Abstract
Group B Streptococcus (GBS, Streptococcus agalactiae) is a pathogen that causes sepsis and meningitis, particularly in newborns, as well as severe infections in the elderly and those at high risk. For many years, the administration of intrapartum antimicrobial prophylaxis (IAP) has been a standard method to prevent neonatal GBS infection. However, IAP may be unsuitable in low-income settings due to its high cost and difficult accessibility to medical institutions. Additionally, IAP may lead to the emergence of antimicrobial-resistant bacteria. Hence, an alternative method for the control of GBS, such as a vaccine, is needed. An effective vaccine will likely prevent the further spread of GBS and be cost-effective compared with IAP. GBS vaccines have been under development for the past two decades, and several candidates have shown potential. In this review, we discuss the current development of GBS vaccines, including types and their implementation in different target populations.
Go to : 
Group B Streptococcus (GBS; Streptococcus agalactiae) is one of the essential Gram-positive bacteria causing neonatal sepsis and meningitis and has become the leading cause of neonatal morbidity and mortality worldwide [1]. GBS commonly appears in healthy women’s reproductive or gastrointestinal tract and can be transmitted to newborns at the time of birth [2]. GBS can occasionally cause serious disease, particularly in infants, but not exclusively, given that diseases caused by GBS were also found in adults. Although there is a regional variation in the prevalence of GBS, about 18% of women worldwide are estimated to be colonized by GBS and may develop an invasive GBS disease [3]. This can lead to stillbirth, maternal, and/or neonatal death. Since it was first identified (Fig. 1), as of 2015, there have been about 2.7 million neonatal deaths, 2.6 million stillbirths, and about 303,000 maternal deaths due to GBS infection, with the highest burden found in low-income settings, particularly African countries [4-7].
Two syndromes exist for GBS disease in neonates: early onset (< 7 days old) and late onset (7-89 days old) [8]. Both early- and late-onset GBS infections can manifest as bacteremia, pneumonia, and meningitis; hence, proper management and prevention are crucial to reduce maternal and neonatal mortality and morbidity risk due to the disease caused by GBS. To date, the most common prevention for GBS infection is through intrapartum antibiotic prophylaxis (IAP), with the most common antibiotics used being penicillin and ampicillin [9,10]. IAP given four or more hours before delivery has been reported to be effective in preventing early-onset disease (EOD) [11]. However, unfortunately, IAP has no impact on late-onset disease (LOD), stillbirths, prematurity due to GBS, and disease in pregnant women [12,13]. Additionally, a study reported that infants exposed to IAP were shown to have a lower abundance of phylum Bacteroidetes in their first week of life, lower Bacteroide and Parabacteroide genera at three months, lower counts of Bifidobacterium spp. at the age of one week, and an increased infection rate of ampicillin-resistant Escherichia coli compared to infants who were not exposed to antibiotics [14-16]. Given the limitation of IAP for the eradication of GBS colonization in pregnant women, the development of GBS vaccines has been identified as a suitable approach for preventing and managing the disease. In this review, we discuss the development of GBS vaccines, their current status, and the possible effectiveness of GBS vaccine administration for the prevention and management of early-onset and late-onset GBS infection based on the updated literature. To give a comprehensive understanding of the possible treatment options and molecules for the development of GBS vaccines, we also discuss the serotypes and the identified virulence factors of GBS, as well as the types of currently developed vaccines and the target implementation of GBS vaccines.
Serotyping is a serologic test based on identifying the differences in specific antigens of microorganisms to classify them into groups [17]. GBS serotyping is crucial for the investigation of possible infection outbreaks as well as to identify possible sources of infection. To date, there are 10 widely known GBS serotypes (Ia, Ib, and II-IX). These serotypes are categorized based on the capsular polysaccharide (CPS) composition. The CPS is considered a major virulence factor that hinders phagocytic clearance and assists GBS in evading the host's defensive mechanism. Serotypes I-V are the most common rectovaginal colonizing GBS in pregnant women worldwide [3]. Meanwhile, serotype III has been reported to be the most frequent serotype that causes disease in infants [18], though it may be less frequent in some South American and Asian countries. Serotypes VI-IX are more commonly identified as causing disease in infants in Asian countries [3].
Apart from serotyping, the more advanced differentiation of GBS was determined based on its genetic variants. The genotyping of GBS revealed extensive variation in the genetic background of GBS, even among the same serotypes. A study in Korea reported that 14 sequence types (STs) of GBS were identified, with ST1 (20.4%), ST17 (19.4%), and ST19 (18.4%) being the most prevalent. Serotype III was the dominant serotype capsule expressed by ST17 and ST19 [19]. Meanwhile, a study in China reported 42 STs among 266 GBS isolates, with ST19 as the most prevalent type. In concordance with the report from the Korean group, serotype III was mainly comprised of ST17 and ST19 [20]. Additionally, through genotyping, several virulence factors of GBS were also identified, e.g., cylE, lmb, scpB, rib, BCA [21,22], cfb, sodA, and dltR [23]. Similarly, several antimicrobial resistance genes were also identified, e.g., lnu(B), lsa(E), aac(6′)aph(2″) [22], ermA, ermB, ermTR, mefA/E, tetM, tetO, and aphA3 [24]. Understanding the serotypes and genotypes of GBS is important not only for the epidemiology of perinatal GBS infection but also in the development of GBS vaccines for neonates and pregnant women, as well as in adults.
The development of GBS vaccines has been initiated for decades [25]. Several predicted factors have slowed the vaccine development, including the complexity of vaccine tests due to the reluctance of individuals to participate, understanding the timing of the vaccination, particularly in pregnant women, and confirming immunity across the placenta, protecting the neonates from GBS infection [26]. Nevertheless, never-ending efforts have brought progress in the development of vaccine for GBS and may become an attractive strategy for GBS disease prevention (Fig. 2).
The CPS of GBS is a structurally conserved protein that is considered a crucial virulence factor and vaccine candidate due to its ability to induce a strong immune response against GBS strains. The first generation of GBS vaccines used unmodified type-specific CPS, i.e., Ia, II, or III. In 1976, a study demonstrated the importance of maternal antibodies against type III CPS for protection against EOD or LOD in neonates. Maternal CPS-specific antibodies can be transferred to the newborn via transplacental transmission, which confers sufficient protection to the newborn against GBS infection [27]. According to Lin et al [28], vaccineinduced specific antibodies (IgG) against GBS Ia with a concentration of ≥ 5µg/mL in mothers are predicted to protect EOD in neonates. Subsequently, an analysis of the maternal CPS-specific antibody for GBS serotypes III and V demonstrated a decrease in neonatal EOD up to 70% if the vaccine-induced maternal CPS-specific antibody concentration is ≥ 1µg/mL [29]. However, only 60% of individuals who received the unmodified CPS vaccine showed an immune response [30].
GBS conjugate polysaccharide vaccines were developed with serotype III coupled to tetanus toxoid to improve the immunogenicity of the specific CPS used in the vaccine. Later, conjugate vaccines based on another nine identified GBS serotypes (Ia, Ib, II, IV-IX) were also developed and tested pre-clinically in animal models [30-32]. The clinical trial of the GBS type III CPS-tetanus toxoid conjugate (III-TT) vaccine was conducted on 100 participating women, and a high level of CPS-specific IgG was observed in the sera from participants [33]. However, this type of vaccine was only effective against specific types of GBS; hence, to provide sufficient protection against other serotypes, CPS–conjugate vaccines need to be multivalent.
A bivalent conjugate polysaccharide vaccine is a conjugate GBS vaccine that targets two different types of CPS. In 2003, a bivalent conjugate polysaccharide GBS vaccine containing GBS type II-TT and type III-TT glycoconjugates was tested in humans [34]. According to the study, bivalent conjugate II-TT/III-TT vaccines were well tolerated and demonstrated a high increase in GBS II- and GBS III-specific IgG. Moreover, the immune serum samples from vaccine recipients promoted the opsonophagocytic against type II and type III GBS in vitro [34]. However, given that there are more than two types of GBS, bivalent conjugate CPS vaccines may not be effective; hence, further investigation and the development of more than two GBS CPSconjugate vaccines were required.
The multivalent vaccine is designed to give protection against multiple serotypes of the microorganism. Given that up-to-date GBS is known to have around 10 different serotypes, with more than three serotypes reported to be the most prevalent at causing disease in infants, pregnant women, or adults [35], a multivariant vaccine is predicted to provide robust immune protection with high effectiveness against multi-serotypes of GBS.
Multivalent GBS conjugate vaccines have been investigated for decades [36] to reduce the GBS disease burden. The GBS5-CV (with or without an adjuvant) was the first multivalent GBS conjugate vaccine deployed in Africa, where GBS disease incidence in infants is the highest worldwide [37]. The study reported that GBS5-CV with or without an adjuvant-induced a robust and functional IgG antibody response against the GBS serotypes Ia, Ib, II, III, and V. Accordingly, GBS5-CV was able to cover 96% of the invasive GBS disease caused by these serotypes in neonates [37].
Given that the CPS/CPS-conjugate GBS vaccines only give serotype-specific protection, the emergence of non-target serotypes could reduce the vaccine's effectiveness. Protein-based vaccines are believed to possess the potential to not only confer protection across all GBS serotypes but possibly reduce concern regarding the emergence of non-target serotypes following vaccine administration [38]. Currently, protein-based vaccines for GBS are in the clinical trial stage. In 2021, an efficacy study of a prototype vaccine consisting of the fused N-terminal domains of the AlphaC and Rib surface protein GBS (GBS-NN) demonstrated a significantly increased antibody concentration in response to two doses of 50 µg of the GBS-NN vaccine in the presence of an adjuvant. Additionally, this study also demonstrated that the vaccine was well tolerated by most of the 240 healthy adult non-pregnant participants [39]. Subsequently, in 2022, a follow-up study demonstrated robust IgG and IgA responses against AlphaC and the Rib surface protein, which are a component of the GBS-NN vaccine, as well as against the heterotypic Alp family members Alp1-Alp3 [40]. This study also demonstrated that protein-based vaccine-induced IgG mediates opsonophagocytic killing and prevents bacterial invasion into epithelial cells [40].
Although GBS is commonly colonized in the vaginal tract of pregnant women, colonization in the elderly has been reported sporadically. Additionally, colonization in pregnant women likely leads to neonatal infection; hence, determining the target of vaccination is also considered a crucial factor in the development of GBS vaccines.
Given the high incidence of GBS infection in infants due to the vertical infection from their mothers, the main target of the GBS vaccine is pregnant women. According to the U.S. Centers for Disease Control and Prevention, around 15% to 40% of pregnant women are colonized with GBS in the vaginal tract or rectum. GBS may also colonize the infants if the mother is colonized with GBS; colonization in infants can occur before or during delivery [41]. However, although pregnant women are considered the main target, several factors, such as lack of awareness and understanding of GBS, may become obstacles in the implementation of the vaccination. Additionally, with the current status of GBS vaccines, which are still in clinical trial phase III, many are doubting the safety of tested vaccines. Hence, for the preparation and implementation of a GBS vaccine, an effort to increase awareness among pregnant women about GBS infections, as well as the benefit for the infant and the actual result of each phase trial to improve the acceptability of currently recommended maternal vaccines should also be considered. Among GBS vaccines that are being developed, the GBS vaccine by MinervaX recently completed its second phase II clinical trial in pregnant women across Denmark, the UK, and South Africa [42].
Compared to the cases in pregnant women and infants, GBS infection cases in adults and the elderly are considered lower. However, a recent study discovered that the incidence and burden of GBS infection in adults and the elderly may be higher than previously thought [43]. Indeed, most studies describing the burden of GBS in adults and the elderly have mainly focused on the result of blood cultures [44]. Nevertheless, studies have reported that GBS also causes infection in the soft tissue, bone, joints, urinary, respiratory tract, and skin [45,46]. Hence, the adult and elderly population is also at great risk of GBS infection. In 2004, a study reported that a type V GBS CPS-conjugate vaccine, administered in 32 healthy adults (65-85 years old), was able to elicit significant increases in type V CPS-specific IgG, IgM, and IgA antibodies, which promoted the opsonophagocytic killing of type V GBS in vitro [47]. Additionally, by April 2023, MinervaX also announced its initiation of phase I clinical trial of the fusion protein-based GBS vaccine in the older adult population aged 55 to 75 years old [48].
Go to : 
Since the first attempt at developing a GBS vaccine, several candidates have completed their initial clinical trial in humans. Additionally, expanding the target of GBS vaccines to the elderly population, including people with a high risk of GBS infection, is a very important step in the battle against GBS. Furthermore, deploying the GBS vaccine in regions where IAP is poorly available may provide a benefit in reducing the risk of GBS infection in neonates. Although, up to date, the licensing of the GBS vaccine remains difficult, awareness regarding the benefit of the GBS vaccine for controlling disease in pregnant women, infants, and non-pregnant adults increased; therefore, more people may participate in clinical trials and accelerate the licensing of GBS vaccines and their implementation for the prevention of severe GBS infection.
Go to : 
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Funding
This research was supported by the National Research Foundation (NRF) of Korea (NRF2021R1I1A3044483 and NRF-2021M3E5E3080382). The funders had no role in the study design, data collection, interpretation, or decision to submit the manuscript for publication.
REFERENCES
1. Kong F, Gowan S, Martin D, James G, Gilbert GL. Serotype identification of group B streptococci by PCR and sequencing. J Clin Microbiol 2002;40:216-26.
.
2. Hanna M and Noor A. Streptococcus group B. In: StatPearls. Treasure Island (FL); StatPearls Publishing, 2020.
.
3. Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B StreStreptococcusptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S100-11.
.
4. Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;4:e98-108.
.
5. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet 2014;384:189-205.
.
6. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430-40.
.
7. Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017;65:S200-19.
.
8. CDC. Group B Strep (GBS). https://www.cdc.gov/groupbstrep/index.html [Online] (last visited on 28 August 2023).
.
9. Braye K, Foureur M, de Waal K, Jones M, Putt E, Ferguson J. Group B streptococcal screening, intrapartum antibiotic prophylaxis, and neonatal early-onset infection rates in an Australian local health district: 2006-2016. PLoS One 2019;14:e0214295.
.
10. Horsley L. CDC updates guidelines for the prevention of perinatal GBS disease. Am Fam Physician 2011;83:1106-10.
.
11. Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol 2013;121:570-7.
.
12. Pintye J, Saltzman B, Wolf E, Crowell CS. Risk factors for late-onset group B streptococcal disease before and after implementation of universal screening and intrapartum antibiotic prophylaxis. J Pediatric Infect Dis Soc 2016;5:431-8.
.
13. Carreras-Abad C, Ramkhelawon L, Heath PT, Le Doare K. A vaccine against group B Streptococcus: recent advances. Infect Drug Resist 2020:1263-72.
.
14. Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome 2017;5:1-10.
.
15. Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG: Int J Obstet Gynaecol 2016;123:983-93.
.
16. Corvaglia L, Tonti G, Martini S, Aceti A, Mazzola G, Aloisio I, et al. Influence of intrapartum antibiotic prophylaxis for group B Streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr 2016;62:304-8.
.
17. CDC. Serotypes and the importance of serotyping Salmonella. https://www.cdc.gov/ salmonella/reportspubs/salmonella-atlas/serotyping-importance.html [Online] (last visited on 28 August 2023).
.
18. Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and metaanalyses. Clin Infect Dis 2017;65:S160-72.
.
19. Kang HM, Lee HJ, Lee H, Jo DS, Lee HS, Kim TS, et al. Genotype characterization of group B Streptococcus isolated from infants with invasive diseases in South Korea. Pediatr Infect Dis J 2017;36:e242-7.
.
20. Yao Z, Jiayin W, Xinyi Z, Ling C, Mingyuan H, Simin M, et al. Identification of group B Streptococcus serotypes and genotypes in late pregnant women and neonates that are associated with neonatal early-onset infection in a South China population. Front Pediatr 2020;8:265.
.
21. Hannoun A, Shehab M, Khairallah MT, Sabra A, Abi-Rached R, Bazi T, et al. Correlation between group B streptococcal genotypes, their antimicrobial resistance profiles, and virulence genes among pregnant women in Lebanon. Int J Microbiol 2009;2009.
.
22. Takahashi T, Maeda T, Lee S, Lee DH, Kim S. Clonal distribution of clindamycin-resistant erythromycin-susceptible (CRES) Streptococcus agalactiae in Korea based on whole genome sequences. Ann Lab Med 2020;40:370-81.
.
23. Udo EE, Boswihi SS, Al-Sweih N. Genotypes and virulence genes in group B Streptococcus isolated in the maternity hospital, Kuwait. Med Princ Pract 2013;22:453-7.
.
24. Zakerifar M, Kaboosi H, Goli HR, Rahmani Z, Peyravii Ghadikolaii F. Antibiotic resistance genes and molecular typing of Streptococcus agalactiae isolated from pregnant women. BMC Pregnancy Childbirth 2023;23:1-13.
.
25. Berner R. Group B Streptococcus vaccines: one step further. Lancet Infect Dis 2021;21:15860.
.
26. GAVI. Routine vaccines, extraordinary impact: Group B Streptococcus (GBS). https://www. gavi.org/vaccineswork/routine-vaccines-extraordinary-impact-group-b-Streptococcus-gbs [Online] (last visited on 30 August 2023).
.
27. Baker CJ and Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976;294:753-6.
.
28. Lin FYC, Philips III JB, Azimi PH, Weisman LE, Clark P, Rhoads GG, et al. Level of maternal antibody required to protect neonates against early-onset disease caused by group B Streptococcus type Ia: a multicenter, seroepidemiology study. J Infect Dis 2001;184:1022-8.
.
29. Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SL, Kasper DL, et al. Maternal antibody at delivery protects neonates from early-onset group B streptococcal disease. J Infect Dis 2014;209:781-8.
.
30. Johri AK, Paoletti LC, Glaser P, Dua M, Sharma PK, Grandi G, et al. Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol 2006;4:932-42.
.
31. Paoletti LC and Kasper DL. Conjugate vaccines against group B Streptococcus types IV and VII. J Infect Dis 2002;186:123-6.
.
32. Wessels M, Paoletti L, Kasper D, DiFabio J, Michon F, Holme K, et al. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest 1990;86:1428-33.
.
33. Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, et al. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest 1996;98:2308-14.
.
34. Baker CJ, Rench MA, Fernandez M, Paoletti LC, Kasper DL, Edwards MS. Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J Infect Dis 2003;188:66-73.
.
35. Ali MM, Asrat D, Fenta DA, Chaka TE, Woldeamanuel Y. Group B Streptococcus colonization rate and serotype distribution among pregnant women and their newborns at Adama Hospital Medical College, Ethiopia. Sci Rep 2020;10:9301.
.
36. Baker C and Edwards M. Group B streptococcal conjugate vaccines. Arch Dis Child 2003;88:375-8.
.
37. Dhar N, Mohamed E, Kirstein F, Williams M, Dorasamy S, van Zyl P, et al. Immune responses against group B Streptococcus monovalent and pentavalent capsular polysaccharide tetanus toxoid conjugate vaccines in Balb/c mice. iScience 2023;26:107380.
.
38. Dominguez K and Randis TM. Toward the development of a protein-based group B Streptococcus vaccine. Cell Rep Med 2022;3:100536.
.
39. Fischer P, Pawlowski A, Cao D, Bell D, Kitson G, Darsley M, et al. Safety and immunogenicity of a prototype recombinant alpha-like protein subunit vaccine (GBS-NN) against Group B Streptococcus in a randomized placebo-controlled double-blind phase 1 trial in healthy adult women. Vaccine 2021;39:4489-99.
.
40. Pawlowski A, Lannergård J, Gonzalez-Miro M, Cao D, Larsson S, Persson JJ, et al. A group B Streptococcus alpha-like protein subunit vaccine induces functionally active antibodies in humans targeting homotypic and heterotypic strains. Cell Rep Med 2022;3:100511.
.
41. CDC. Group B streptococcal infections: the Division of Bacterial and Mycotic Diseases National Center for Infectious Diseases Centers for Disease Control and Prevention. https:// wonder.cdc.gov/wonder/prevguid/p0000422/p0000422.asp [Online] (last visited on 26 August 2023).
.
42. MinervaX. MinervaX commences first phase 1 clinical study of novel GBS vaccine in older adults. https://www.minervax.com/minervax-commences-first-phase-1-clinical-study-ofnovel-gbs-vaccine-in-older-adults/ [Online] (last visited on 30 August 2023).
.
43. McLaughlin JM, Peyrani P, Furmanek S, Khan FL, Quinn A, Jodar L, et al. The burden of adults hospitalized with group B streptococcal infection. J Infect Dis 2021;224:1170-8.
.
44. CDC. Active bacterial core surveillance report, emerging infections program network, group B Streptococcus, 2009. https://www.cdc.gov/abcs/reports-findings/survreports/gbs09.pdf [Online] (last visited on 31 August 2023).
.
45. Smith E, Khan M, Reingold A, Watt J. Group B Streptococcus infections of soft tissue and bone in California adults, 1995–2012. Epidemiol Infect 2015;143:3343-50.
.
46. Lamagni TL, Keshishian C, Efstratiou A, Guy R, Henderson KL, Broughton K, et al. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin Infect Dis 2013;57:682-8.
.
47. Palazzi DL, Rench MA, Edwards MS, Baker CJ. Use of type V group B streptococcal conjugate vaccine in adults 65–85 years old. J Infect Dis 2004;190:558-64.
.
48. Arena CT. MinervaX initiates Phase I GBS vaccine trial in older people. https://www. clinicaltrialsarena.com/news/minervax-phase-i-gbs-trial-older-people/ [Online] (last visited on 1 September 2023).
.
Go to : 
 | Fig. 1.Timeline of GBS discovery to the time it was considered the predominant pathogen causing sepsis and meningitis in neonates. GBS, Group B Streptococcus. |
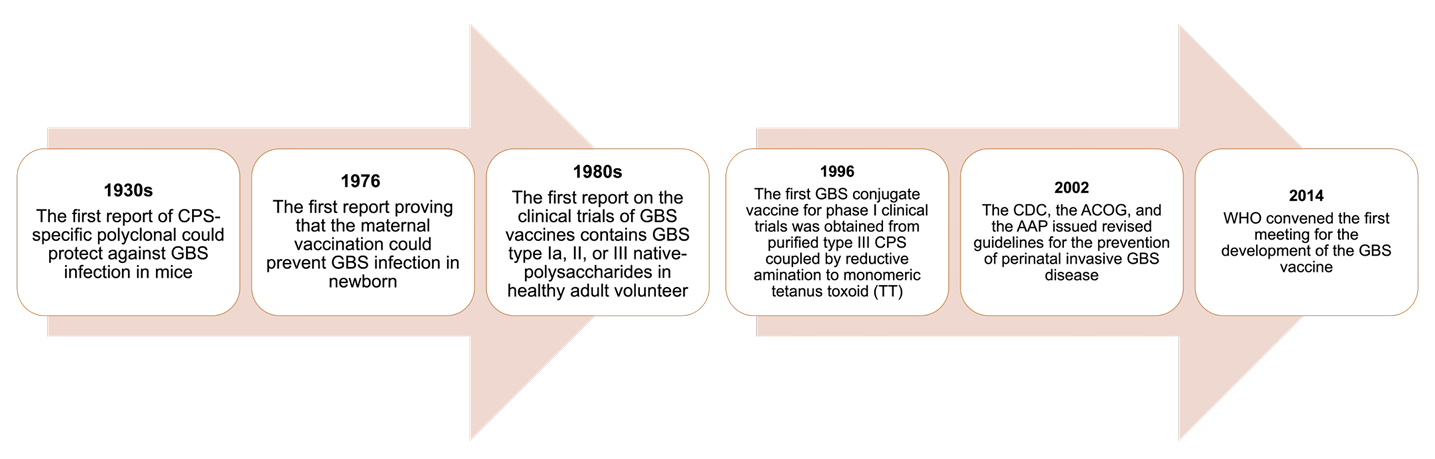 | Fig. 2.Timeline depicting the discovery of factors crucial for GBS vaccine development. CPS, capsular polysaccharide; GBS, Group B Streptococcus; CDC, Centers for Disease Control and Prevention; ACOG, the American College of Obstetricians and Gynecologists; AAP, the American Academy of Pediatrics; WHO, World Health Organization. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download