Abstract
Background: To solve the difficulty in determining the appropriate treatment regimen for patients infected with extensively drug-resistant Acinetobacter baumannii (XDRAB), it is necessary to develop various strategies to increase the therapeutic effect of antimicrobial agents. The purpose of this study was to select the treatment combination showing the greatest antimicrobial effect among seven candidate antimicrobial substances. Methods: Seven strains of XDRAB were used in this study. The composition of the treatment consisted of colistin as the base and one of the seven antimicrobial substances, doripenem, minocycline, tigecycline, linezolid, fusidic acid, vancomycin, or alyteserin E4K peptide. The interaction between the drugs in each combination was evaluated by measuring the synergy rates using time-kill analysis. Results: The synergy rates of the seven combinations tested in the time-kill assay in this study were as follows, in descending order from the combination with the highest synergy rate: colistin + minocycline (57.1%), colistin + alyteserin E4K (50.0%), colistin + tigecycline (42.9%), colistin + vancomycin (28.6%), colistin + doripenem (14.3%), colistin + fusidic acid (14.3%), and colistin + linzolid (0%). None of the combinations showed antagonism. The three combinations showing bactericidal activity and the rates of their bactericidal activity were colistin + alyteserin E4K combination (33.3%), colistin + minocycline (14.3%), and colistin + vancomycin (14.3%). Conclusion: The colistin + minocycline and colistin + alyteserin E4K treatment combinations, which showed high synergy rates, can be considered as promising candidates for future in vivo experiments evaluating combination therapies.
[in Korean]
Supplementary materials
The Data Supplement is available with this article at https://doi.org/10.5145/ACM.2022.25.4.3.
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
REFERENCES
1. Conlon JM, Mechkarska M, Arafat K, Attoub S, Sonnevend A. Analogues of the frog skin peptide alyteserin-2a with enhanced antimicrobial activities against Gram-negative bacteria. J Pept Sci 2012;18:270-5.
.
2. Conlon JM, Sonnevend A, Pál T, Vila-Farrés X. Efficacy of six frog skin-derived antimicrobial peptides against colistin-resistant strains of the Acinetobacter baumannii group. Int J Antimicrob Agents 2012;39:317-20.
.
3. Feizabadi MM, Fathollahzadeh B, Taherikalani M, Rasoolinejad M, Sadeghifard N, Aligholi M, et al. Antimicrobial susceptibility patterns and distribution of blaOXA genes among Acinetobacter spp. Isolated from patients at Tehran hospitals. Jpn J Infect Dis 2008;61:274-8.
.
4. Higgins PG, Wisplinghoff H, Krut O, Seifert H. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin Microbiol Infect 2007;13:1199-201.
.
5. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrugresistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81.
.
6. Phee LM, Betts JW, Bharathan B, Wareham DW. Colistin and fusidic acid, a novel potent synergistic combination for treatment of multidrug-resistant Acinetobacter baumannii Infections. Antimicrob Agents Chemother 2015;59:4544-50.
.
7. Oleksiuk LM, Nguyen MH, Press EG, Updike CL, O'Hara JA, Doi Y, et al. in vitro responses of Acinetobacter baumannii to two-and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother 2014;58:1195-9.
.
8. Mao J, Li T, Zhang N, Wang S, Li Y, Peng Y, et al. Dose optimization of combined linezolid and fosfomycin against Enterococcus by using an in vitro pharmacokinetic/pharmacodynamic model. Microbiol Spectr 2021;9:e00871-21.
.
9. Colton B, McConeghy KW, Schreckenberger PC, Danziger LH. I.V. minocycline revisited for infections caused by multidrug-resistant organisms. Am J Health Syst Pharm 2016;73:279-85.
.
10. Zhanel GG, Baudry PJ, Tailor F, Cox L, Hoban DJ, Karlowsky JA. Determination of the pharmacodynamic activity of clinically achievable tigecycline serum concentrations against clinical isolates of Escherichia coli with extended-spectrum β-lactamases, AmpC β-lactamases and reduced susceptibility to carbapenems using an in vitro model. J Antimicrob Chemother 2009;64:824-8.
.
11. Gordon NC, Png K, Wareham DW. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 2010;54:5316-22.
.
12. Kim UJ, Kim CM, Jang SJ, Lee SB, Cho SS, Jeong SH, et al. Evaluation of synergistic effect of combined multidrug-resistant Acinetobacter baumannii treatment with linalool and colistin on to expand candidate for therapeutic option. Ann Clin Microbiol 2020;23:11-20.
.
13. Park GC, Choi JA, Jang SJ, Jeong SH, Kim CM, Choi IS, et al. in vitro interactions of antibiotic combinations of colistin, tigecycline, and doripenem against extensively drugresistant and multidrug-resistant Acinetobacter baumannii. Ann Lab Med 2016;36:124-30.
.
14. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; CLSI document M100. Wayne; PA: 2018.
.
15. Tan TY, Yong Ng LS, Tan E, Huang G. in vitro effect of minocycline and colistin combinations on imipenem-resistant Acinetobacter baumannii clinical isolates. J Antimicrob Chemother 2007;60:421-3.
.
16. March GA and Bratos MA. A meta-analysis of in vitro antibiotic synergy against Acinetobacter baumannii. J Microbiol Methods 2015;119:31-6.
.
17. Principe L, D'Arezzo S, Capone A, Petrosillo N, Visca P. in vitro activity of tigecycline in combination with various antimicrobials against multidrug resistant Acinetobacter baumannii. Ann Clin Microbiol Antimicrob 2009;8:18.
.
18. Peck KR, Kim MJ, Choi JY, Kim HS, Kang CI, Cho YK, et al. in vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii, including colistin- or tigecycline-resistant isolates. J Med Microbiol 2012;61:353-60.
.
19. Li J, Yang X, Chen L, Duan X, Jiang Z. in vitro activity of various antibiotics in combination with tigecycline against Acinetobacter baumannii: a systematic review and meta-analysis. Microb Drug Resist 2017;23:982-93.
.
20. Vidaillac C, Benichou L, Duval RE. in vitro synergy of colistin combinations against colistinresistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 2012;56:4856-61.
.
21. Pankuch GA, Seifert H, Appelbaum PC. Activity of doripenem with and without levofloxacin, amikacin, and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn Microbiol Infect Dis 2010 Jun;67:191-7.
.
22. Principe L, Capone A, Mazzarelli A, D'Arezzo S, Bordi E, Caro AD, et al. in vitro activity of doripenem in combination with various antimicrobials against multidrug-resistant Acinetobacter baumannii: possible options for the treatment of complicated infection. Microb Drug Resist 2013;19:407-14.
.
23. Ma XL, Guo YZ, Wu YM, Gong WT, Sun J, Huang Z. in vivo bactericidal effect of colistinlinezolid combination in a murine model of MDR and XDR Acinetobacter baumannii pneumonia. Sci Rep 2020;10:17518.
.
24. Oliva A, Garzoli S, De Angelis M, Marzuillo C, Vullo V, Mastroianni CM, et al. Invitro evaluation of different antimicrobial combinations with and without colistin against carbapenem-resistant Acinetobacter baumannii. Molecules 2019;24:886.
.
25. Zusman O, Avni T, Leibovici L, Adler A, Lena Friberg, Stergiopoulou T, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 2013;57:5104-11.
.
26. Liu CB, Shan B, Bai HM, Tang J, Yan LZ, Ma YB. Hydrophilic/hydrophobic characters of antimicrobial peptides derived from animals and their effects on multidrug resistant clinical isolates. Dongwuxue Yanjiu 2015;36:41-7.
.
Table 1
Results of time-kill assay against two-drug combinations of colistin and various antibiotics or a peptide for seven extensively drugresistant A. baumannii clinical isolates
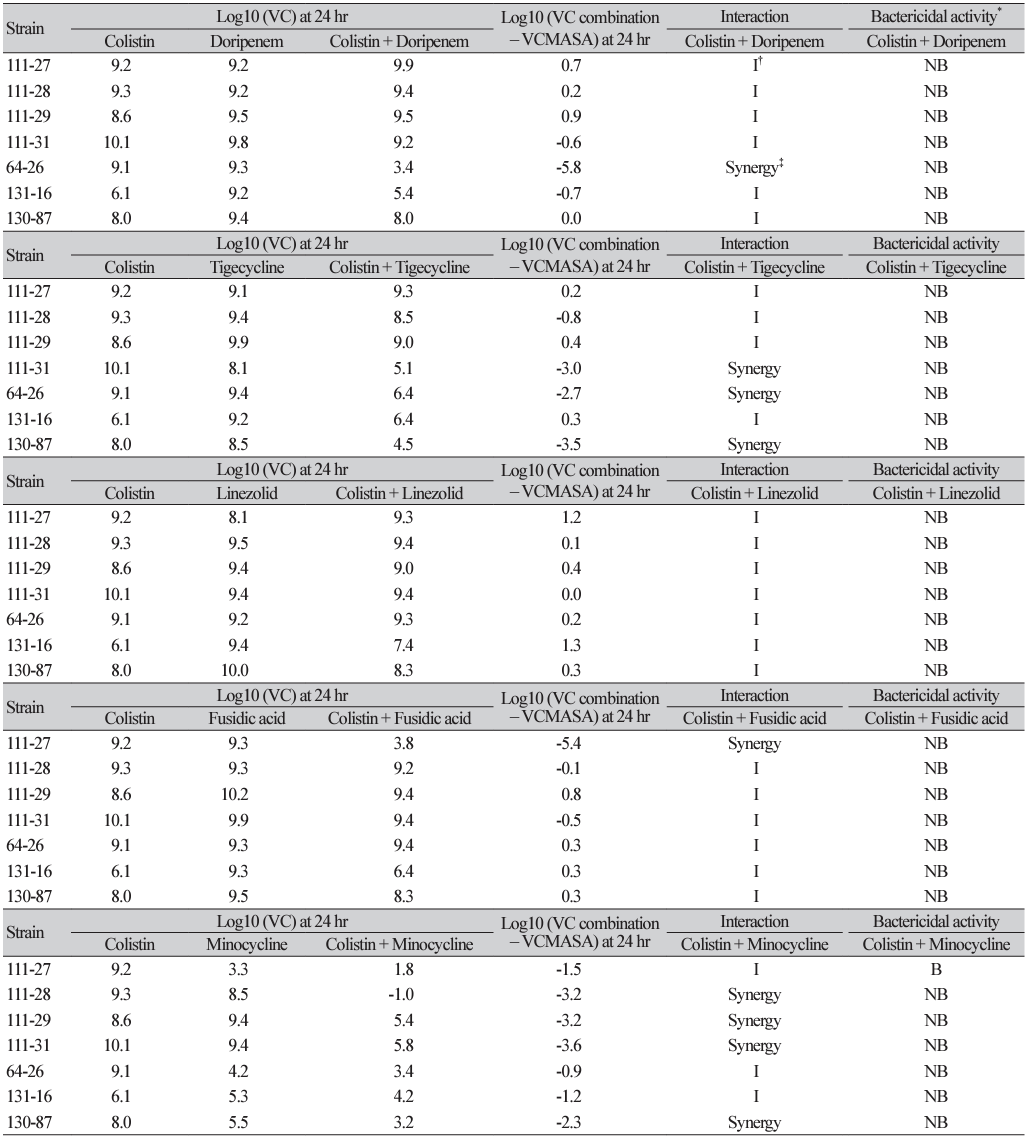
Table 1
Results of time-kill assay against two-drug combinations of colistin and various antibiotics or a peptide for seven extensively drugresistant A. baumannii clinical isolates (continued)
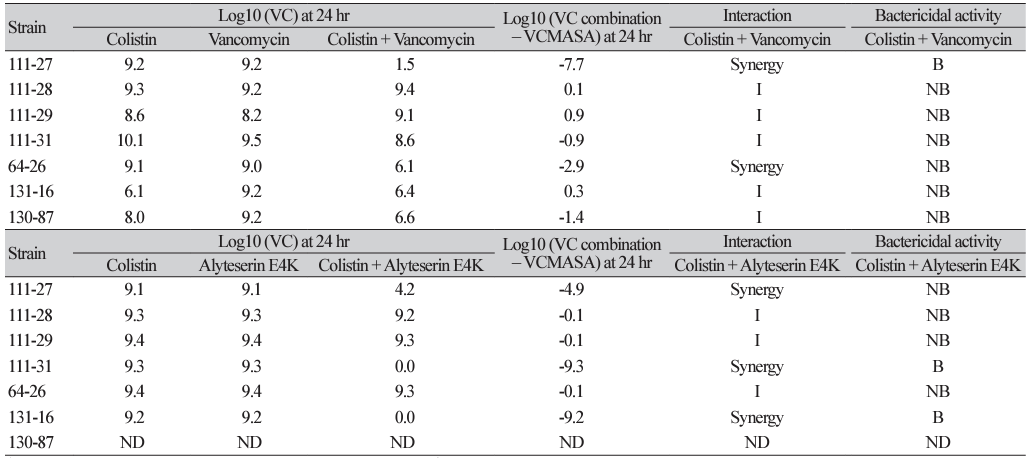
*≥3 log10 CFU/mL reduction compared with the initial inoculums; † <2 log10 change in CFU/mL at 24 hr with the combination compared with the most active single agent; ‡≥2 log10 CFU/mL reduction with the combination compared with the most active single agent of 24hr.
Abbreviation: VC, viable colony count; I, Indifference; NB, non-bactericidal; B, bactericidal; ND, not done; MASA, most active single agent.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download