Abstract
Gemella sanguinis is a gram-positive, facultatively anaerobic coccus bacterium that has rarely been reported as a cause of infective endocarditis. A 41-year-old male patient with mitral valve prolapse visited the outpatient clinic presenting with fever. Transthoracic echocardiography and transesophageal echocardiography revealed myxomatous change and vegetation of the mitral valve. We isolated G. sanguinis from the patient’s blood, cultured it in both aerobic and anaerobic blood culture bottles, and identified it using matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS; bioMérieux, France) and 16s rRNA sequencing. The isolated G. sanguinis was highly susceptible to penicillin and vancomycin and intermediately susceptible to erythromycin, clindamycin, and levofloxacin. Following the American Heart Association recommendations, this highly penicillin-sensitive isolate was eradicated with ceftriaxone and gentamicin, and the patient recovered and was discharged. To the best of our knowledge, this is the first reported case in Korea where G. sanguinis, the causative agent of endocarditis, was identified using MALDI-TOF MS and 16s rRNA sequencing and was treated with only antibiotics and without surgical valve replacement.
Go to : 
Gemella sanguinis is a gram-positive, catalase-negative, facultative anaerobic coccus bacterium. It was first identified as a new species in 1998 [1]. Based on phylogenetic and phenotypic evidence, it was proposed that the unknown bacterium be classified as G. sanguinis sp. nov.. It is known to be a normal f lora of the human oropharynx, gastrointestinal tract, and urogenital tract and has rarely been reported as a cause of infective endocarditis (IE) [2–6]. IE is a disease of the endocardial surface of the heart that has high morbidity and mortality. Infection typically involves the cardiac valves (native or prosthetic) or an indwelling cardiac device [7].
In this report, we describe a case of G. sanguinis isolated from a patient with endocarditis. To the best of our knowledge, this case is the first report of endocarditis caused by G. sanguinis in Korea, except for one previously reported in an abstract [6].
Go to : 
A 41-year-old male patient visited our outpatient clinic with a fever of up to 38.0°C for two weeks. Other symptoms included chills, sweating, and headache, which were especially severe at night. He had a mitral valve prolapse discovered incidentally when he was 20 years and had been receiving dental treatment for braces in the past year. Physical examination revealed a blood pressure of 126/57 mmHg, with a pulse rate of 96 beats/min. Complete blood count revealed a hemoglobin count of 12.4 g/dL, a hematocrit count of 38.1%, a total white blood cell count of 11.90×109/L (neutrophils, 85.8%), and a platelet count of 290×109/L. The erythrocyte sedimentation rate was 23 mm/h (1–10 mm/h), and C-reactive protein levels were 8.1 mg/dL (0–0.5 mg/dL).
One set of blood cultures was completed in the emergency room and two sets of blood cultures were completed during hospitalization. All blood cultures were inoculated into BACT/ALERT® FA Plus (aerobic) bottles and BACT/ALERT® FN Plus (anaerobic) bottles (bioMérieux, Marcy l’Etoile, France) and incubated in BACT/ALERT® VIRTUO® (bioMérieux, Marcy l’Etoile, France). After 19 hours of incubation, bacterial growth was detected in both aerobic and anaerobic blood culture bottles. Gram-stained smears of the cultured bottles revealed singles, pairs, or short chains of gram-positive cocci. The colonies on blood agar plate were small, circular, non-pigmented, and translucent to opaque (Fig. 1). The colonies did not grow on the MacConkey agar plates. The VITEK 2 system (bioMérieux, Durham, NC, USA; software version), with a gram positive ID card, also identified the isolate as G. sanguinis. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; bioMérieux, Marcy l’Etoile, France) identified the strain as G. sanguinis (confidence value: 99.9%). For confirmation, 16S rRNA sequencing with the primers 5′-AGA GTT TGA TCM TGG CTC AG-3′(27F) and 5′-CGG TTA CCT TGT TAC GAC TT-3′(1492R) was performed. A Basic Local Alignment Search Tool similarity search of the GenBank database of 1420-basepair sequences of the 16S rRNA gene matched G. sanguinis with a similarity of 99.58% (1414/1420), followed by Gemella taiwanensis (98.31%, 1396/1420) and Gemella haemolysans (98.24%, 1395/1420).
Transthoracic echocardiography revealed diffuse prolapse of both mitral valves (MV) with moderate-tosevere mitral regurgitation. A mobile mass-like lesion with a size of 7.7×4.9 mm on the anterior leaflet tip was observed, and it was determined that transesophageal echocardiography (TEE) was required for accurate evaluation. A TEE was performed, and echogenic thickening, measuring up to 4 mm in the MV anterior leaflet medial segment, was found to have potential myxomatous change and vegetation.
Using the modified Duke criteria, the patient met two major (evidence of endocardial involvement and echocardiogram positive for IE) and two minor (predisposing heart condition and positive blood culture) criteria, and was diagnosed with IE [4].
Antimicrobial susceptibility testing was performed using the Sensititre gram-positive plate, GPALL1F panel (TREK Diagnostic Systems, Inc., East Grinstead, UK). Minimal inhibitory concentrations (MICs) were 0.5 μg/mL for erythromycin, 2 μg/mL for gentamicin, 0.5 μg/mL for clindamycin, 0.06 μg/mL for penicillin, 0.5 μg/mL for vancomycin, and 4 μg/mL for levofloxacin (Table 1).
While the patient was hospitalized, antibiotic therapy was initiated with a combination of intravenous (IV) ceftriaxone and gentamicin. One week after treatment with IV antibiotics, G. sanguinis was not detected in the blood culture. The C-reactive protein decreased to 1.9 mg/dL, the white blood cell count was lowered to 9.60×109/L (neutrophils, 71.9%), and the fever subsided. After six weeks, antibiotic treatment was stopped, and the patient followed-up at the outpatient clinic.
Go to : 
This is the first report in Korea of a causative agent of endocarditis, G. sanguinis, being identified and confirmed using MALDI-TOF MS and 16s rRNA sequencing. Other than IE, G. sanguinis infections rarely occur. In most cases, it affects the native valves with predisposing factors, including underlying heart disease, poor dental hygiene, dental procedures, diabetes mellitus, and end-stage renal disease [5,8–10]. Our patient had predisposing factors including a history of recent dental procedures and a structural abnormality of the mitral valve.
This isolate was not initially identified in the VITEK 2 system; however, G. sanguinis was found when reculturing the isolate. It is believed that the failed ID result was because testing was delayed since the laboratory is closed on weekends; thus, biochemical reactions in each well of the VITEK 2 ID card did not properly occur because of decreased activity of the isolate.
In this case, the isolate was treated with ceftriaxone combined with gentamicin, according to the decision of the infectious disease specialist. The isolate was highly susceptible to penicillin and vancomycin and intermediately susceptible to erythromycin, clindamycin, and levofloxacin according to the 2016 Clinical and Laboratory Standards Institute (M45-ED3) criteria for Gemella species [11]. However, there was no interpretation standard for the MIC of gentamicin and the isolate was not tested for ceftriaxone. According to American Heart Association’s recommendations, combination therapy with penicillin G or ceftriaxone and gentamicin can be used to treat an infection of highly susceptible Gemella species (penicillin MIC ≤ 0.12 μg/ mL) [4,8].
In most cases, surgical valve replacement is performed along with antibiotic treatment [5,8–10]; however, this is a rare case of successful treatment with antimicrobial agents without valve replacement surgery. We demonstrated that the accurate identification of G. sanguinis was possible through MALDI-TOF MS and 16s rRNA sequencing.
Go to : 
Ethics statement
The obtainment of informed consent was waived since it is a retrospective chart review.
REFERENCES
1. Collins MD, Hutson RA, Falsen E, Sjöden B, Facklam RR. Description of Gemella sanguinis sp. nov., isolated from human clinical specimens. J Clin Microbiol 1998;36:3090–3.
.
2. Geller SA. Infective endocarditis: a history of the development of its understanding. Autops Case Rep 2013;3:5–12.
.
3. Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective endocarditis: a contemporary review. Mayo Clin Proc 2020;95:982–97.
.
4. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435–86.
.
5. Mugunthan M, Bhalla S, Shete V, Grover N. Gemella sanguinis: a rare cause of native valve endocarditis in a child. Med J Armed Forces India 2016;72S:84–6.
.
6. Lee SH, Cho DK, Lee HM, Lee SC, Kim IT, Park HB, et al. Infective endocarditis caused by Gemella sanguinis in a hemodialysis patient. Korean J Med 2009;77:899
.
7. Emmanouilidou G, Voukelatou P, Vrettos I, Aftzi V, Dodos K, Koumpouli D, et al. A case report of successful conservative treatment for infective endocarditis caused by Gemella sanguinis. Case Rep Infect Dis 2019;2019.
.
8. Maraki S, Plevritaki A, Kofteridis D, Scoulica E, Eskitzis A, Gikas A, et al. Bicuspid aortic valve endocarditis caused by Gemella sanguinis: case report and literature review. J Infect Public Health 2019;12:304–8.
.
9. Yang CH and Tsai KT. Gemella sanguinis endocarditis: first case report in Taiwan and review of the literature. J Formos Med Assoc 2014;113:562–5.
.
10. Sideris AC, Zimmermann E, Ogami T, Avgerinos DV. A rare case of isolated mitral valve endocarditis by Gemella sanguinis: case report and review of the literature. Int J Surg Case Rep 2020;69:51–4.
.
11. Clinical and Laboratory Standards Institute (CLSI). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; CLSI guideline M45. Wayne; PA: 2016.
.
Go to : 
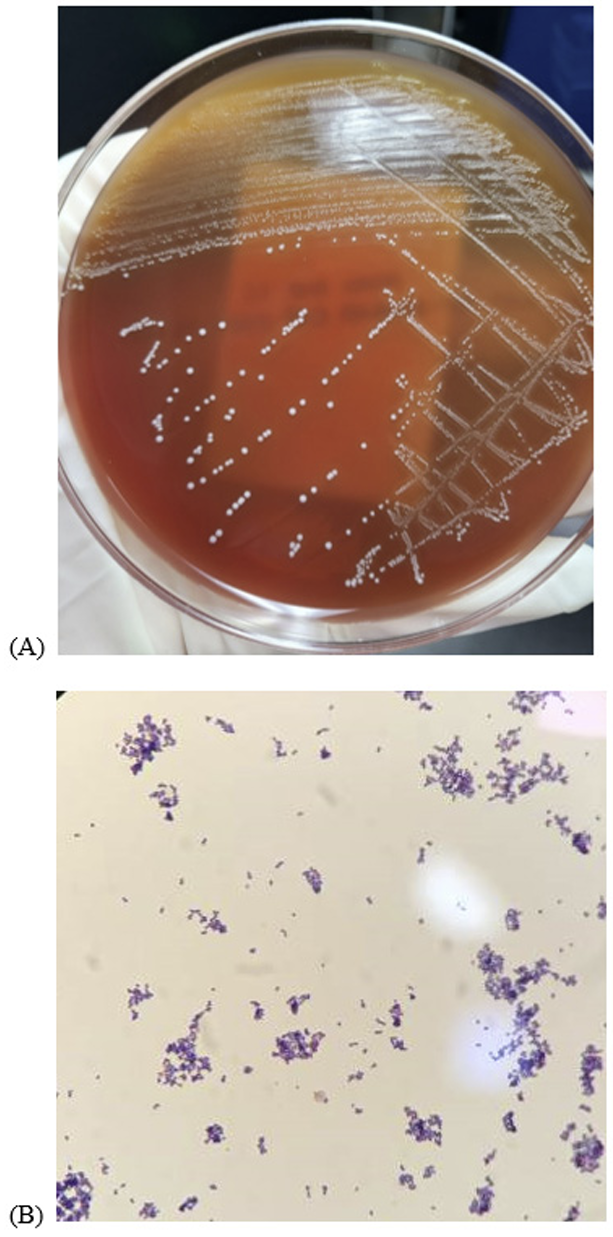 | Fig. 1.Morphological characterization of the Gemella sanguinis clinical isolates. (A) Small, circular, nonpigmented and translucent to opaque bacterial colonies grew on a blood agar plate. (B) Microscopic f inding of Gemella sanguinis shows singles, pairs or short chains of gram-positive cocci (Gram stain, 1,000×). |
Table 1
Antimicrobial susceptibility of Gemella sanguinis isolated from the patient’s blood culture
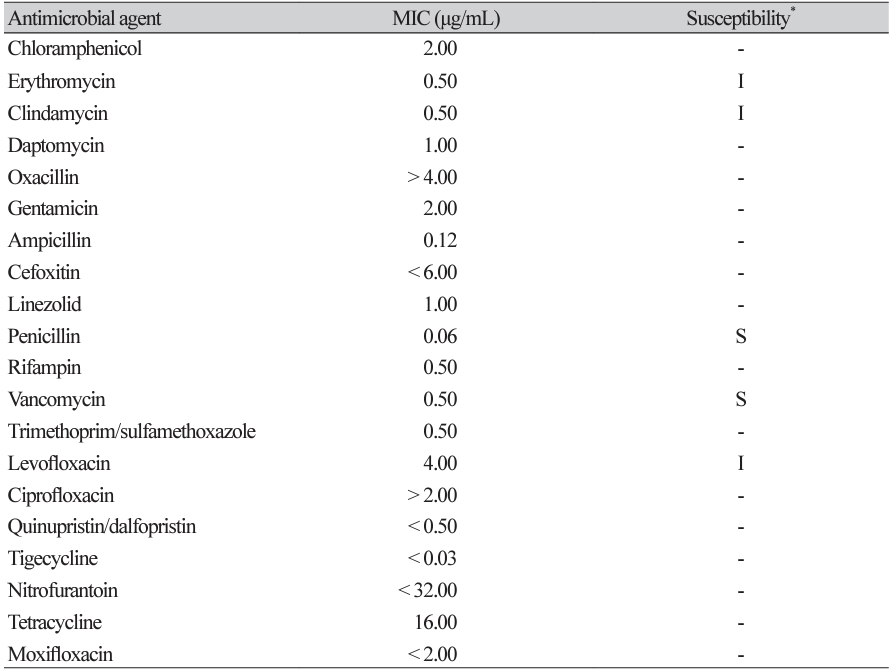
*The drug susceptibility of Gemella sanguinis in the present case was determined using the Sensititre gram positive plate, GPALL1F panel, and the 2016 Clinical and Laboratory Standards Institute (M45-ED3) criteria for Gemella species.
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; I, intermediate.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download