Abstract
Clostridioides difficile infection (CDI) is a common healthcare-associated infection that is expected to increase with the increases in the elderly population, immunocompromised patients treated with chemotherapy and immunosuppressive drugs, antimicrobial-resistant bacteria, and invasive medical technologies. Accurate diagnosis is critical for proper treatment and management of CDI. Clinical laboratories typically use four methods to diagnose CDI: C. difficile culture, toxin detection using immunoassays, detection of glutamate dehydrogenase using immunoassays, and detection of toxin A/B gene. Each CDI diagnostic test has strengths and limitations, and varies in performance. Guidelines for CDI diagnosis have been developed by organizations that include the European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America/Society for Healthcare Epidemiology of America, and American College of Gastroenterology. Certain other countries have guidelines. In Korea, surveys on CDI diagnosis performed in 2015 and 2018 revealed a shift in CDI testing in clinical laboratories in Korea. It is necessary to develop standardized diagnostic guidelines for CDI appropriate for the Korean context.
Clostridioides difficile (formerly Clostridium difficile) infection (CDI) is a common healthcare-associated infection that most often presents as diarrhea in patients treated with antibiotics. Severe cases can lead to sepsis or toxic megacolon, which can be fatal [1-3]. The United States Centers for Disease Control and Prevention considers C. difficile as an urgent threat to healthcare [4]. In 2017, there were an estimated 12,800 deaths, compared to 14,000 deaths per year in 2013 in United States. The decline in the number of cases is thought to be a result of continued appropriate infection control, antibiotic use, and diagnostic testing. However, C. difficile remains an urgent threat, particularly in terms of healthcare costs, which are estimated to be $1 billion annually in United States.
Unlike in other countries, infections caused by ribotype 027, which has a more severe clinical presentation, are not common in Korea [5]. During 2020-2021, the incidence of CDI in nine tertiary hospitals was reported to be 5.9 cases per 10,000 patient-days [6]. National Health Insurance claims data from Korea indicate the evolution of CDI in Korean medical institutions from 2006 to 2015 [7]. During that time, the number of infections increased by approximately 19 times and infection rate increased by approximately 10 times, especially among the elderly and patients with severe comorbidities. The incidence of CDI is expected to increase with the increases in the elderly population, immunocompromised patients treated with chemotherapy and immunosuppressive drugs, antimicrobial-resistant bacteria, and the development of invasive medical technologies.
Accurate diagnosis is critical for proper treatment and management of CDI. Various methods are used to diagnose CDI, and the large number of diagnostic tests reflect the difficulty of diagnosing CDI [8,9]. Tests for the diagnosis of CDI detect C. difficile strains, toxins A and B, toxin genes (tcdA and tcdB), and glutamate dehydrogenase (GDH) secreted by C. difficile strains. The various detection techniques include culture, latex agglutination, immunochromatography, enzyme immunoassay (EIA), nucleic acid amplification test (NAAT), cell cytotoxicity neutralization assay (CCNA), and toxigenic culture (TC). According to a 2018 survey of diagnostic laboratories and specialists in Korea [10], toxin EIA and NAAT were the most commonly performed CDI diagnostic tests, with a significant decrease in the number of laboratories performing cultures and a significant increase in the number of laboratories performing GDH tests compared with the 2015 survey [11]. Most institutions were using a combination of two or more tests, and the combination of tests varied across institutions.
In this review, we discuss the laboratory diagnosis of CDI, including guidelines and the current status of practice in Korea.
Reference methods include CCNA and TC [8,9]. The CCNA evaluates the cytopathic effect of a toxin by culturing a cell line and determining whether it is neutralized by an antitoxin. TC is a two-step process that involves culturing C. difficile and determining toxin production in the colonies. Methods for detecting toxins include cell line culture, toxin EIA, or gene detection. These two methods have different targets; CCNA detects toxins and TC detects toxigenic C. difficile. Due to the differences in the two reference methods, the results may show inconsistency, and the performance may vary. When evaluating a test, the performance of the test can differ based on the reference method against which it is compared. For example, it is logical to compare the toxin EIA with the CCNA, as both target the toxin; however, it performs unfavorably when compared to TC. In one systematic review and meta-analysis study, the pooled sensitivity of toxin EIA was 83% (95% confidence interval 76%-88%) compared with CCNA, whereas it was lower at 57% (51%-63%) compared with TC [12]. Both reference methods are laborious and time-consuming, making them difficult to perform in clinical laboratories.
Clinical laboratories typically use four methods to diagnose CDI: C. difficile culture, toxin detection using immunoassays, GDH detection using immunoassays, and toxin A/B gene detection [2,8,9,12].
C. difficile culture is performed on a patient's diarrheal stool for 48-72 hours of anaerobic culture on cycloserine cefoxitine fructose agar (CCFA), Clostridium difficile selective agar (CDSA), or commercially available chromogenic media. In the 2018 survey in Korea, commercially available chromogenic media were the most commonly used media [10]. Isolated strains can be identified using automated methods. Even if C. difficile is isolated, there may be strains that do not produce toxins. Therefore, toxin secretion should be confirmed by immunoassays or PCR for the toxin gene. Culturing alone cannot be used to diagnose CDI because it cannot identify toxin-producing strains.
Toxins A and B are detected using C. difficile toxin EIA, which is based on the principles of enzyme-linked f luorescent immunoassay (ELFA) or enzyme-linked immunosorbent assay (ELISA). The toxin EIA is the most commonly used CDI diagnostic test in Korean laboratories, with the highest number of tests performed [10]. The problem with toxin EIA is its low sensitivity, which is reported to be approximately 60%-80% for commonly used reagents in Korea [13]. Therefore, it is not recommended as a single test for CDI diagnosis.
The detection of GDH, an antigen secreted by C. difficile, can be used as a surrogate marker for the presence or absence of C. difficile strains and as a screening test because of its high sensitivity, although its specificity for toxin-producing strains is low. Compared to other CDI diagnostic tests, the GDH test was introduced relatively recently in Korea and is still relatively underutilized; however, its use is gradually increasing [10,11]. The significance of the GDH test is similar to that of C. difficile culture; therefore, it is often compared to culture when evaluating GDH assays.
Several commercialized NAATs, including PCR, have been developed and used to detect toxin A and B genes (tcdA and tcdB). They primarily target toxin B, with a few targeting the toxin A gene. In 2019, the C. difficile toxin gene test was designated as an item that receives additional reimbursement in Korea if the result is reported within 4–6 hours from the prescription of the test to the report of the result using an integrated automated diagnostic kit, recognizing the importance of rapid and accurate diagnostic tests. Moreover, there are products that can detect the C. difficile toxin gene along with other diarrhea-causing pathogens using multiplex PCR techniques. Although NAATs are highly sensitive, there is a potential for false positives, and the possibility of carrier status should be considered in the case of a positive NAAT result. NAATs cannot distinguish between infection and colonization, leading to a possible overdiagnosis. While certain guidelines previously recommended NAAT alone, more recent guidelines recommend toxin EIA to confirm the presence of toxins. Toxin testing should be included in the diagnostic workup, rather than NAAT alone, especially if there are no established referral criteria for C. difficile testing.
Several studies have reported on the systematic review and meta-analysis of the performance of CDI diagnostic tests [12-14]. In June 2014, a systematic review and meta-analysis of data from 2009 onwards showed that the performance varied depending on whether the standard method was CCNA or TC, with toxin EIA having an overall sensitivity of approximately 80% when compared to CCNA but a lower sensitivity of ≤ 60% when compared to TC [12]. The specificity of the toxin EIA was good (> 99%), whereas those of GDH and NAAT were slightly lower (approximately 95%). The specificity was particularly poor compared to that of CCNA. Similarly, in August 2018, a systematic review and meta-analysis of post-2014 data was conducted [13]. The results were not significantly different from those of previous studies. However, in this study, the NAAT had a lower sensitivity of 90%. In this study, GDH and toxin EIA were analyzed separately using automated equipment; the sensitivity of the automated method was significantly higher than that of the non-automated method (p< 0.01). For toxin EIA, low sensitivity is a major problem that can be overcome using an automated method.
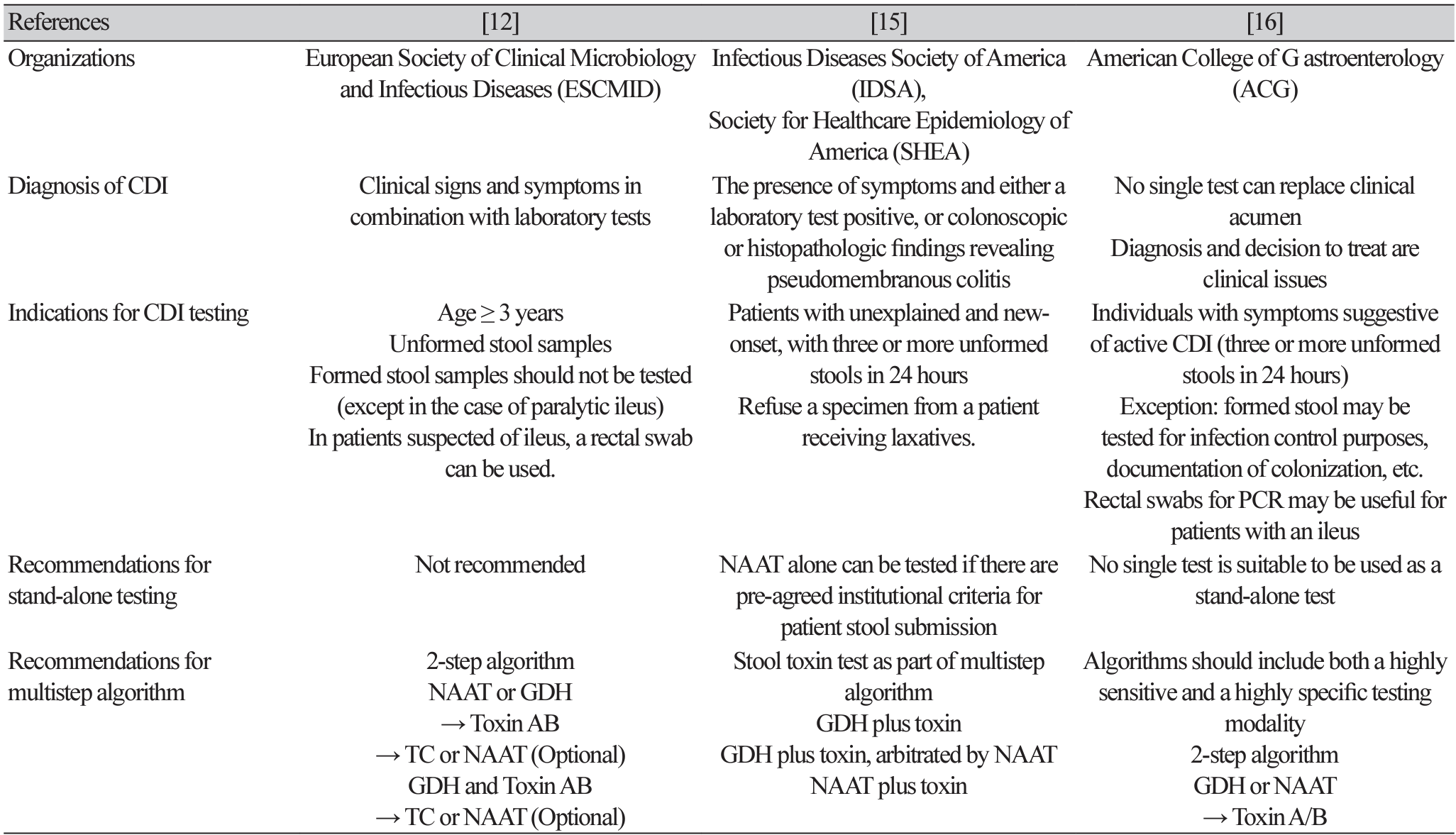
Guidelines for the diagnosis of CDI have been published by several organizations and countries, most by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [12], Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA) [15], and the American College of Gastroenterology (ACG) [16] (Table 1).
For the diagnosis of CDI, all three guidelines include symptoms, with the ACG guidelines emphasizing clinical judgment. In addition to laboratory tests, IDSA guidelines mention colonoscopic and histopathological findings. In terms of the indications for testing, they all emphasize testing in symptomatic patients, that is, those with unformed stools. The ESCMID and ACG guidelines state that a rectal swab is acceptable if ileus is present, and the ACG guidelines state that formed stools may be tested exceptionally for infection control or to confirm colonization.
The consensus is that standalone testing is not appropriate in most cases and is not recommended. However, the IDSA guidelines note that NAAT alone can be tested if there are institutional criteria for specimen submission. All guidelines recommend a multistep algorithm. Each test has strengths and weaknesses, and no one test is perfect; therefore, a combination of two or more tests should be used to diagnose CDI. The ACG guidelines provide a good explanation for this; the algorithm should include both a test with high sensitivity and one with high specificity.
The ESCMID guidelines suggest two specific algorithms. First, step 1 is to test for NAAT or GDH, which have high sensitivity. If positive, step 2 is toxin EIA, which has high specificity. If the toxin test is positive, CDI can be diagnosed. If the toxin test is negative, a clinical evaluation should be performed or the possibility of carriage should be considered. Another algorithm tests both GDH and toxins simultaneously in step 1. If both tests are negative, the diagnosis is not CDI. If both tests are positive, the diagnosis is CDI and no further testing is needed. If GDH is positive, but the toxin is negative, NAAT or TC can be considered. The IDSA guidelines prioritize institutional-level clinical and laboratory agreement on specimen criteria, such as testing only in patients with unexplained and new-onset of three or more unformed stools in 24 hours, and rejecting specimens from patients who have received laxatives. If there is no such agreement, the multistep algorithm should include a toxin test. If there is agreement, the NAAT may be used as a stand-alone test. The ACG guidelines recommend the ESCMID guidelines' two-step testing algorithm. First, the stool should be tested with a highly sensitive NAAT or GDH test, followed by a more specific toxin, EIA, in the second test.
Surveys on CDI diagnoses were conducted in Korea in 2015 [11] and 2018 [10]. The main focus of the surveys was to determine how clinical laboratories in hospitals test for CDI. Over time, there has been a shift in CDI testing in clinical laboratories in Korea, likely due to changes in laboratory and healthcare environments, including the introduction of new tests and changing perceptions of CDI.
According to a 2018 survey, toxin EIA was the most common test method (84.3%), followed by NAAT (58.4%), culture (36%), and GDH (25.8%). In terms of the test method combinations, toxin EIA, NAAT, and culture were the most common (22.5%). Comparing 2018 to 2015 (Table 2), toxin EIAs were the most common tests performed by organizations in both 2015 and 2018, with over 80% of the organizations performing them. NAAT was performed by approximately 60% of institutions in 2018, although the percentage of institutions performing NAAT decreased slightly in 2018, while culture showed a significant decrease in the percentage of institutions performing it in 2018 compared to 2015 (64.9% vs. 36.0%, P = 0.004). In contrast, GDH was introduced relatively recently in Korea compared to other tests. In 2015, when it was just undergoing evaluation as a new medical technology, only one institution was testing for GDH. However, in 2018, GDH testing had increased significantly to 25.8% of the surveyed laboratories. The most common combination of tests was toxin EIA, NAAT, and culture in both 2015 and 2018; however, the proportion decreased significantly in 2018 (40.4% vs. 22.5%, P = 0.036). The second most common combination was toxin EIAs and NAATs, in 2015 and 2018. In 2015, the combination of toxin EIA and culture was 15.8%, which decreased to 5.6% by 2018. In 2018, more than 10% of the institutions performed toxin EIA and GDH, with nine of the 11 institutions being relatively small hospitals with 500 beds or less. A simple test exist that uses the principle of immunochromatography to detect both toxins and GDH simultaneously, and it is believed that many laboratories have adopted it because it is easy to perform, even in small laboratories. However, there were still more than 15% of laboratories testing for the toxin by EIA alone in 2015 and 2018, which is not an appropriate testing strategy.
Because each CDI diagnostic test has its strengths and limitations, varies in performance, and no single test is perfect, many guidelines recommend combining tests or using multistep algorithms. Guidelines for CDI diagnosis have been developed by organizations, such as ESCMID, IDSA/SHEA, and ACG, and other countries have guidelines. These guidelines are often revised as advances in diagnostic technology require revisions and changes in the diagnostic criteria. In Korea, there are no official CDI diagnostic criteria or guidelines. Each organization or medical staff will use international guidelines or synthesize several guidelines to develop their own guidelines. It is necessary to develop standardized diagnostic guidelines for CDI appropriate for the Korean context.
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Conflicts of interest
Hae-Sun Chung has been an editor-in-chief of the Annals of Clinical Microbiology since January 2022. However, she was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
REFERENCES
1. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825-34.
2. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015;313:398-408.
3. Czepiel J, Drozdz M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, et al. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis 2019;38:1211-21.
4. CDC. Antibiotic resistance threats in the United States, 2019. https://www.cdc.gov/ drugresistance/biggest-threats.html [Online] (last visited on 22 February 2024).
5. Byun JH, Kim H, Kim JL, Kim D, Jeong SH, Shin JH, et al. A nationwide study of molecular epidemiology and antimicrobial susceptibility of Clostridioides difficile in South Korea. Anaerobe 2019;60:102106.
6. Kim J, Kim B, Lee CS, Kim ES, Lee SH, Kim YS, et al. Active surveillance of Clostridioides difficile infections in 10 tertiary hospitals. Infect Chemother 2021;53 Suppl 2:S264.
7. Son KJ, Kim YA, Park YS. The trend of Clostridioides difficile infection in Korean hospitals with the analysis of nationwide sample cohort. Ann Clin Microbiol 2020;23:241-9.
8. Martinez-Melendez A, Camacho-Ortiz A, Morfin-Otero R, Maldonado-Garza HJ, VillarrealTrevino L, Garza-Gonzalez E. Current knowledge on the laboratory diagnosis of Clostridium difficile infection. World J Gastroenterol 2017;23:1552-67.
9. Planche T and Wilcox MH. Diagnostic pitfalls in Clostridium diff icile infection. Infect Dis Clin North Am 2015;29:63-82.
10. Chung HS, Park JS, Shin BM, Yoo HM, Kim H, Cho J, et al. Nationwide survey for current status of laboratory diagnosis of Clostridioides diff icile infection in Korea. J Korean Med Sci 2022;37:e38.
11. Chung HS, Park JS, Shin BM. Laboratory diagnosis of Clostridium diff icile infection in Korea: the first national survey. Ann Lab Med 2019;39:317-21.
12. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium diff icile infection. Clin Microbiol Infect 2016;22 Suppl 4:S63-81.
13. Chung HS, Park JS, Shin BM. Laboratory diagnostic methods for Clostridioides difficile infection: the first systematic review and meta-analysis in Korea. Ann Lab Med 2021;41:17180.
14. Kraft CS, Parrott JS, Cornish NE, Rubinstein ML, Weissfeld AS, McNult P, et al. A laboratory medicine best practices systematic review and meta-analysis of nucleic acid amplification tests (NAATs) and algorithms including NAATs for the diagnosis of Clostridioides (Clostridium) difficile in adults. Clin Microbiol Rev 2019;32:e00032-18.
15. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:987-94.
16. Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021;116:1124-47.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download