Abstract
Purpose
Intraoperative neurophysiological monitoring (IONM) has been introduced in thyroid surgery to prevent injury of the recurrent laryngeal nerve (RLN). However, its effectiveness remains controversial in robotic thyroidectomy (RT). This study aimed to compare the surgical outcome of RT in patients with and without the application of IONM.
Methods
This retrospective case-control study included 100 patients who underwent total thyroidectomy via robotic bilateral axillo-breast approach in a tertiary center. A study group of 50 patients who had IONM during RT was compared to a control group of 50 patients who underwent RT with nerve visualization alone.
Results
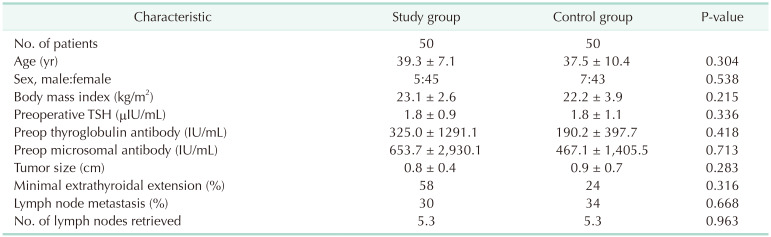
The sex ratio (4:45 vs. 7:43, P = 0.538), mean age (39.3 ± 7.1 years vs. 37.5 ± 10.4 years, P = 0.304), and body mass index (23.1 ± 2.6 kg/m2
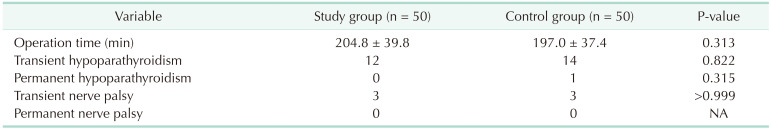
vs. 22.2 ± 3.9 kg/m2, P = 0.215) were comparable between the IONM and control groups. Pathologic features including tumor size (0.8 cm vs. 0.9 cm, P = 0.283), extrathyroidal extension (58.0% vs. 24.0%, P = 0.316), lymph node metastasis (30% vs. 34%, P = 0.668), and number of lymph nodes (5.3 vs. 5.3, P = 0.668) showed no differences. There was no permanent RLN palsy, postoperative bleeding, and wound complications. Transient hypoparathyroidism was observed in 12 (24.0%) and 14 (28.0%), permanent hypoparathyroidism in 0 (0%) and 1 (2.0%), and transient RLN palsy was observed in 3 (6.0%) and 3 (6.0%), respectively.
The number of institutions conducting minimally invasive thyroid surgery has rapidly grown globally since its introduction in 1996 [1]. An important impetus towards this change was brought about by the application of da Vinci robotic surgical system (Intuitive Surgical, Inc.) to the various minimally invasive thyroidectomy procedures [23]. The greatest advantage of the robotic system is that it has allowed surgeons to retain the cosmetic superiority of minimally invasive operations while overcoming the limitations of conventional laparoscopic operations by allowing precise dissection in a confined operation field, which leads to the increasing application of robot assistance in expert-centered features including 3-dimensional (3D) high-definition magnified view from 10 to 12 times, operator-controlled camera, articulating instruments with increased range of motion, motion scaling, ergonomic designs, and tremor filtration allowing more surgeons to join along with the trend of minimally invasive thyroidectomy with less difficulty.
Thyroidectomy, however, is a procedure that requires meticulous handling of the recurrent laryngeal nerve (RLN), among all other structures. Although many articles report the safety of RLN preservation in robotic thyroidectomy (RT) [456], the evidence is inconclusive even among meta-analyses; while some report a statistically nonsignificant increase in transient RLN injury compared to conventional open procedures [17], 1 study reveals a statistically significant difference in transient RLN injury after RT [8]. Several factors may cause such results. Minimally invasive surgery depends heavily on energy-based devices which easily cause thermal damage. Furthermore, a downside of the robotic system is the lack of tactile sensation, which leaves the RLN prone to traction injury. Traction and thermal damage together comprise 88% of the cause of vocal cord palsy after thyroidectomy [9]. The intraoperative neurophysiological monitoring (IONM) [10] is therefore applied by many surgeons performing RT to monitor the nerve function during operation and therefore prevent possible nerve injuries [111213].
The effectiveness of IONM in protecting RLN in conventional open or endoscopic thyroidectomy has been reported [1014]. However, its utility in the RT setting yet lacks consensus; while reports of reduced time needed for voice recovery do exist, the number of cases and reports is too small to arrive at a conclusion [101516]. The objective of this study was to demonstrate our initial experience of IONM application and its advantage in improving surgical outcomes in the RT setting by comparing surgical outcomes of RT patients with and without the application of IONM.
The Institutional Review Board of Ewha Womans University Medical Center approved this retrospective cohort study (No. 2022-07-032), and informed consent was exempted due to its retrospective nature.
This retrospective case-control study included 100 patients who underwent total thyroidectomy using a robot-assisted bilateral axillo-breast approach (BABA) from March 2016 to May 2017. Previous studies have demonstrated the detailed surgical procedures of BABA RT [17] and it will not be discussed in this article.
Because of the limited number of IONM systems, IONM was performed on the basis of the availability of equipment. During the study period, 50 consecutive patients in which IONM was not utilized were categorized into the study group. The control group included another 50 consecutive patients in which IONM was utilized. As there were no selection criteria for IONM, we included all eligible patients who underwent BABA RT. Patients in the study group have undergone BABA RT with the intraoperative IONM nerve monitoring system (Medtronic Xomed) to identify the RLN. The Da Vinci Si system was used for the operation and the nerve integrity monitor (NIM version 3.0 with standard reinforced electromyography tube, Medtronic Xomed) was used for nerve monitoring. We used the robotic instrument itself (hook bovies) as a stimulating probe, by connecting it to the IONM system with a custom-made cable as previously described [18].
Clinicopathological characteristics and postoperative surgical outcome data pertaining to RLN palsy, hypoparathyroidism, bleeding, and wound problems were collected. The primary endpoint of this study was the transient RLN palsy rate.
IBM SPSS Statistics ver. 22.0 (IBM Corp.) and R software ver. 4.1.2 (The R Foundation) were used for statistical analyses. The Student t-tests were used to compare continuous variables. Categorical variables were compared using the chi-square tests. Comparisons demonstrating a P-value less than 0.05 were considered statistically significant.
Among the 100 patients recruited in this study, 50 received RT with IONM (study group) and the other 50 underwent RT with nerve visualization only (control group) (Table 1). There were 5 male and 45 female patients in the IONM group and 7 male and 43 female patients in the control group (P = 0.538). The mean age was 39.3 ± 7.1 years and 37.5 ± 10.4 years (P = 0.304), and the body mass index was 23.1 kg/m2 and 22.2 kg/m2 (P = 0.215) for the IONM and control group, respectively. The mean preoperative thyroid-stimulating hormone, thyroglobulin antibody, and microsomal antibody titer for the IONM and control group was 1.8 ± 0.9 µIU/mL and 1.8 ± 1.1 µIU/mL, 325.0 ± 1,291.1 U/mL and 190.2 ± 397.7 U/mL, and 653.7 ± 2,930.1 U/mL and 467.1 ± 1,405.5 U/mL, respectively. There were no statistically significant differences in the final pathology reports in features such as tumor size (0.8 cm vs. 0.9 cm, P = 0.283), presence of minimal extrathyroidal extension (58.0% vs. 24.0%, P = 0.316), number of lymph nodes retrieved (5.3 vs. 5.3, P = 0.963), and number of patients with lymph node metastasis (15 [30.0%] vs. 17 [34.0%], P = 0.668) between the IONM and control group, respectively. Furthermore, there were no differences in the distribution of tumor stage according to the 8th edition of the AJCC (American Joint Committee on Cancer) Cancer Staging Manual between both groups.
A comparison of the surgical outcomes of both groups demonstrated that there were no significant differences between both groups (Table 2). The mean operation time was 204.8 ± 39.8 minutes and 197.0 ± 37.4 minutes (P = 0.313) for the IONM and control groups, respectively. There were no cases of permanent RLN palsies, postoperative bleeding, or wound complications in either group. Transient hypoparathyroidism was observed in 12 (24.0%) and 14 patients (28.0%) (P = 0.822), permanent hypoparathyroidism was observed in 0 (0%) and 1 person (2.0%) (P = 0.315), and transient RLN palsy was observed in 3 (6.0%) and 3 patients (6.0%) (P > 0.999), respectively, in the IONM and control groups.
The advantages of RT, after more than 10 years of being incorporated into clinical practice, have been repeatedly reported in large-scale studies with participants counting up to the thousands [51920]. The superior cosmetic outcome, magnified view, 3D vision, operator-controlled camera, articulating instruments, motion scaling, ergonomic designs, and tremor filtration offered by the robotic system factor out the physical and mental duress under which thyroid surgeons must meticulously preserve anatomic structures such as the RLN. Moreover, the long-term surgical outcome of these reports indicates that, with proper patient selection, RT is oncologically as safe as a conventional counterpart. Such long-term evidence and tangible advantages that the robotic system offers have led to an increase in the number of approaches and cases of robotic thyroidectomies conducted [21].
There are, however, concerns that RT—because of its lack of tactile sensation—could increase the risk of RLN injury by thermal and traction damage which comprise almost 90% of the causes [9]. There have been reports that RT, compared to its conventional counterpart, results in a higher rate of transient RLN injury. In a recent meta-analysis, Lang et al. [8] reported that RT has an odds ratio of 2.444 (95% confidence interval, 1.178–5.068; P = 0.016), compared to open procedures, for transient RLN injuries to occur. In order to prevent such events from happening, surgeons from many institutions have started applying IONM when performing RT.
There are several supposed advantages of applying IONM to RT [10]. First is the expedited RLN identification, especially for recurrent or large tumor cases. The second is the ability to quantitatively assess the postthyroidectomy nerve function in the operation room. Thirdly, surgeons can rely on the IONM signal during risky procedures such as the dissection of the Berry ligament, the use of energy-based devices near the nerve, and the traction of the gland. Finally, it may assist surgeons with little experience with RT to overcome the steep learning curve. The protective effect of IONM, however, has not been comprehensively established as it has been in the open and endoscopic procedures due to a limited number of reported cases to draw solid conclusion from [10]. On the contrary, some argue that applying IONM could increase operation time, result in interference with the energy-based surgical instruments, and not enhance the performance of surgeons’ experience in RT. Furthermore, some adverse effects have been reported during carotid sheath while placing the continuous IONM probe such as vessel injury, hemodynamic instability, and reversible neuropraxia [22].
According to our results, IONM did not significantly reduce the rate of adverse surgical outcomes such as transient/permanent laryngeal nerve palsies, transient/permanent hypoparathyroidism, postoperative bleeding, prolonged operation time, and wound infection. That the surgeon who performed all the operations in this study was highly experienced in RT could offer an explanation for the lack of difference. It is noteworthy, however, that applying the IONM in an RT setting resulted in similar postoperative results to its counterpart without increasing the length of operation time. According to Lang et al. [8], RT had a statistically significant increased overall operation time of 55.8 minutes compared to that of open thyroidectomy. This is attributed to the extensive skin flap formation to secure a visual of the operation field and docking of the robotic system. With the RT already taking up much time, allocating more of it for IONM configuration could dissuade surgeons from applying IONM. Our results show that this is not the case. This implies that IONM could give surgeons the aforementioned intangible advantages during operation without delaying surgery [10].
Some limitations exist within our study. First, it was a retrospective study with a relatively small sample size. Therefore, evaluation of the actual voice outcomes such as voice range profile or GRBAS (grade, roughness, breathiness, asthenia, and strain) scale was not documented. Secondly, the instrument version allowed us to use the intermittent IONM function only so we could not assess the effect or adverse events of continuous IONM. Third, RT from our institution included the robotic BABA approach only and, therefore, this result could not be generalized for robotic transaxillary or transoral thyroidectomy procedures. Finally, all the operations were performed by a surgeon experienced in RT; therefore, we could not assess the advantages of IONM which would be emphasized by novice surgeons.
In conclusion, the initial results of our institutional study did not demonstrate a clear advantage of IONM in RT. However, with prospective research with more patients, current intangible advantages of the IONM may be elucidated concerning RT surgical outcomes.
Notes
References
1. Jackson NR, Yao L, Tufano RP, Kandil EH. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck. 2014; 36:137–143. PMID: 23471784.
2. Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009; 146:1048–1055. PMID: 19879615.
3. Lee KE, Rao J, Youn YK. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech. 2009; 19:e71–e75. PMID: 19542833.
4. Kwon H, Yi JW, Song RY, Chai YJ, Kim SJ, Choi JY, et al. Comparison of bilateral axillo-breast approach robotic thyroidectomy with open thyroidectomy for Graves’ disease. World J Surg. 2016; 40:498–504. PMID: 26754077.
5. Kim MJ, Nam KH, Lee SG, Choi JB, Kim TH, Lee CR, et al. Yonsei experience of 5000 gasless transaxillary robotic thyroidectomies. World J Surg. 2018; 42:393–401. PMID: 28879559.
6. Pan JH, Zhou H, Zhao XX, Ding H, Wei L, Qin L, et al. Robotic thyroidectomy versus conventional open thyroidectomy for thyroid cancer: a systematic review and meta-analysis. Surg Endosc. 2017; 31:3985–4001. PMID: 28337546.
7. Son SK, Kim JH, Bae JS, Lee SH. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015; 22:3022–3032. PMID: 25634780.
8. Lang BH, Wong CK, Tsang JS, Wong KP, Wan KY. A systematic review and meta-analysis comparing surgically-related complications between robotic-assisted thyroidectomy and conventional open thyroidectomy. Ann Surg Oncol. 2014; 21:850–861. PMID: 24271160.
9. Dionigi G, Wu CW, Kim HY, Rausei S, Boni L, Chiang FY. Severity of recurrent laryngeal nerve injuries in thyroid surgery. World J Surg. 2016; 40:1373–1381. PMID: 26817650.
10. Dionigi G, Kim HY, Wu CW, Lavazza M, Materazzi G, Lombardi CP, et al. Neuromonitoring in endoscopic and robotic thyroidectomy. Updates Surg. 2017; 69:171–179. PMID: 28439772.
11. Lörincz BB, Möckelmann N, Busch CJ, Hezel M, Knecht R. Automatic periodic stimulation of the vagus nerve during single-incision transaxillary robotic thyroidectomy: feasibility, safety, and first cases. Head Neck. 2016; 38:482–485. PMID: 26540014.
12. Lombardi CP, Raffaelli M, Princi P, Lulli P, Rossi ED, Fadda G, et al. Safety of video-assisted thyroidectomy versus conventional surgery. Head Neck. 2005; 27:58–64. PMID: 15459914.
13. Terris DJ, Anderson SK, Watts TL, Chin E. Laryngeal nerve monitoring and minimally invasive thyroid surgery: complementary technologies. Arch Otolaryngol Head Neck Surg. 2007; 133:1254–1257. PMID: 18086968.
14. Yang S, Zhou L, Lu Z, Ma B, Ji Q, Wang Y. Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. Int J Surg. 2017; 39:104–113. PMID: 28130189.
15. Lee HY, Lee JY, Dionigi G, Bae JW, Kim HY. The efficacy of intraoperative neuromonitoring during robotic thyroidectomy: a prospective, randomized case-control evaluation. J Laparoendosc Adv Surg Tech A. 2015; 25:908–914. PMID: 26575249.
16. Bae DS, Kim SJ. Intraoperative neuromonitoring of the recurrent laryngeal nerve in robotic thyroid surgery. Surg Laparosc Endosc Percutan Tech. 2015; 25:23–26. PMID: 25238177.
17. Kim SJ, Lee KE, Oh BM, Oh EM, Bae DS, Choi JY, et al. Intraoperative neuromonitoring of the external branch of the superior laryngeal nerve during robotic thyroid surgery: a preliminary prospective study. Ann Surg Treat Res. 2015; 89:233–239. PMID: 26576402.
18. Ji YB, Ko SH, Song CM, Sung ES, Lee BJ, Wu CW, et al. Feasibility and efficacy of intraoperative neural monitoring in remote access robotic and endoscopic thyroidectomy. Oral Oncol. 2020; 103:104617. PMID: 32126516.
19. Lee KE, Kim E, Koo do H, Choi JY, Kim KH, Youn YK. Robotic thyroidectomy by bilateral axillo-breast approach: review of 1,026 cases and surgical completeness. Surg Endosc. 2013; 27:2955–2962. PMID: 23436099.
20. Kandil E, Hammad AY, Walvekar RR, Hu T, Masoodi H, Mohamed SE, et al. Robotic thyroidectomy versus nonrobotic approaches: a meta-analysis examining surgical outcomes. Surg Innov. 2016; 23:317–325. PMID: 26525401.
21. Chang EH, Kim HY, Koh YW, Chung WY. Overview of robotic thyroidectomy. Gland Surg. 2017; 6:218–228. PMID: 28713692.
22. Terris DJ, Chaung K, Duke WS. Continuous vagal nerve monitoring is dangerous and should not routinely be done during thyroid surgery. World J Surg. 2015; 39:2471–2476. PMID: 26138874.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download