Abstract
We conducted environmental cultures for carbapenemase-producing Enterobacterales (CPE) to evaluate the environmental contamination around patients with CPE. CPE was detected in the environmental cultures of four of the nine intensive care unit (ICU) inpatients with CPE. All four isolates were collected from sink surfaces in isolation rooms within the ICU. CPE isolates from the environment differed from those isolated from patients and had different carbapenemases. Even though CPE isolates from the environment of the ICU were not associated with CPE isolates from patients, the repeated isolation of CPE from sinks over several months is alarming.
The global prevalence of carbapenemase-producing Enterobacterales (CPE) has been increasing recently, posing a substantial threat to infection control [1,2]. In particular, the importance of environmental factors in CPE transmission has been recognized, and the potential for transmission through the environment has been demonstrated in outbreak cases [3]. Consequently, environmental management is important in CPE infection control.
Environmental culture involves collecting and culturing specimens from air, water, and environmental surfaces. When conducted properly, environmental culture can support epidemiological investigations and aid in determining the effectiveness of infection control measures; however, if conducted improperly, it can lead to wastage of clinical microbiology laboratory and infection control resources, and misleading data can lead to incorrect infection control measures. The purpose of environmental cultures is twofold: to monitor compliance with hygienic standards and detect the presence or absence of specific healthcare-associated infectious pathogens. The second is generally undertaken during outbreak investigations [4]. In cases involving carbapenem-resistant Enterobacterales (CRE), the Korean infection control guidelines recommend environmental culture testing in the event of an outbreak [5].
However, few studies have investigated environmental cultures for CPE in Korea [6,7]. In the present study, we conducted environmental cultures for CPE to evaluate the environmental contamination around patients with CPE.
Nine intensive care unit (ICU) inpatients with CPE isolated from clinical specimens referred for culture between March 2017 and October 2017 were included. Clinical information such as sex, age, ward, medical department, comorbidities, hospitalization history, and clinical microbiology results were collected by reviewing electronic records. The specimens were collected from bed railings/controls, headboards, intravenous poles, call buttons, telephones, bedside tables, chairs, sinks, light switches, door handles, bathroom door handles, bathroom light switches, bathroom-assisted handles, toilet handles, bathroom sinks, and toilet seats. Additionally, specimens were collected from infusion pumps, monitor controls, monitor control touch screens, monitor cables, and ventilator controls. Culture for CPE detection was performed by incubating 10 μg meropenem disks in trypticase soy broth overnight followed by overnight incubation in MacConkey agar in accordance with the Centers for Disease Control and Prevention laboratory guidelines [8]. Identification of isolates and antimicrobial susceptibility testing were performed using the VITEK2 system (bioMérieux, Durham, NC, USA). RAPIDEC CARBA NP (bioMérieux, Marcy-l'Étoile, France) and Xpert Carba-R (Cepheid, Sunnyvale, CA, USA) tests were conducted to detect and genotype the carbapenemases.
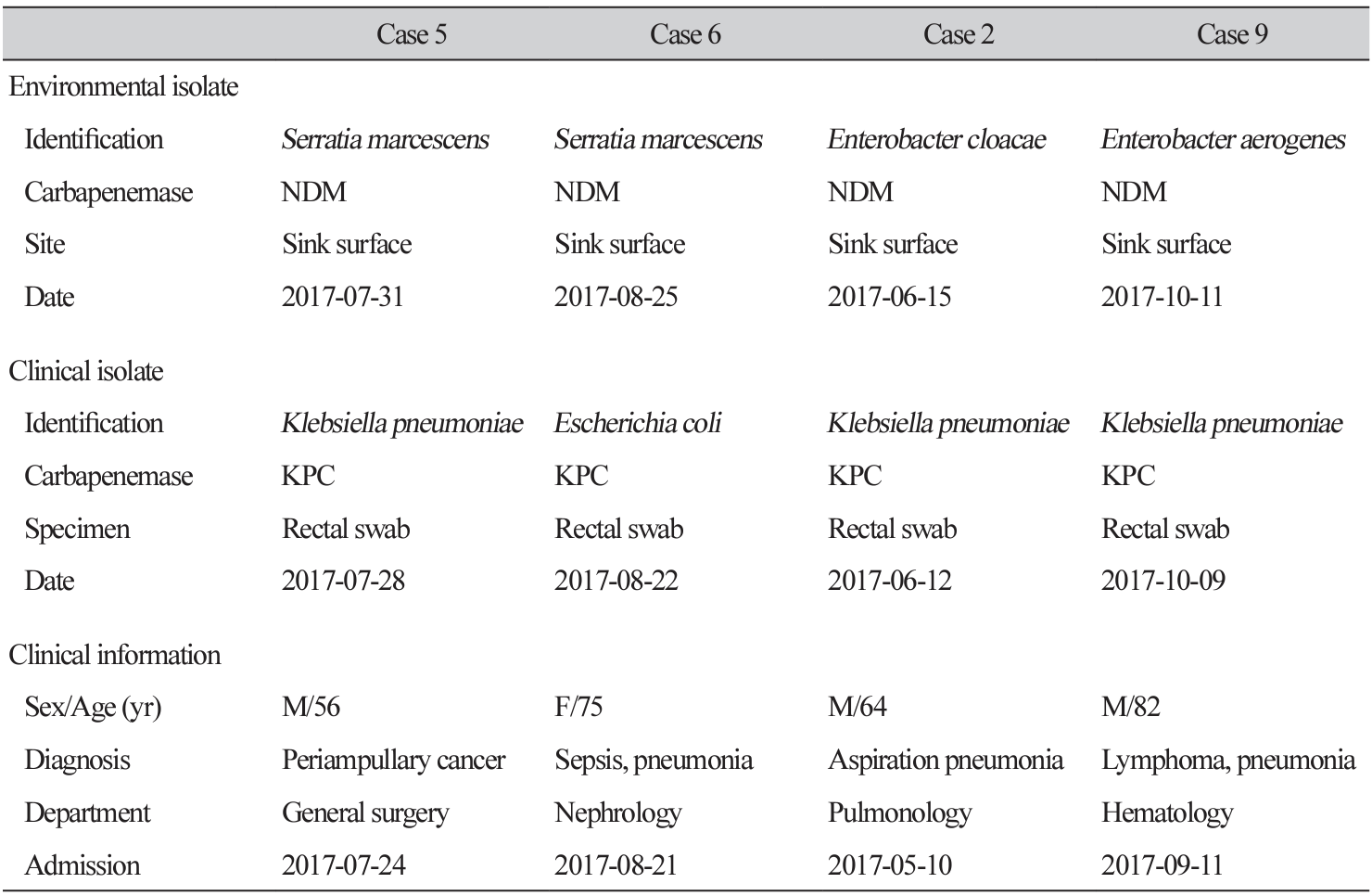
CPE was detected in the environmental cultures of four of the nine cases (Table 1). All four isolates were collected from sink surfaces in isolation rooms within the ICU (Fig. 1). Environmental isolates were identified as Serratia marcescens (n = 2), Enterobacter cloacae (n = 1), and Enterobacter aerogenes (n = 1). All four isolates harbored blaNDM. CPE isolates from the environment differed from those isolated from patients and had different carbapenemases. In all cases, Acinetobacter baumannii and Pseudomonas aeruginosa were detected at various environmental sites (data not shown). In Case 2, environmental cultures were conducted again after the source was disinfected, and CPE was not isolated.
Associations between water environments in hospitals and healthcare-associated infections have been reported [3,9]. Healthcare water environments, including potable water, faucets, sink surfaces, and wastewater drainage systems such as drains, sink/shower traps, toilets, and drainage pipes, can serve as reservoirs for nosocomial pathogens such as drug-resistant Enterobacterales, Pseudomonas spp., and A. baumannii [3]. This study found that sink surfaces were a major source of contamination by multidrug-resistant organisms, including CPE and P. aeruginosa. Similarly, previous reports have shown that sinks are a reservoir for CPE, are associated with CPE in patients, and are a transmission source [6,10,11].
Reports indicate that CPEs in the environment are associated with CPEs isolated from patients and may even cause outbreaks [6,12,13]. However, in this study, the carbapenemase genes of CPEs isolated from the environment differed from those of CPEs isolated from patients (the environmental isolates were all blaNDM, and the patient isolates were blaKPC), suggesting that the environmental and patient isolates were unrelated. Environmental cultures were performed in most cases shortly after isolation was initiated. Considering the timing of these procedures, environmental isolates were presumed to be present in the environment, regardless of the occurrence of CPE carriers.
This study has several limitations. The study included a small number of cases. In addition, the original study plan was to perform environmental cultures where the patient was staying when the CPE was isolated; however, this was possible only in one case. In other cases, the environmental culture was performed at the location where the patient was isolated after CPE isolation (isolation room in the ICU). This was due to the time required to identify the target cases, as it typically took more than 3 days to report CPE during the study period, and we were unable to delay patient isolation and disinfection for research purposes because of the priority of patient care and infection control. Further studies are needed to demonstrate the horizontal transmission of blaNDM among environmental isolates.
In the present study, we detected CPEs carrying blaNDM in sinks in the ICU, which were not associated with the isolation of CPEs from patients. However, the repeated isolation of CPE from sinks over several months is alarming. More aggressive disinfection and environmental surveillance are needed to prevent contamination by CPE and other multidrug-resistant organisms in ICUs.
Ethics statement
This study was approved by the Institutional Review Board of the Ewha Womans University Mokdong Hospital (IRB No. EUMC 2016-11-026). The informed consent was waived for the nature of this study.
Conflicts of interest
Hae-Sun Chung has been an editor-in-chief of the Annals of Clinical Microbiology since January 2022. However, she was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
Acknowledgements
I would like to thank the staff of the Infection Control Office of Ewha Womans University Mokdong Hospital for their excellent work in collecting specimens for environmental cultures and the staff of the Department of Laboratory Medicine of Ewha Womans University Mokdong Hospital for their excellent work in performing microbiological experiments.
Funding
This work was supported by the Research Fund (2016) of the Korean Society of Clinical Microbiology.
REFERENCES
1. Hansen GT. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect Dis Ther 2021;10:75-92.
.
2. Caliskan-Aydogan O and Alocilja EC. A review of carbapenem resistance in Enterobacterales and its detection techniques. Microorganisms 2023;11:1491.
.
3. Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospitalacquired infections-a systematic review of the literature. Clin Infect Dis 2017;64:1435-44.
.
4. Kizny Gordon AE, Mathers AJ, Cheong EYL, Gottlieb T, Kotay S, Walker AS, et al. The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospitalacquired infections-a systematic review of the literature. Clin Infect Dis 2017;64:1435-44.
.
5. Korea Disease Control and Prevention Agency. Healthcare-associated infections guidance (2023). Osong; KDCA: 2023.
.
6. Kim SH, Kim GR, Kim EY, Jeong J, Kim S, Shin JH. Carbapenemase-producing Enterobacterales from hospital environment and their relation to those from patient specimens. J Infect Public Health 2022;15:241-4.
.
7. Shi HJ, Kim JH, Kim NY, Lee JB, Eom JS. Environmental culture of bacteria at the intensive care unit of a tertiary hospital in Korea: a consideration for improving medical environmental safety and healthcare-associated infection. Korean J Healthc assoc Infect Control Prev 2020;25:105-14.
.
8. Centers for Disease Control and Prevention. Laboratory protocol for detection of carbapenemresistant or carbapenemase-producing, Klebsiella spp. and E. coli from rectal swabs. Atlanta; CDC: 2008.
.
9. Yiek WK, Coenen O, Nillesen M, van Ingen J, Bowles E, Tostmann A. Outbreaks of healthcare-associated infections linked to water-containing hospital equipment: a literature review. Antimicrob Resist Infect Control 2021;10:77.
.
10. De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H, et al. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control 2017;6:24.
.
11. Feng Y, Wei L, Zhu S, Qiao F, Zhang X, Kang Y, et al. Handwashing sinks as the source of transmission of ST16 carbapenem-resistant Klebsiella pneumoniae, an international high-risk clone, in an intensive care unit. J Hosp Infect 2020;104:492-6.
.
12. Zhang Y, Yu S, Chen C, Sun F, Zhou L, Yao H, et al. Comprehensive surveillance and sampling reveal carbapenem-resistant organism spreading in tertiary hospitals in China. Infect Drug Resist 2022;15:4563-73.
.
13. Yan Z, Zhou Y, Du M, Bai Y, Liu B, Gong M, et al. Prospective investigation of carbapenemresistant Klebsiella pneumonia transmission among the staff, environment and patients in five major intensive care units, Beijing. J Hosp Infect 2019;101:150-7.
.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download