Abstract
Human taeniasis is presumed to have almost disappeared from Korea. Recently, we incidentally detected a Taenia saginata infection in an 8-year-old boy undergoing lymphoma diagnosis. The patient had been suffering for 4 months from intensifying snoring and obstructive sleep apnea. A neck computed tomography scan revealed a nasopharyngeal mass, and malignant B-cell lymphoma was supported by punch biopsy. On day 6 of the lymphoma workup period, the patient experienced anal itching, and two proglottids were detected in his stool. The patient had experienced four or five similar episodes within the past 2 years. He self-reported a history of raw beef and fish consumption and no history of traveling abroad. Laboratory findings revealed mild eosinophilia (eosinophil count: 791/μL). Two proglottids exhibited movement and possessed more than 15 branched uterine structures. Long segments approximately 84 cm in length were expelled after praziquantel treatment. Sequencing of the cytochrome oxidase 1 gene confirmed T. saginata, ruling out related Taenia species. After treatment, no proglottids or ova were detected in his stool, and the patient finally started chemotherapy for lymphoma. This case highlights the importance of timely diagnosis of hidden taeniasis in low-frequency endemic regions.
Human taeniases are distributed worldwide, and they are caused by three Taenia species, namely, T. solium, T. asiatica, and T. saginata. According to a recent report of USA, prevalence of taeniasis almost 9%, which is very similar to previous reports of taeniasis prevalence (0.01%-10%) from Europe [1,2]. In Korea, human taeniasis was relatively common in the wide localities until the 1980s. However, national surveys recently reported a Taenia egg prevalence as low as 0.02% in 1997, a figure that eventually dropped to 0%–0.004% in 2004–2013 [3]. Thereafter, only five sporadic cases were detected during the 2006–2011 period. Human taeniasis is presumed to have almost disappeared from Korea. However, in 2018, a mini-outbreak of T. saginata-induced taeniasis occurred [4,5]. Therefore, whether human taeniasis has been eliminated or underestimated in Korea remains unclear. Recently, we incidentally encountered a case of T. saginata infection during lymphoma diagnosis.
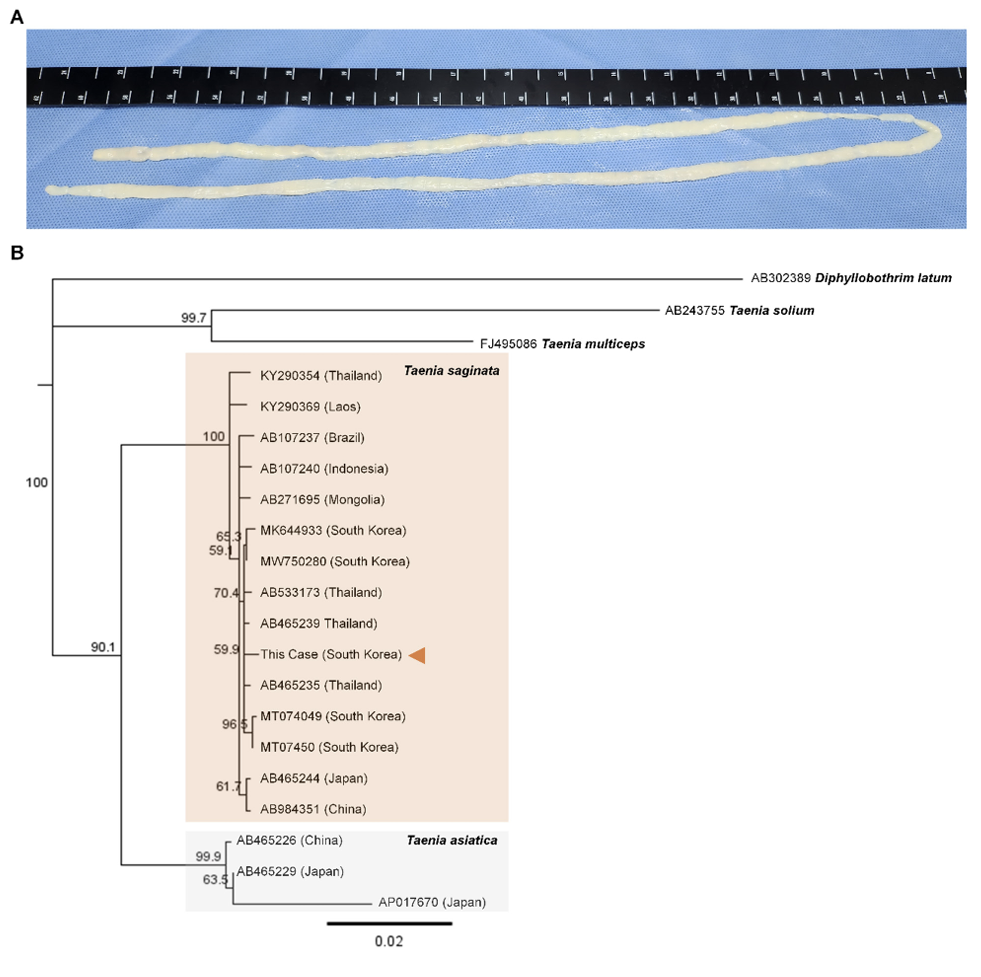
An 8-year-old boy suffered from worsening snoring and obstructive sleep apnea that had begun 4 months prior. The patient developed a fever and persistent yellowish nasal discharge, leading to a diagnosis of acute otitis media at a local clinic. Fever and nasal symptoms did not improve, irrespective of 6 weeks of antibiotic treatment. Moreover, the patient was developing hearing impairment. Neck computed tomography was performed, revealing a nasopharyngeal mass and hypertrophy of both palatine tonsils. A punch biopsy of the nasopharyngeal mass confirmed a diagnosis of malignant B-cell lymphoma. The patient was subsequently transferred to our hospital for further evaluation and treatment. On hospital day 6, the patient experienced anal itching, and two parasite segments were detected in his stool. Two years prior, the patient visited Jeju-do, consumed seafood dishes, and thereafter experienced four or five episodes of the emergence of this parasite. The patient and all his family members received early albendazole treatment; however, the symptoms recurred intermittently. Nonetheless, the patient presented no abdominal symptoms, such as nausea, vomiting, abdominal pain, diarrhea, or constipation. The patient had a self-reported history of raw beef and fish consumption and no history of traveling abroad. Laboratory findings revealed a mild degree of eosinophilia (eosinophil count: 791/μL). Two segments that had passed out in his stool were submitted to our clinical laboratory. The segments exhibited movement, and they were pressed between two microscope slides and examined macroscopically without staining. More than 15 branched uterine structures were observed in the segments. However, Taenia species ova were not identified upon stool examination using the formalin–ether concentration method. T. saginata-induced taeniasis was tentatively diagnosed based on the proglottid morphology. After praziquantel (10 mg/kg) treatment, long parasite segments approximately 84 cm in length were expelled (Fig. 1A). To identify the tapeworm species, we conducted a molecular analysis of the proglottid segments. Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and subsequently used as a template for polymerase chain reaction (PCR) analysis. The mitochondrial cytochrome c oxidase subunit I (cox1) gene was targeted for PCR amplification. The PCR primers were as follows: Tsag cox 1 F (5′- GAG GAA ATT GTG AAG TTA CTG CTA-3′) and Tsag cox 1 R (5′- ATG ATG CAA AAG GCA AAT AAA CCT-3′). PCR was performed using a 30-μL reaction mixture containing 15 μL Smart 2 × PCR Pre-Mix (SolGent Co., Ltd., Daejeon, Korea), 2 μL template DNA, 10 μM of each primer, and 11 μL distilled water, as described in a previous study [4,6]. The amplification process comprised pre-denaturation (94°C for 3 min), 40 cycles of denaturation (94°C for 60 s), annealing (52°C for 60 s), extension (72°C for 60 s), and final extension (72°C for 10 min). The PCR products were sent to Cosmogenetech (Seoul, Korea) for direct sequencing using the same PCR primers. The 1750-bp cox1 sequence had a 100%, 95.9%, and 92.7% match with the T. saginata (accession no. AB465235), T. asiatica (accession no. AP017670), and T. multiceps (accession no. JX535570) sequences, respectively. DNA sequences were aligned using the CLUSTAL W computer program [7]. Phylogenetic trees were constructed using the neighbor-joining method, and genetic distances were computed via the Tamura–Nei method using the Geneious computer program [8]. The neighbor-joining tree based on GenBank sequences indicated that our specimen was in the same phylogenetic group as T. saginata, but not in the same group as T. asiatica, T. multiceps, or T. solium (Fig. 1B). No T. saginata proglottids or ova were detected in his stool, and the patient subsequently commenced chemotherapy for lymphoma.
Certain researchers have suggested that human taeniasis is now almost nonexistent in Korea. However, several cases of infection are potentially overlooked, especially in areas where taeniasis was previously ubiquitous. Furthermore, in our case, the patient originated from Gyeongsang-do, where an outbreak of human taeniasis occurred in 2018 [5]. Taeniasis may be underdiagnosed, considering that raw fish or beef consumption is deeply rooted in the traditional customs of rural residents in Korea [9]. In such scenarios, patients with taeniasis are likely to hide their symptoms, despite experiencing the passing out of parasite segments several times. Typically, these patients repeatedly take albendazole purchased from a pharmacy instead of visiting a hospital [4]. This behavior is potentially influenced by a previous National Deworming Campaign in Korea over the past years that focused on soil-transmitted helminthiases and emphasized the repeated administration of albendazole to control parasitic infections. Patients expect albendazole to eliminate the parasite from their bodies; however, albendazole cannot completely resolve taeniasis. Repeated administration of albendazole without consulting a doctor could delay proper diagnosis. Collectively, this tendency of hiding their symptoms and self-medicating with albendazole complicates the timely diagnosis of taeniasis. The patient did not voluntarily disclose any previous episodes of passing out parasite segments. Fortunately, two segments were passed out in his stool before intensive chemotherapy for the treatment of malignant lymphoma. Therefore, we were able to accurately diagnose subclinical taeniasis via morphological and molecular diagnostic modalities. The present case highlights the importance of the timely diagnosis of subclinical taeniasis in low endemic regions, such as Korea.
Ethics statement
The study was approved by the Institutional Review Board of Asan Medical Center (No. 2023-1441) and the requirement for informed consent was waived because of the retrospective nature of the study.
Funding
This study was supported by grants from the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (Grant no 2022R1C1C1002741).
REFERENCES
1. Dorny P and Praet N. Taenia saginata in Europe. Vet Parasitol 2007;149:22–4.
.
2. Braae UC, Thomas LF, Robertson LJ, Dermauw V, Dorny P, Willingham AL, et al. Epidemiology of Taenia saginata taeniosis/cysticercosis: a systematic review of the distribution in the Americas. Parasit Vectors 2018;11:518.
.
3. Korea Centers for Disease Control and Prevention. National survey of intestinal parasitic infections in Korea. 8th report; 2013. KCDC 2014;1:89–94.
.
4. Won EJ, Shin JH, Lee YJ, Kim MJ, Kang SJ, Jung SI, et al. Four Taeniasis saginata cases diagnosed at a university hospital in Korea. Korean J Parasitol 2019;57:313–8.
.
5. Song SM, Yun HS, VanBik D, Chang HH, Lee SA, Kim SW, et al. Ten cases of Taenia saginata infection confirmed by analysis of the internal transcribed spacer 1 rDNA region in the Republic of Korea. Korean J Parasitol 2019;57:417–22.
.
6. Jeon HK, Kim KH, Eom KS. Molecular identification of Taenia specimens after long-term preservation in formalin. Parasitol Int 2011;60:203–5.
.
7. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994;22:4673–80.
.
8. Saitou N and Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406–25.
.
9. Yiek WK, Coenen O, Nillesen M, van Ingen J, Bowles E, Tostmann A. Outbreaks of healthcare-associated infections linked to water-containing hospital equipment: a literature review. Antimicrob Resist Infect Control 2021;10:77.
.
10. Chai JY. Human taeniasis in the Republic of Korea: hidden or gone? Korean J Parasitol 2013;51:9–17.
.
Fig. 1.
Morphological and molecular findings of Taenia saginata found in this case. (A) After praziquantel (10 mg/kg) treatment, long parasite segments approximately 84 cm in length were expelled. (B) The 1750-bp cox1 sequence was yielded from our specimen and the neighbor-joining tree showed that our specimen (indicated by arrow head) was in the same phylogenetic group as T. saginata, but not in the same group as T. asiatica, T. multiceps, or T. solium.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download