 (Fig. 2E). Additionally, our analysis revealed that 5-Aza treatment significantly reduced the expression levels of SNAI1 and TWIST1 transcription factors (Fig. 2E), consequently leading to a reduction in COL1A1 transcription (Fig. 2F).
Collectively, our findings reaffirm the substantial influence of 5-Aza on the preservation of hepatic progenitor cell traits in hCdHs, while concurrently mitigating EMT-driven fibrogenesis. Notably, our investigation also unveiled the adverse consequences of the combined treatment of 5-Aza with NaB. This combination treatment triggered an exacerbation of EMT, which in turn activated SNAI1 and TWIST1, ultimately culminating in cellular senescence.
Our investigation extended to a comprehensive analysis of the pivotal role of the transcription factor SOX9 to the hepatic progenitor traits of mCdHs. Utilizing novel CRISPRi techniques, we executed a precise and deliberate knockdown of SOX9 expression at the transcriptional level. To achieve this, we delivered the catalytic inactive CRISPR-associated 9 protein fused with Krüppel associated box (KRAB) domain (dCas9-KRAB) plasmid alongside the corresponding SOX9-transcription start site targeting sgRNA via electroporation (Fig. 3A, B).
Following the transfection, we monitored the cellular responses for 3–5 days. Firstly, we confirmed that the dCas9-KRAB transduction without any sgRNA (sgRNA–) did not cause any significant negative impact on the cell proliferation rate and phenotype (‘a’ in Fig. 3C). Intriguingly, we observed no distinctive alterations in the cell morphology post-transfection (‘a’ in Fig. 3C). Nevertheless, a significant change of the rate of cell proliferation was observed with the most notable effects found in cells targeted with sgRNA #4 and #5 (‘b’ in Fig. 3C).
Furthermore, we conducted RT-PCR analyses to provide quantitative insights into the transcriptional impact of our SOX9 knockdown strategy. Importantly, we observed a significant reduction in SOX9 transcription levels, particularly in cells targeted with sgRNA #4 and #5 (Fig. 3D). This notable downregulation of SOX9 corresponded with a concurrent reduction in the expression of several key genetic markers characteristic of mCdHs, including alpha fetoprotein (AFP), keratin 7 (KRT7), EpCAM, and cluster of differentiation 44 (CD44) (Fig. 3D). Moreover, it also exhibited a substantial influence on the expression of hepatic markers such as albumin and hepatocyte nuclear factor 4 alpha (HNF4A) (Fig. 3E). Taken together, these findings indicated the essential role of SOX9 to the hepatic progenitor traits of mCdHs.
The initial findings in this study are centered around the reprogramming of hPHs into proliferative hepatic progenitor-like cells (hCdHs). This process is significant in the context of regenerative medicine in the hepatobiliary area, where the generation of functional hepatic cells from readily available sources, such as hPHs, has substantial therapeutic potential. The success achieved in reprogramming hPHs into hCdHs with the combination of growth factors (HGF, A83-01, and CHIR9902) and epidrugs (5-Aza and NaB) is a particularly noteworthy breakthrough in the regenerative medicine field.
The supplementation of epidrugs appears to play a critical role in the success of this reprogramming strategy. Epidrugs have been recognized for their capacity to modulate gene expression by influencing DNA methylation and histone modifications [24], as well as being recently used to clinically treat various liver diseases like fibrosis and nonalcoholic steatohepatitis [25]. In this context, their use is associated with enhanced proliferation of hCdHs and a marked inhibition of EMT. The emergence of early-stage colonies within just 2 days of epidrug supplementation underscores their importance in this process. The control of EMT is of great significance since it affects the transformation of epithelial cells into mesenchymal cells, a process relevant to both development and tissue repair.
The investigation into the long-term maintenance of hCdHs following epidrugs treatment sheds light on an important aspect of regenerative medicine—sustaining the desired cellular characteristics over time. Here, the study revealed that while 5-Aza treatment allows for the maintenance of epithelial and hepatic progenitor traits in hCdHs, combinational NaB treatment presents a unique challenge. High concentrations of NaB led to a fibrotic phenotype and, notably, cellular senescence. These findings underlined the need for cautious consideration of epidrugs selection, dosage, and their long-term effects when devising strategies for the in vitro maintenance of hepatic progenitor cells.
The observed impact of NaB on cellular senescence is particularly intriguing. Cellular senescence, often characterized by the irreversible loss of cell proliferation and cell-cycle arrest [26], is a phenomenon relevant not only in regenerative medicine but also in aging and disease processes. The data presented here provide insight into the dual-edged nature of epidrugs interventions. While they can be beneficial for enhancing cellular reprogramming and inhibiting EMT by partially resetting the cellular epigenetic memory, particularly DNA methylation as seen with 5-Aza treatment, they may also exert deleterious effects, as evidenced by the senescence and decreases in hepatic progenitor markers induced by high concentrations of NaB.
The third section of this study delves into the important role of the transcription factor SOX9 in shaping the hepatic progenitor characteristics of mCdHs. Utilizing CRISPRi, SOX9 expression was methodically knocked down at the transcriptional level. The results of this experiment showed a cascade of significant effects. While no substantial changes in cell morphology were observed post-transfection, an evident shift was noticed in cell proliferation, particularly in cells targeted with sgRNA #4 and #5. This implies a direct association between SOX9 expression and the regulation of cell proliferation, which is in line with the crucial role of SOX9 in liver cancer stem cells’ self-renewal, pluripotency, and tumorigenicity [27].
The consequences of this downregulation were further characterized by transcriptomic analysis. It led to a decrease in the expression of several important genetic markers characteristic of mCdHs, including AFP, EpCAM, CD44, and KRT7. Moreover, the expression of hepatic markers such as albumin and HNF4A was also considerably reduced. These findings solidify the crucial role of SOX9 in determining and maintaining liver regeneration by hepatic progenitor cells [28], offering crucial insights into the molecular mechanisms underlying liver regeneration, hepatocyte differentiation, and long-term mCdHs maintenance. Further studies may be required to understand the essential role of additional transcription factors other than SOX9 to the CdHs.
In summary, the results of this study bring to the forefront several key considerations in the realm of regenerative medicine. This emphasizes the importance of epidrugs, especially 5-Aza, in the reprogramming and maintenance of hepatic progenitor cells while also cautioning against their potential adverse effects. Moreover, the pivotal role of SOX9 in maintaining hepatic progenitor characteristics highlights a promising avenue for further research in understanding and manipulating the fate of these cells. Overall, these findings contribute significantly to the broader field of regenerative medicine and hold potential for novel approaches in clinical and therapeutic settings. Further studies should aim to uncover the intricate molecular epigenetic mechanisms underlying these processes and explore their clinical applications in greater and broader detail. In particular, it has been reported that HBV infection results in aberrant hypermethylation that causes fibrosis and even primary liver cancer.
ACKNOWLEDGEMENTS
Hanyang University Medical Center and Samsung Medical Center provided human liver tissues.
Notes
Fund/Grant Support: This research is funded by grants from Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C1234).
References
1. Oh SH, Swiderska-Syn M, Jewell ML, Premont RT, Diehl AM. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J Hepatol. 2018; 69:359–367. PMID: 29758331.
2. Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim SJ, et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J Hepatol. 2019; 70:97–107. PMID: 30240598.
3. Thorgersen EB, Barratt-Due A, Haugaa H, Harboe M, Pischke SE, Nilsson PH, et al. The role of complement in liver injury, regeneration, and transplantation. Hepatology. 2019; 70:725–736. PMID: 30653682.
4. Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007; 282:22089–22101. PMID: 17513865.
5. Lu K, Liu G, Yang L, Liu F, Gao L, Shi J, et al. Sustainable inflammation transforms hepatic cells by causing oxidative stress injury and potential epithelial-mesenchymal transition. Int J Oncol. 2016; 49:971–980. PMID: 27315196.
6. Xue ZF, Wu XM, Liu M. Hepatic regeneration and the epithelial to mesenchymal transition. World J Gastroenterol. 2013; 19:1380–1386. PMID: 23538893.
7. Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. Epigenetic regulation: a new frontier for biomedical engineers. Annu Rev Biomed Eng. 2017; 19:195–219. PMID: 28301736.
8. Kelly TK, De Carvalho DD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotechnol. 2010; 28:1069–1078. PMID: 20944599.
9. Paluch BE, Naqash AR, Brumberger Z, Nemeth MJ, Griffiths EA. Epigenetics: a primer for clinicians. Blood Rev. 2016; 30:285–295. PMID: 26969414.
10. Bedi U, Mishra VK, Wasilewski D, Scheel C, Johnsen SA. Epigenetic plasticity: a central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget. 2014; 5:2016–2029. PMID: 24840099.
11. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008; 14:818–829. PMID: 18539112.
12. Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018; 154:46–56. PMID: 29107021.
13. Dhawan A, Chaijitraruch N, Fitzpatrick E, Bansal S, Filippi C, Lehec SC, et al. Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children. J Hepatol. 2020; 72:877–884. PMID: 31843649.
14. Ho CM, Chen YH, Chien CS, Ho SL, Chen HL, Hu RH, et al. Hepatocyte and mesenchymal stem cell co-transplantation in rats with acute liver failure. Korean J Transplant. 2020; 34:100–108. PMID: 35769351.
15. Mu N, Liu HB, Meng QH, Du DW, Jiang Y, Hu HZ. The differentiation of human multipotent adult progenitor cells into hepatocyte-like cells induced by coculture with human hepatocyte line L02. Ann Surg Treat Res. 2015; 88:1–7. PMID: 25553318.
16. Buisson EM, Park SH, Kim M, Kang K, Yoon S, Lee JE, et al. Transplantation of patient-specific bile duct bioengineered with chemically reprogrammed and microtopographically differentiated cells. Bioeng Transl Med. 2021; 7:e10252. PMID: 35079629.
17. Kim Y, Hong SA, Yu J, Eom J, Jang K, Yoon S, et al. Adenine base editing and prime editing of chemically derived hepatic progenitors rescue genetic liver disease. Cell Stem Cell. 2021; 28:1614–1624. PMID: 33951479.
18. Salas-Silva S, Kim Y, Kim TH, Kim M, Seo D, Choi J, et al. Human chemically-derived hepatic progenitors (hCdHs) as a source of liver organoid generation: application in regenerative medicine, disease modeling, and toxicology testing. Biomaterials. 2023; 303:122360. PMID: 38465578.
19. Nie YZ, Zheng YW, Taniguchi H. Improving the repopulation capacity of elderly human hepatocytes by decoding aging-associated hepatocyte plasticity. Hepatology. 2022; 76:1030–1045. PMID: 35243665.
20. Zhang W, Qu J, Liu GH, Belmonte JC. The ageing epigenome and its rejuvenation. Nat Rev Mol Cell Biol. 2020; 21:137–150. PMID: 32020082.
21. Tanimizu N, Ichinohe N, Yamamoto M, Akiyama H, Nishikawa Y, Mitaka T. Progressive induction of hepatocyte progenitor cells in chronically injured liver. Sci Rep. 2017; 7:39990. PMID: 28051157.
22. Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009; 136:2325–2333. PMID: 19403103.
23. Kim M, Kim Y, Silva ES, Adisasmita M, Kim KS, Jung YK, et al. Enhancing generation efficiency of liver organoids in a collagen scaffold using human chemically derived hepatic progenitors. Ann Hepatobiliary Pancreat Surg. 2023; 27:342–349. PMID: 37661098.
24. Falahi F, Sgro A, Blancafort P. Epigenome engineering in cancer: fairytale or a realistic path to the clinic? Front Oncol. 2015; 5:22. PMID: 25705610.
25. Mann DA. Epigenetics in liver disease. Hepatology. 2014; 60:1418–1425. PMID: 24633972.
26. Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004; 23:2919–2933. PMID: 15077154.
27. Liu C, Liu L, Chen X, Cheng J, Zhang H, Shen J, et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology. 2016; 64:117–129. PMID: 26910875.
28. Pastore N, Huynh T, Herz NJ, Calcagni’ A, Klisch TJ, Brunetti L, et al. TFEB regulates murine liver cell fate during development and regeneration. Nat Commun. 2020; 11:2461. PMID: 32424153.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–3 can be found via https://doi.org/10.4174/astr.2024.106.5.274.
Fig. 1
Effect of epigenetic modulating drugs (epidrugs) on the cellular reprogramming of human primary hepatocytes (hPHs) generation into human chemically-derived hepatic progenitors (hCdHs). (A) Scheme of the generation of hCdHs perfused hPHs with the treatment of epidrugs 5-azacytidine (5-Aza) and sodium butyrate (NaB). (B) Brightfield imaging of the reprogramming process of hCdHs with epidrugs treatment with different drug concentrations (5-Aza, 0.5 µM; NaB, 0–100 µM). Scale bars, 100 µm. (C–E) Relative messenger RNA (mRNA) expression level of hepatic progenitor markers (EpCAM, SOX9) (C), epithelial-mesenchymal transition-related transcription factor (TWIST1, SNAI1) (D), and mesenchymal marker (COL1A1) (E). Values are presented as mean ± standard error of mean in triplicates (n = 3). HAC, HGF, A83-01, CHIR-99021; HGF, hepatocyte growth factor; EpCAM, epithelial cell adhesion molecule; SOX9, SRY-box transcription factor 9; SNAI1, Snail family transcriptional repressor 1; TWIST1, twist family bHLH transcription factor 1; COL1A1, collagen type I alpha 1 chain; NS, not significant. *P < 0.05 and ****P < 0.001, by 2-tailed t-test.
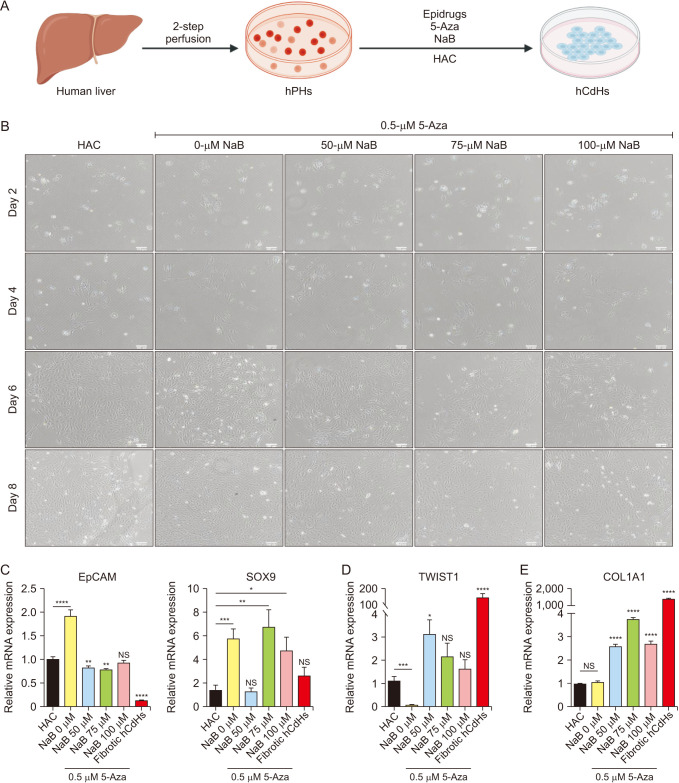
Fig. 2
Epigenetic modulating drugs (epidrugs) inhibit epithelial-mesenchymal transition (EMT)-driven fibrogenesis by regulating the EMT transcription factors SNAI1 and TWIST1. (A) Brightfield imaging of human chemically-derived hepatic progenitors (hCdHs) and fibrotic hCdHs. (B) Schematic image of the treatment of 5-azacytidine (5-Aza) and sodium butyrate (NaB) on the long-term maintenance and culture of hCdHs. (C) Brightfield imaging of the long-term culture and expansion of hCdHs treated with epidrugs with different drug concentrations on day 2 and day 5 after passaging. Scale bars, 100 µm. (D–F) Relative messenger RNA (mRNA) expression level of hepatic progenitor markers (EpCAM, SOX9) (D), EMT-related transcription factor (TWIST1, SNAI1) (E), and mesenchymal marker (COL1A1) (F). Values are presented as mean ± standard error of mean in triplicates (n = 3). HAC, HGF, A83-01, CHIR-99021; HGF, hepatocyte growth factor; EpCAM, epithelial cell adhesion molecule; SOX9, SRY-box transcription factor 9; SNAI1, Snail family transcriptional repressor 1; TWIST1, twist family bHLH transcription factor 1; COL1A1, collagen type I alpha 1 chain; NS, not significant. *P < 0.05 and ****P < 0.001, by 2-tailed t-test.
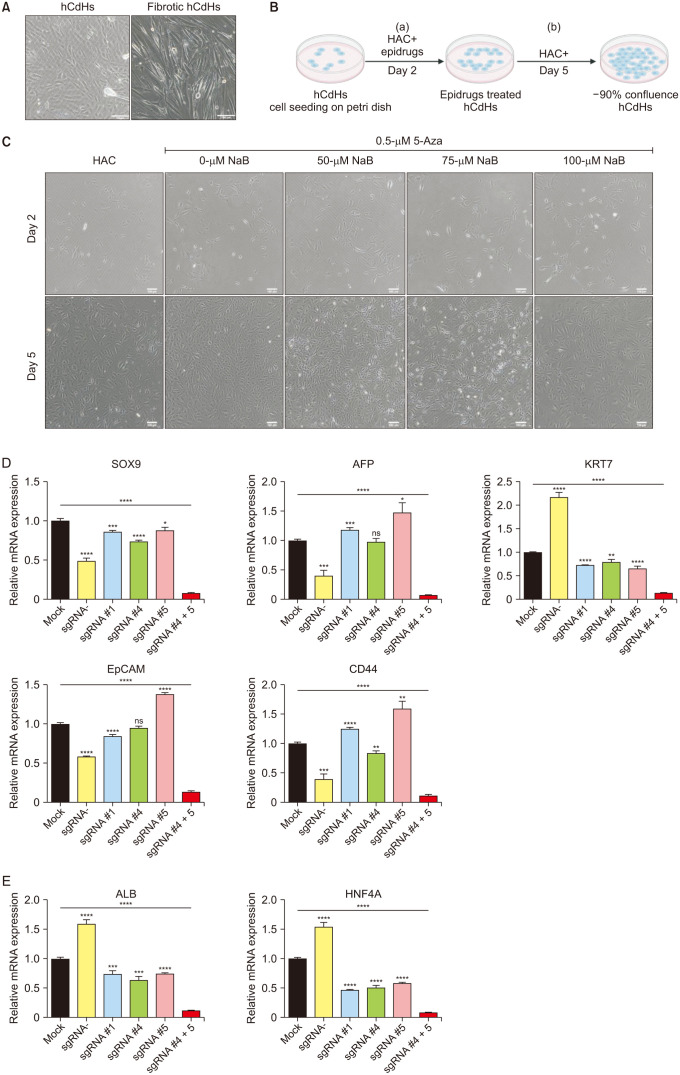
Fig. 3
Clustered regularly interspaced short palindromic repeats-interference (CRISPRi)-mediated SOX9 knockdown demonstrates SOX9 essential role as a transcription factor of mouse chemically-derived hepatic progenitors (mCdHs). (A) Illustration of the SOX9 repression by CRISPRi electroporation. (B) SOX9 genome map shows the single guide RNA (sgRNA) target site (sgRNA#1, #4, and #5) relative to its transcription start site. (C) Brightfield imaging of the impact of SOX9 repression by CRISPRi with and without sgRNA transfection on mCdHs. Scale bars, 100 µm (a) and 500 µm (b). Relative messenger RNA (mRNA) expression level of hepatic progenitor markers (SOX9, AFP, KRT7, EpCAM, CD44) (D) and hepatic marker (ALB, HNF4A) (E). Values are presented as means ± standard error of mean in triplicates (n = 3). AFP, alpha-fetoprotein; KRT7, keratin 7; EpCAM, epithelial cell adhesion molecule; CD44, cluster of differentiation 44; ALB, albumin; HNF4A, hepatocyte nuclear factor 4 alpha. *P < 0.05 and ****P < 0.001, by 2-tailed t-test.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download