Abstract
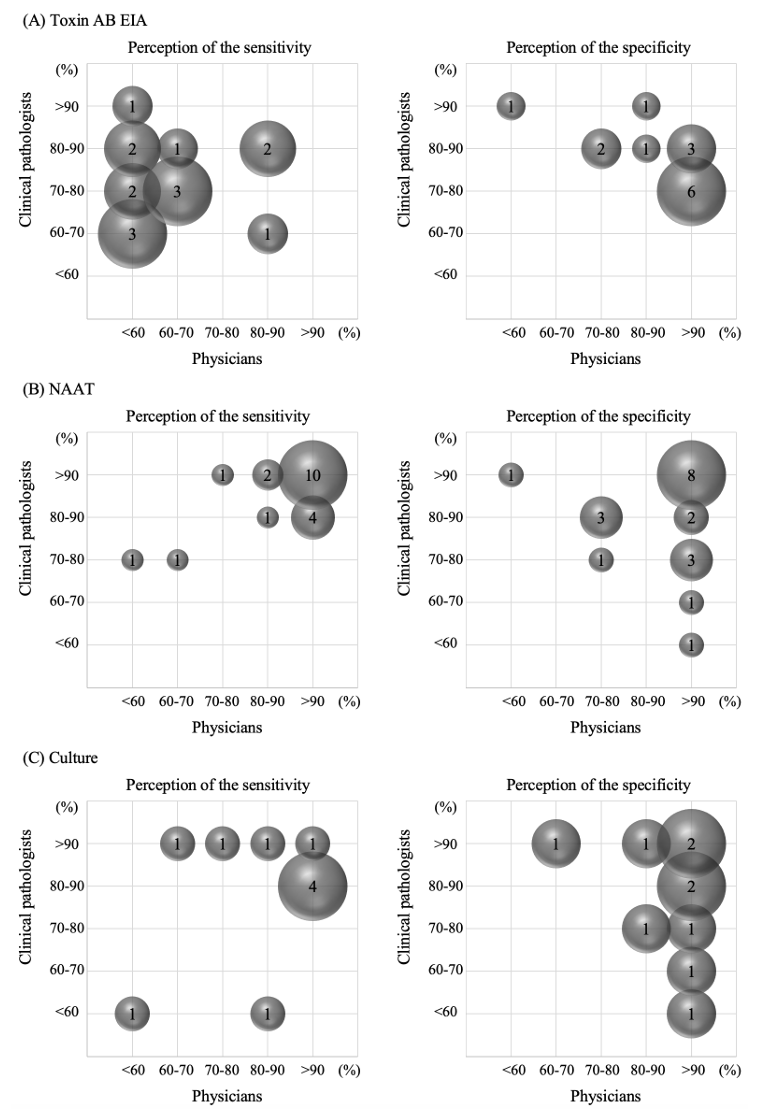
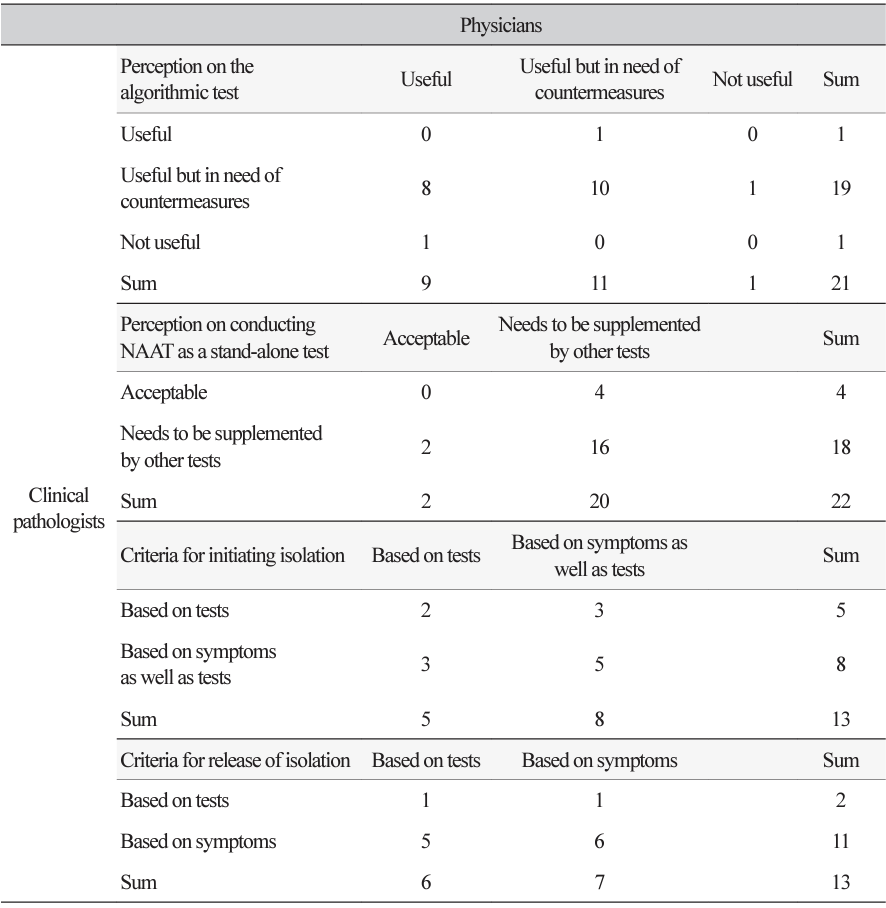
Background: This study aimed to investigate perceptions of Clostridioides difficile infection (CDI) diagnostic tests among physicians who prescribe CDI diagnostic tests as part of providing direct patient care. Methods: In August 2018, we provided a 12-question survey of gastroenterologists (the most common referral source for CDI testing) to 35 medical institutions in Korea, asking them about their perceptions of CDI diagnosis and testing. Results: A comparison of the perceptions of physicians and clinical pathologists (CPs) found that physicians had a lower perceived sensitivity of the toxin AB enzyme immunoassay test. For nucleic acid amplification tests, physicians exhibited a perception of higher assay sensitivity and specificity than CPs. The specificity of culture tests was generally perceived as high by physicians, whereas CPs regarding expressed mixed opinions. All but one physician and one CPs found the algorithmic test useful. Concerning the CDI isolation criteria, physicians commenced patient isolation by concurrently assessing both test results and clinical symptoms, rather than exclusively relying upon test results. Among CPs, 84.6% said they could rely on symptoms to determine when to release a patient from isolation, while 46.2% of physicians said they would rely on test results. Conclusion: This study provides useful information on the status of laboratory diagnosis of CDI in Korea and what needs to be improved, which will help to standardize and improve laboratory diagnosis of CDI in Korea.
[in Korean]
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Inje University Sanggye Paik Hospital (IRB No. SGPAIK-2018-10-010), which waived the requirement for informed consent.
Conflicts of interest
Hae-Sun Chung has been an editor-in-chief of the Annals of Clinical Microbiology since January 2022. However, she was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2017NP280600).
REFERENCES
1. Leffler DA and Lamont JT. Clostridium difficile infection. N Engl J Med 2015;372:1539-48.
.
2. Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015;313:398-408.
.
3. Biswas R, Dudani H, Lakhera P, Pal AK, Kurbah P, Bhatia D, et al. Challenges and future solutions for detection of Clostridioides difficile in adults. Ann Gastroenterol 2023;36:369-77.
.
4. Crobach MJT, Baktash A, Duszenko N, Kuijper EJ. Diagnostic guidance for C. difficile infections. Adv Exp Med Biol 2018;1050:27-44.
.
5. Chung HS, Park JS, Shin BM, Yoo HM, Kim H, Cho J, et al. Nationwide survey for current status of laboratory diagnosis of Clostridioides difficile Infection in Korea. J Korean Med Sci 2022;37:e38.
.
6. Chung HS, Park JS, Shin BM. Laboratory diagnosis of Clostridium difficile infection in Korea: the first national survey. Ann Lab Med 2019;39:317-21.
.
7. Crobach MJT, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016;22:S63-81.
.
8. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018;66:987-94.
.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download