Abstract
Purpose
This study was performed to analyze the association between age and outcomes of carotid endarterectomy (CEA) by comparing postoperative outcomes between octogenarians and younger patients.
Methods
From November 1994 to December 2022, 1,585 internal carotid arteries of 1,434 patients were enrolled. Patients were stratified into 2 groups: octogenarians (≥80 years old) and non-octogenarians (<80 years old). Primary endpoints were early (≤30 days) outcomes of ipsilateral stroke, any stroke, myocardial infarction, death, and major adverse cardiovascular events (MACE). We also compared overall any stroke and death between the 2 groups.
Results
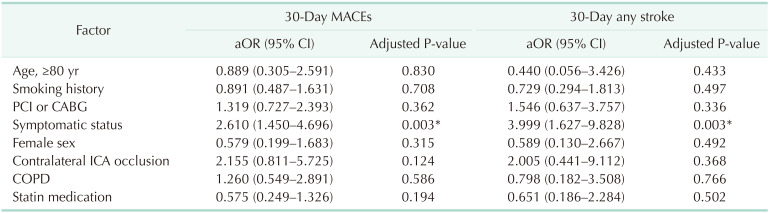
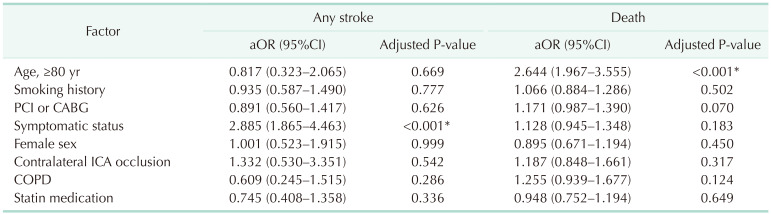
One of 132 octogenarians (0.8%) and 17 of 1,453 non-octogenarians (1.1%) experienced ipsilateral stroke within 30 days. Thirty-day MACE occurred in 4 of 132 octogenarians (3%) and 44 of 1,453 non-octogenarians (3%). There were no significant differences in any early (≤30 days) outcomes. Symptomatic status was associated with increased 30-day MACE (odds ratio [OR], 2.610; 95% confidence interval [CI], 1.450–4.696; P = 0.003) and 30-day any stroke (OR, 3.999; 95% CI, 1.627–9.828; P = 0.003). Symptomatic status was also associated with overall any stroke (hazard ratio [HR], 2.885; 95% CI, 1.865–4.463; P < 0.001), but age of ≥80 years was not associated with 30-day MACE, 30-day any stroke, or overall stroke. Age of ≥80 years was only associated with overall survival (HR, 2.644; 95% CI, 1.967–3.555; P < 0.001).
According to the Global Burden of Diseases, Injury, and Risk Factor Study, stroke is the second leading cause of death and the third leading cause of disability worldwide [1]. Extracranial internal carotid stenosis is a major cause of ischemic stroke, estimated to cause 8%–15% of cases, and it is characterized by a high rate of recurrence [2]. Carotid endarterectomy (CEA) decreases the chance of ischemic stroke in asymptomatic patients and prevents recurrent stroke in symptomatic patients. Current stroke prevention guidelines recommend CEA over maximal medical therapy for asymptomatic low-risk patients with stenosis of 70%–99% [3]. The guidelines also recommend CEA over carotid artery stenting (CAS) for symptomatic low-risk surgical patients with stenosis of 50%–99% [3].
Elderly patients are characterized by a limited life expectancy and more frequent medical comorbidities compared to younger patients; these factors reduce benefits and increase the operative risk of CEA. In the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial, age of ≥80 years was a high-risk factor for CEA [4]. Additional studies also reported elderly patients to have a slightly higher risk of complications than younger patients [56]. However, it is uncertain whether advanced age itself is a risk factor for CEA, and previous stroke prevention guidelines do not specify this issue [378].
The aim of the study is to analyze the association between age and outcomes of CEA by comparing postoperative outcomes between octogenarians and younger patients.
This study was approved by the Institutional Review Board of Samsung Medical Center in Seoul, Korea (No. 2023-12-130). Given the retrospective nature of the study, the need for informed consent was waived.
We retrospectively analyzed the vascular surgery registry from a single tertiary referral center. From November 1994 to December 2022, 1,642 internal carotid arteries underwent elective CEA. All patients underwent preoperative duplex ultrasonography or CT angiography, and the degree of stenosis was calculated according to the North American Symptomatic Carotid Endarterectomy Trial criteria. In duplex ultrasound, the degree of stenosis was calculated by measuring the peak systolic velocity and end-diastolic velocity. We included patients with symptomatic moderate to high grade (50%–99%) stenosis or asymptomatic high grade (70%–99%) stenosis as an indication for CEA. Symptomatic carotid stenosis was defined as an acute transient ischemic attack or neurologic event lasting for 24 hours or more within 6 months. We excluded those treated with CEA with coronary artery bypass graft (CABG) or aorta surgery (n = 55), CEA after stent restenosis (n = 1), and CEA after iatrogenic internal carotid artery injury (n = 1). Finally, a total of 1,585 arteries of 1,434 patients were enrolled, of which 132 belonged to octogenarians and 1,453 to non-octogenarians.
To analyze the association between age and outcome, patients were stratified into octogenarians (≥80 years old) and younger patients (<80 years old). We collected the following baseline characteristics: age; sex; medical comorbidities including hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, chronic kidney disease, and chronic obstructive pulmonary disease (COPD); history of neck radiation therapy; preoperative symptomatic status; and statin medication. Primary endpoints were early (≤30 days) outcomes of ipsilateral stroke, any stroke, myocardial infarction (MI), death, and major adverse cardiovascular events (MACE). MACE was defined as composite outcomes including transient ischemic attack, any stroke, MI, and death. Secondary endpoints were overall complications; long-term any stroke and survival. We also compared overall complications including any stroke and death between the 2 groups. We defined postoperative stroke as an acute symptomatic neurologic defect lasting for 24 hours or more with consistent cerebral ischemia with evidence on a radiologic image. We defined postoperative MI as symptoms consistent with ischemia with an increased cardiac enzyme or ST abnormality on an electrocardiogram. Additional surgical complications were compared between octogenarians and non-octogenarians: cerebral hyperperfusion syndrome, cranial nerve palsy (transient or permanent), and postoperative bleeding.
IBM SPSS Statistics ver. 27.0 (IBM Corp.) was used and we considered P < 0.05 as significant. In univariable analysis, the chi-square and Fisher exact tests were used for comparison of categorical variables. The statistically significant variables in univariable analysis and clinically significant variables were used in logistic regression analysis and the Cox proportional hazards model. Survival analysis for freedom from stroke rates and overall survival was conducted by a Kaplan-Meier survival analysis, and the differences were analyzed by a log-rank test.
Table 1 shows the demographics and comorbidities of the 110 octogenarians (7.7%) and 1,324 non-octogenarians (92.3%). Octogenarians were more likely to have COPD (18.2% vs. 11.3%, P = 0.033), statin medication (95.5% vs. 89.9%, P =0.033), and to be symptomatic (57.3% vs. 31.2%, P < 0.001) and were less likely to have percutaneous coronary intervention (PCI) or CABG (26.4% vs. 38.2%, P = 0.014) and smoking history (27.3% vs. 49.4%, P < 0.001) compared with non-octogenarians. Table 2 shows preoperative anatomical features and intraoperative procedures. There were no significant differences in the proportion of severe stenosis (≥70%), contralateral carotid occlusion, level of upper border, and surgical technique. Table 3 shows the follow-up durations and early complications of the 2 groups. The median follow-up duration was shorter in octogenarians than in non-octogenarians (31 months [interquartile range (IQR), 11–58 months] vs. 56 months [IQR, 22–107 months]). One of 132 octogenarians (0.8%) and 17 of 1,453 non-octogenarians (1.1%) suffered from ipsilateral stroke within 30 days. MACE occurred in 4 octogenarians (3.0%) and 44 non-octogenarians (3.0%). There were no significant differences in any other early (≤30 days) complications. Table 4 shows factors associated with 30-day MACE and any stroke in the 2 groups. Symptomatic status was associated with increased 30-day MACE (odds ratio [OR], 2.610; 95% confidence interval [CI], 1.450–4.696; P = 0.003) and 30-day any stroke (OR, 3.999; 95% CI, 1.627–9.828; P = 0.003). Table 5 shows factors associated with overall any stroke and death. Symptomatic status was also associated with overall any stroke (hazard ratio [HR], 2.885; 95% CI, 1.865–4.463; P < 0.001), but age of ≥80 years was not associated with 30-day MACE, 30-day any stroke, and overall stroke. Age of ≥80 years was only associated with overall survival (HR, 2.644; 95% CI, 1.967–3.555; P < 0.001).
Although advanced age is associated with higher frailty and operative risk, it is uncertain whether advanced age itself is a high-risk factor for CEA [569]. Only a few studies have analyzed the associations between age and outcomes of CEA in Asian populations. Jeong et al. [10] reported no significant difference in the risks of perioperative MACE and the 4-year overall incidence of cardiovascular events between younger and elderly patients in Korea. However, the age threshold was 60 years, which was not old enough to represent elderly patients.
In our study, age of ≥80 years was not associated with increased postoperative risk of stroke. And symptomatic status, a well-known risk factor for postoperative risk of stroke, showed a similar trend, but contralateral carotid stenosis did not [1112]. In the demographic analysis, octogenarians were more likely to be symptomatic and less likely to have PCI or CABG compared with younger patients. This is contrary to the common belief that elderly patients have more underlying medical comorbidities than younger patients. There may have been a bias due to our choice of best medical treatment (BMT) or CAS rather than CEA for asymptomatic octogenarians with severe cardiovascular comorbidities and other high operative risk factors, which may have increased the calculated proportion of symptomatic patients in octogenarians. It is also possible that asymptomatic octogenarians who are treated with BMT later undergo CEA due to the development of symptoms.
In asymptomatic carotid artery stenosis, the guideline recommends a < 3% perioperative risk of stroke and death to achieve benefit for the patients [3]. In our study including both symptomatic and asymptomatic patients, the 30-day any stroke rate was low at 0.8% in octogenarians and 1.4% in non-octogenarians, and there was no 30-day mortality. CEA is a comparable procedure for octogenarians with acceptable operative risk, and old age is not a contraindication for CEA. Over the past few decades, the role of BMT has been expanding with the development of antiplatelet, antihypertensive, and cholesterol-lowering drugs. Reiff et al. [13] reported that CEA plus BMT was not superior to BMT alone regarding the risk of any stroke, death within 30 days, or ipsilateral stroke during a 5-year observation period. For octogenarians with severe comorbidities, BMT can be an effective treatment option.
In symptomatic carotid stenosis, CAS, a minimally invasive technique, is usually preferred for patients with severe medical comorbidities or other risk factors for CEA, such as prior neck irradiation, post-endarterectomy restenosis, or surgically inaccessible severe lesion [141516]. However, elderly patients might be unsuitable for CAS because greater systemic atherosclerosis could exist along the catheter route of CAS due to aging [17]. In the 2021 American Heart Association/American Stroke Association guidelines, the recommendation was updated to consider CEA rather than CAS in symptomatic patients older than 70 years [18]. CEA should be more frequently recommended for octogenarians due to its acceptable operative risk. For elderly patients with severe comorbidities, alternative treatments like transcarotid artery revascularization are needed for better outcomes [19].
Seo et al. [20] reported an analysis of the Korean nationwide database of the Health Insurance Review and Assessment Service from 2007 to 2016, with 3,892 (24.2%) and 12,173 patients (75.8%) with CEA and CAS, respectively. In the United States Medicare beneficiaries, 937,111 (80%) patients underwent CEA and 231,077 (20%) underwent CAS from 1999 to 2014 [21]. Although CEA is recommended as a first-line treatment option for carotid stenosis, CAS has been preferred in Korea and is performed 3 times more frequently than CEA. Considering the situation in Korea, which is becoming a severely aged population, CEA could be a treatment option for elderly patients with acceptable operative risk.
The present study had several limitations. First, it was a retrospective study performed in a single center and the total number of the octogenarians was small compared with the younger patients. This imbalance in the number of each group may have caused bias. Second, elderly patients with severe comorbidities often undergo BMT instead of CEA in our hospital, and they were excluded from the analysis. Further research is needed to prove the secondary prevention effect of stroke in elderly patients by comparing surgery and non-surgery groups. Third, the assessment for operative risk was unclear and the choice of treatment option was based on the subjective surgeon decision. Despite these limitations, the operative outcomes of CEA performed by skilled surgeons in octogenarians were comparable with those of younger patients. Our study suggests that CEA may be a good treatment option for patients with acceptable operative risk regardless of age.
In conclusion, CEA would be a safe and effective treatment for octogenarians with low 30-day complications and long-term stroke rates, comparable with that of younger counterparts. Advanced age is not a contraindication for CEA.
References
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380:2095–2128. PMID: 23245604.
2. Krawisz AK, Carroll BJ, Secemsky EA. Risk stratification and management of extracranial carotid artery disease. Cardiol Clin. 2021; 39:539–549. PMID: 34686266.
3. AbuRahma AF, Avgerinos ED, Chang RW, Darling RC 3rd, Duncan AA, Forbes TL, et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J Vasc Surg. 2022; 75(1S):4S–22S. PMID: 34153348.
4. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004; 351:1493–1501. PMID: 15470212.
5. Schneider JR, Jackson CR, Helenowski IB, Verta MJ, Wilkinson JB, Kim S, et al. A comparison of results of carotid endarterectomy in octogenarians and nonagenarians to younger patients from the Mid-America Vascular Study Group and the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2017; 65:1643–1652. PMID: 28259574.
6. Nantakool S, Chuatrakoon B, Orrapin S, Leung R, Howard DP, Rerkasem A, et al. Influences of age and gender on operative risks following carotid endarterectomy: a systematic review and meta-analysis. PLoS One. 2023; 18:e0285540. PMID: 37163559.
7. Glousman BN, Sebastian R, Macsata R, Kuang X, Yang A, Patel D, et al. Carotid endarterectomy for asymptomatic carotid stenosis is safe in octogenarians. J Vasc Surg. 2020; 71:518–524. PMID: 31471235.
8. Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: executive summary. J Vasc Surg. 2011; 54:832–836. PMID: 21889705.
9. Fahad S, Shirsath S, Metcalfe M, Elmallah A. Carotid endarterectomy in the very elderly: short-, medium-, and long-term outcomes. Vasc Specialist Int. 2023; 39:28. PMID: 37748930.
10. Jeong MJ, Kwon SU, Kim MJ, Han Y, Kwon TW, Cho YP. Effects of patient age on outcomes after carotid endarterectomy: a retrospective, single-center study in Korea. Medicine (Baltimore). 2019; 98:e16781. PMID: 31393403.
11. Ball S, Ball A, Antoniou GA. Editor’s choice: prognostic role of pre-operative symptom status in carotid endarterectomy: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2020; 59:516–524. PMID: 32081531.
12. Jeong MJ, Kwon H, Kim MJ, Han Y, Kwon TW, Cho YP. Effect of severe contralateral carotid stenosis or occlusion on early and late outcomes after carotid endarterectomy. Ann Surg Treat Res. 2019; 97:202–209. PMID: 31620394.
13. Reiff T, Eckstein HH, Mansmann U, Jansen O, Fraedrich G, Mudra H, et al. Carotid endarterectomy or stenting or best medical treatment alone for moderate-to-severe asymptomatic carotid artery stenosis: 5-year results of a multicentre, randomised controlled trial. Lancet Neurol. 2022; 21:877–888. PMID: 36115360.
14. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236. PMID: 24788967.
15. Kang J, Woo SY, Yang SS, Park YJ, Kim DI, Jeon P, et al. Treatment results of carotid endarterectomy and carotid artery stenting for patients with radiation-induced carotid stenosis. Ann Surg Treat Res. 2022; 103:112–118. PMID: 36017138.
16. Mendez-Sosa MA, Contreras-Jimenez E, Anaya-Ayala JE, Miranda-Ramirez MW, Lopez-Pena G, Arzola LH, et al. Surgical management of a type II extracranial internal carotid aneurysm near to the skull base. Vasc Specialist Int. 2021; 37:27. PMID: 34349047.
17. Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G, et al. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet. 2016; 387:1305–1311. PMID: 26880122.
18. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021; 52:e364–e467. PMID: 34024117.
19. Park JK, Woo SY, Park YJ, Kim DI, Kim GM, Seo WK, et al. The Impact of age and outcomes of high-risk patients on carotid revascularization. Vascular. 2023; 17085381231155035. PMID: 36794829.
20. Seo KD, Lee KY, Suh SH. Comparison of long term prognosis between carotid endarterectomy versus stenting; a Korean population-based study using national insurance data. Neurointervention. 2019; 14:82–90. PMID: 31450880.
21. Lichtman JH, Jones MR, Leifheit EC, Sheffet AJ, Howard G, Lal BK, et al. Carotid endarterectomy and carotid artery stenting in the US medicare population, 1999-2014. JAMA. 2017; 318:1035–1046. PMID: 28975306.
Fig. 1
The Kaplan-Meier survival curve of the freedom from stroke rates in octogenarians and non-octogenarians who underwent carotid endarterectomy.
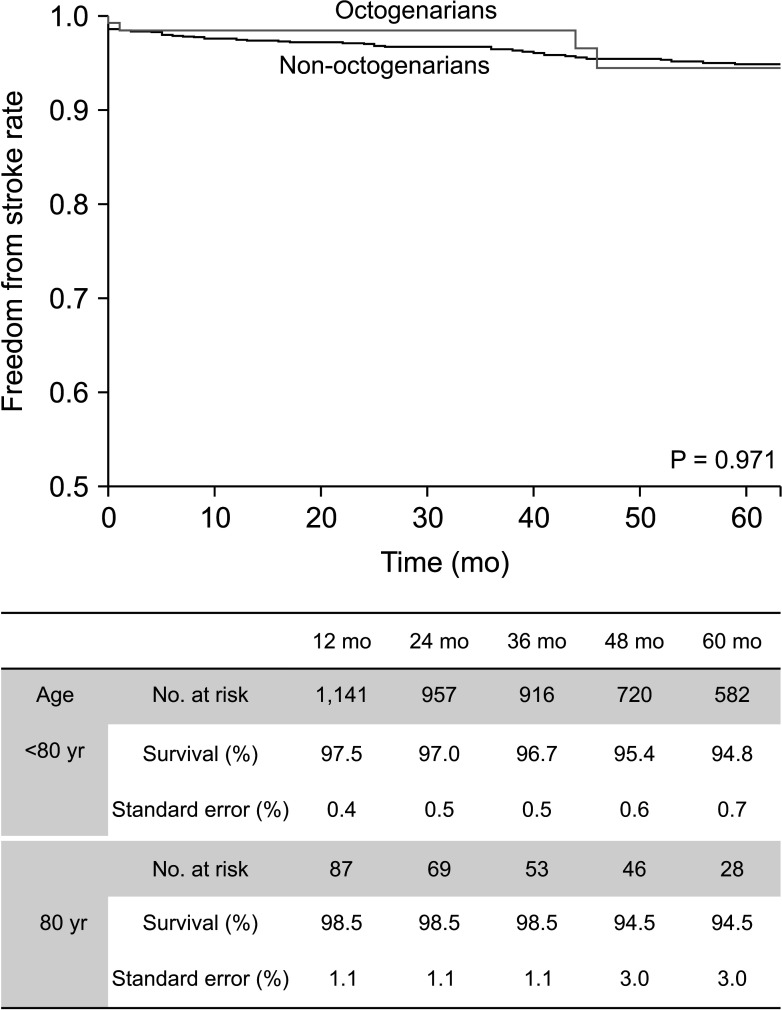
Fig. 2
The Kaplan-Meier survival curve of the overall survival in octogenarians and non-octogenarians who underwent carotid endarterectomy.
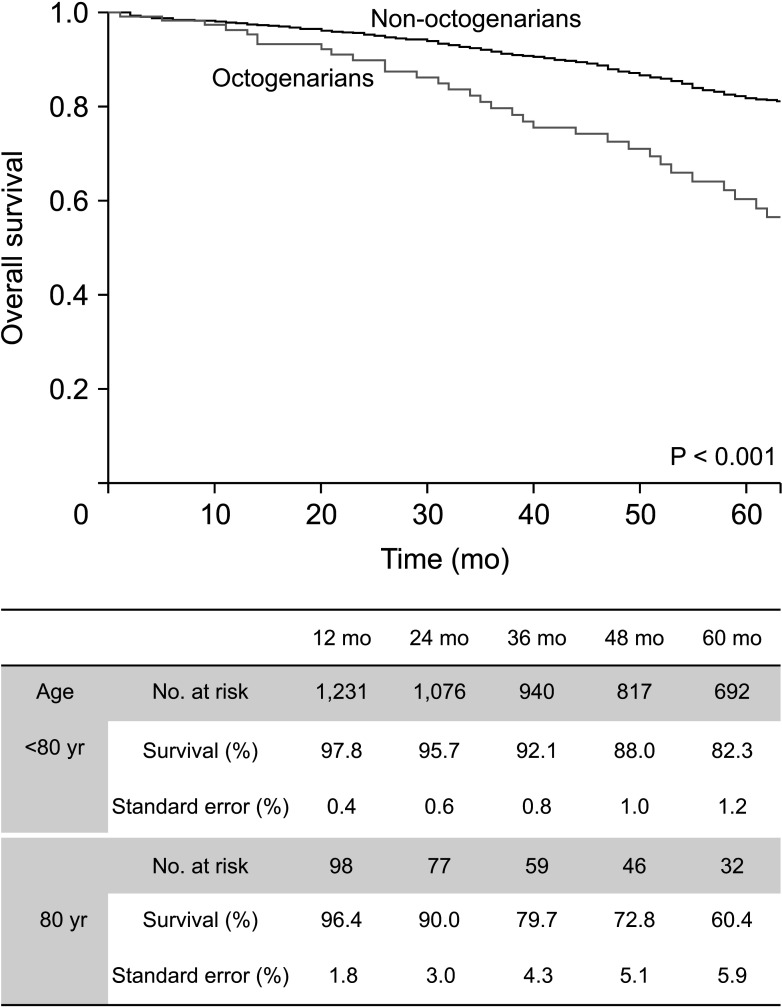
Table 1
Demographics and comorbidities of octogenarians and non-octogenarians who underwent carotid endarterectomy between 1994 to 2022
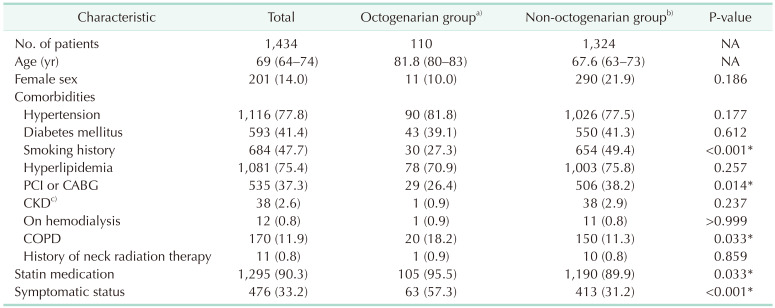
Values are presented as number only, median (interquartile range), or number (%).
NA, not applicable; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
a)≥80 years old, b)<80 years old. c)Creatinine ≥2 mg/dL.
*P < 0.05, statistically significant.
Table 2
Anatomical features and surgical technique of octogenarians and non-octogenarians who underwent carotid endarterectomy
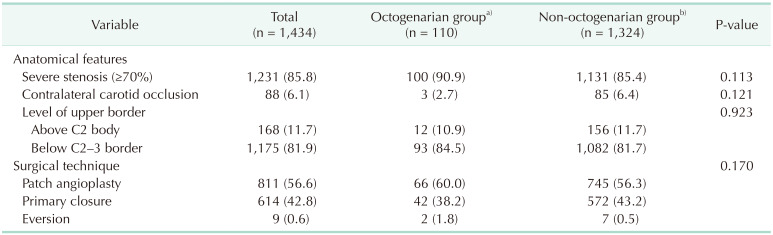
Table 3
Univariable analysis of early (≤30 days) and late outcomes of octogenarians and non-octogenarians who underwent carotid endarterectomy
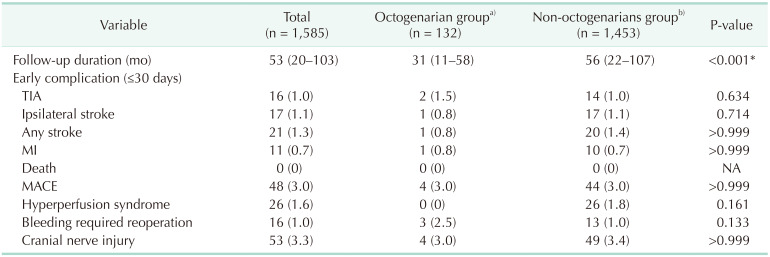
Table 4
Factors associated with the occurrence of MACEs and any stroke within 30-days after carotid endarterectomy




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download