Abstract
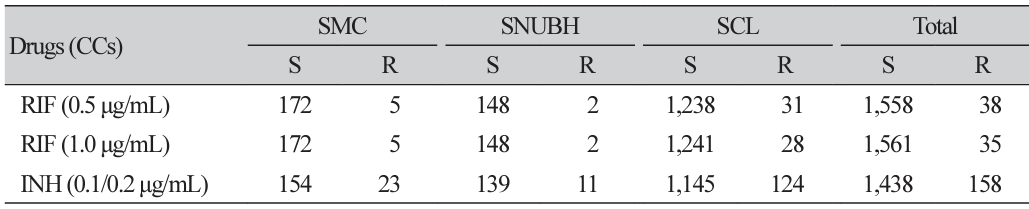
Background: Rifampin plays an important role in tuberculosis treatment. In recent years, the introduction of molecular testing techniques has enabled the rapid detection of rifampin resistance, leading to discrepancies with conventional methods. The World Health Organization (WHO) has analyzed mutations in the rpoB gene that induce rifampin resistance and identified certain mutations causing borderline resistance, which are often undetected using conventional tests. Consequently, the WHO has lowered the rifampin resistance criterion concentration from 1.0 to 0.5 μg/mL in 7H10 and MGIT 960. The present study aimed to evaluate the impact of this change in critical concentration on the detection of borderline rifampin resistance in Mycobacterium tuberculosis. Methods: Tuberculosis strains submitted for antituberculosis drug susceptibility testing from May 2021–2022 were analyzed. Three institutions participated; the Seoul Clinical Laboratories used the agar proportion method, whereas the Samsung Medical Center and Seoul National University Bundang Hospital utilized the MGIT 960 system to test both the original and revised concentrations. Mutations were confirmed through rpoB gene sequencing for strains showing discrepancies. Results: A total of 1,596 valid susceptibility tests were conducted during the study period. Rifampin resistance was detected in 35 cases (2.19%) at 1.0 μg/mL and in 38 cases (2.38%) at 0.5 μg/mL, whereas isoniazid resistance was observed in 158 cases (9.90%). Among the three rifampin discrepancy strains, one harbored an H445L mutation, whereas the remaining two exhibited an L452P mutation. These mutations were classified as borderline resistant. Conclusion: Applying the new rifampin critical concentration resulted in a 0.19% increase in resistance rate and an 8.57% increase in detection cases. Additionally, despite testing with large number of rifampin-susceptible strains, no false resistance results were obtained. Therefore, the application of the new critical concentration is considered beneficial for the management of rifampin-resistant tuberculosis..
[in Korean]
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
REFERENCES
1. WHO. WHO operational handbook on tuberculosis Module 4: Treatment – drug-susceptible tuberculosis treatment. Geneva; World Health Organization, 2022.
.
2. WHO. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). Geneva; World Health Organization, 2021.
.
3. WHO. WHO consolidated guidelines on tuberculosis Module 3: Diagnosis - tests for tuberculosis infection. Geneva; World Health Organization, 2022.
.
4. WHO. Global tuberculosis report 2022. Geneva; World Health Organization, 2022.
.
5. Gurbanova E, Mehdiyev R, Blondal K, Tahirli R, Mirzayev F, Hillemann D, et al. Mitigation of discordant rifampicin-susceptibility results obtained by Xpert Mycobacterium tuberculosis/ rifampicin and mycobacterium growth indicator tube. Microb Drug Resist 2017;23:1045–52.
.
6. Mokaddas E, Ahmad S, Eldeen HS, Al-Mutairi N. Discordance between Xpert MTB/RIF assay and Bactec MGIT 960 culture system for detection of rifampin-resistant Mycobacterium tuberculosis isolates in a country with a low tuberculosis (TB) incidence. J Clin Microbiol 2015;53:1351–4.
.
7. Miotto P, Cabibbe AM, Borroni E, Degano M, Cirillo DM. Role of disputed mutations in the rpoB gene in interpretation of automated liquid MGIT culture results for rifampin susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 2018;56:e01599-17.
.
8. Sinha P, Srivastava GN, Tripathi R, Mishra MN, Anupurba S. Detection of mutations in the rpoB gene of rifampicin-resistant Mycobacterium tuberculosis strains inhibiting wild type probe hybridization in the MTBDR plus assay by DNA sequencing directly from clinical specimens. BMC Microbiol 2020;20:284.
.
9. WHO. Tuberculosis laboratory biosafety manual. Geneva; World Health Organization, 2018.
.
10. WHO. WHO operational handbook on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detention, 2021 update. Geneva; World Health Organization, 2021.
.
11. WHO. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’; practical considerations. Geneva; World Health Organization, 2014.
.
12. Al-Mutairi NM, Ahmad S, Mokaddas E, Eldeen HS, Joseph S. Occurrence of disputed rpoB mutations among Mycobacterium tuberculosis isolates phenotypically susceptible to rifampicin in a country with a low incidence of multidrug-resistant tuberculosis. BMC Infect Dis 2019;19:3.
.
13. Van Deun A, Aung KJM, Hossain A, De Rijk P, Gumusboga M, Rigouts L, et al. Disputed rpoB mutations can frequently cause important rifampicin resistance among new tuberculosis patients. Int J Tuberc Lung Dis 2015;19:185–90.
.
14. Ocheretina O, Escuyer VE, Mabou MM, Royal-Mardi G, Collins S, Vilbrun SC, et al. Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One 2014;9:e90569.
.
15. Van Ingen J, Aarnoutse R, De Vries G, Boeree MJ, Van Soolingen D. Low-level rifampicinresistant Mycobacterium tuberculosis strains raise a new therapeutic challenge. Int J Tuberc Lung Dis 2011;15:990–2.
.
16. Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 2014;52:2157–62.
.
17. Van Deun A, Aung KJM, Bola V, Lebeke R, Hossain MA, De Rijk WB, et al. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 2013;51:2633–40.
.
18. Van Deun A, Decroo T, Aung KJM, Hossain MA, Gumusboga M, De Rijk WB, et al. Mycobacterium tuberculosis borderline rpoB mutations: emerging from the unknown. Eur Respir J 2021;58:2100783.
.
19. Gonzalo X, Claxton P, Brown T, Montgomery L, Fitzgibbon M, Laurenson I, et al. True rifampicin resistance missed by the MGIT: prevalence of this pheno/genotype in the UK and Ireland after 18 month surveillance. Clin Microbiol Infect 2017;23:260–3.
.
20. Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol 2009;47:3501–6.
.
21. Yu HJ, Kim TY, Kim G, Shim HJ, Kang OK, Kim S, et al. Performance evaluation of the BACTEC MGIT 960 system for rifampin drug-susceptibility testing of Mycobacterium tuberculosis using the current WHO critical concentration. J Clin Microbiol 2023;61:e01086-22.
.
Table 2
Conventional DST results and rpoB gene sequencing data of three rifampin resistant isolates with new critical concentration
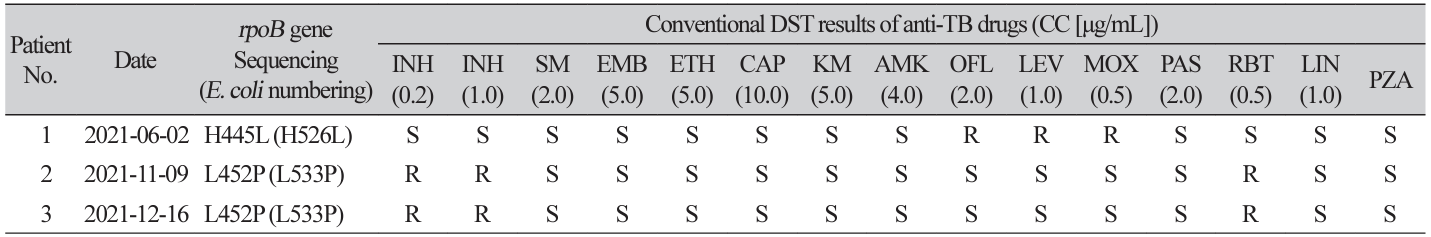
Abbreviations: DST, drug susceptibility testing; TB, tuberculosis; CC, critical concentration; INH, isoniazid; SM, streptomycin; EMB, ethambutol; ETH, ethionamide; CAP, capreomycin; KM, kanamycin; AMK, amikacin; OFL, ofloxacin; LEV, levofloxacin; MOX, moxifloxacin; PAS, para-aminosalicylic acid; RBT, rifabutin; LIN, linezolid; PZA, pyrazinamide; S, susceptible; R, resistant.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download