Introduction
Bordetella hinzii is an oxidase-positive, motile gram-negative rod first described by Vandamme et al. [1] in 1995. This organism is a commensal organism of poultry, and a rare opportunistic pathogen of respiratory infections [2,3], bacteremia [4,5] and cholangitis [6] in humans. B. hinzii has been isolated from respiratory specimens in patients with cystic fibrosis [7,8] and malignancy [9]. A few cases of B. hinzii bacteremia are reported in immunocompromised patients [4,5]. In addition, two cases of infective endocarditis and respiratory infection have been reported in immunocompetent patients [2,10]. Here, we describe the first case of bacteremia caused by B. hinzii in Korea.
Case report
A 59-year-old man was referred to a pulmonology clinic after the detection of a 2.8-cm-sized mass in the left lower lobe suspected of lung cancer detected at a health promotion center a month ago. The patient had hypertension, a past pulmonary tuberculosis, and a smoking history of 10 pack years. Chest computed tomography (CT) of the lung mass suggested that it was a post-inflammatory lesion with mucoid impaction (Fig. 1). Bronchoscopy revealed no endobronchial lesion. Bacterial cultures of sputum and bronchial wash specimens produced only normal microbiota. Acid fast bacilli (AFB) culture and real-time PCR for Mycobacterium tuberculosis complex of bronchial wash specimens were all negative.
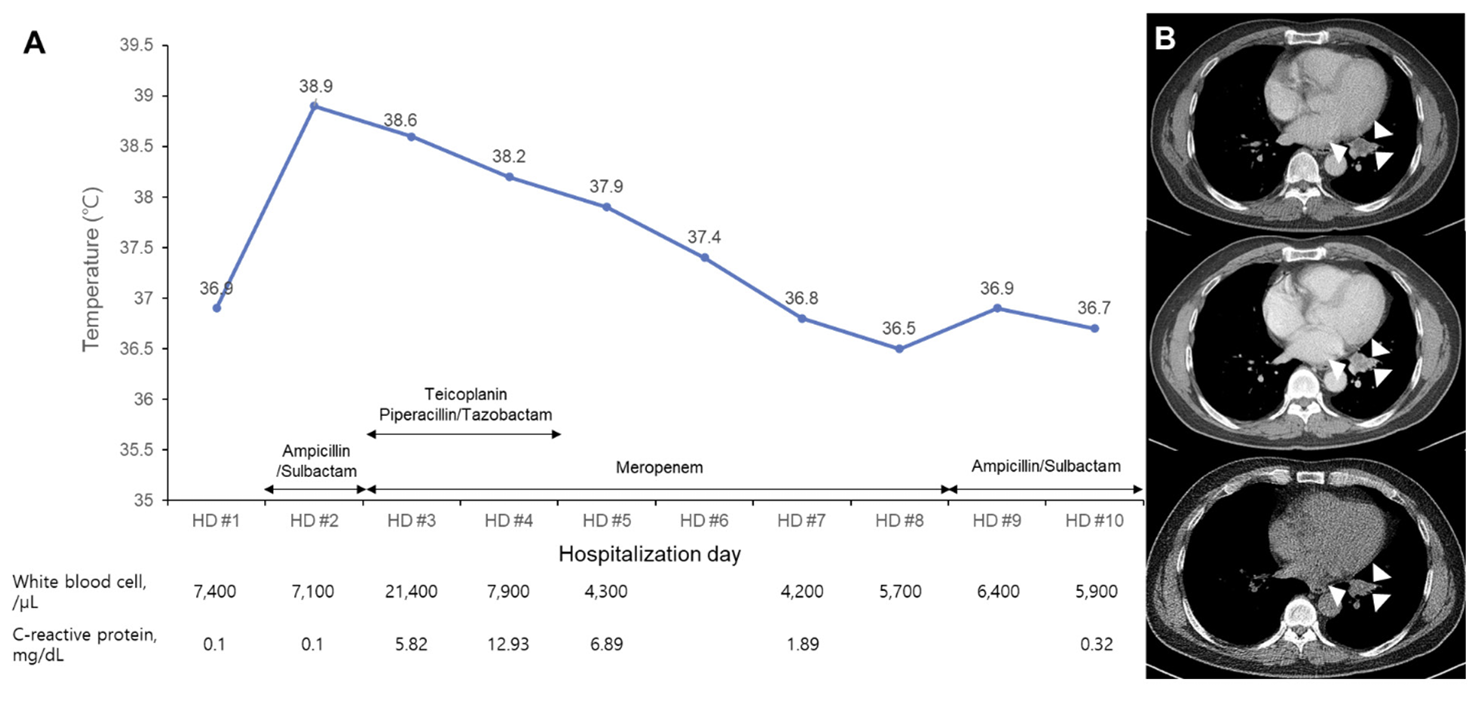
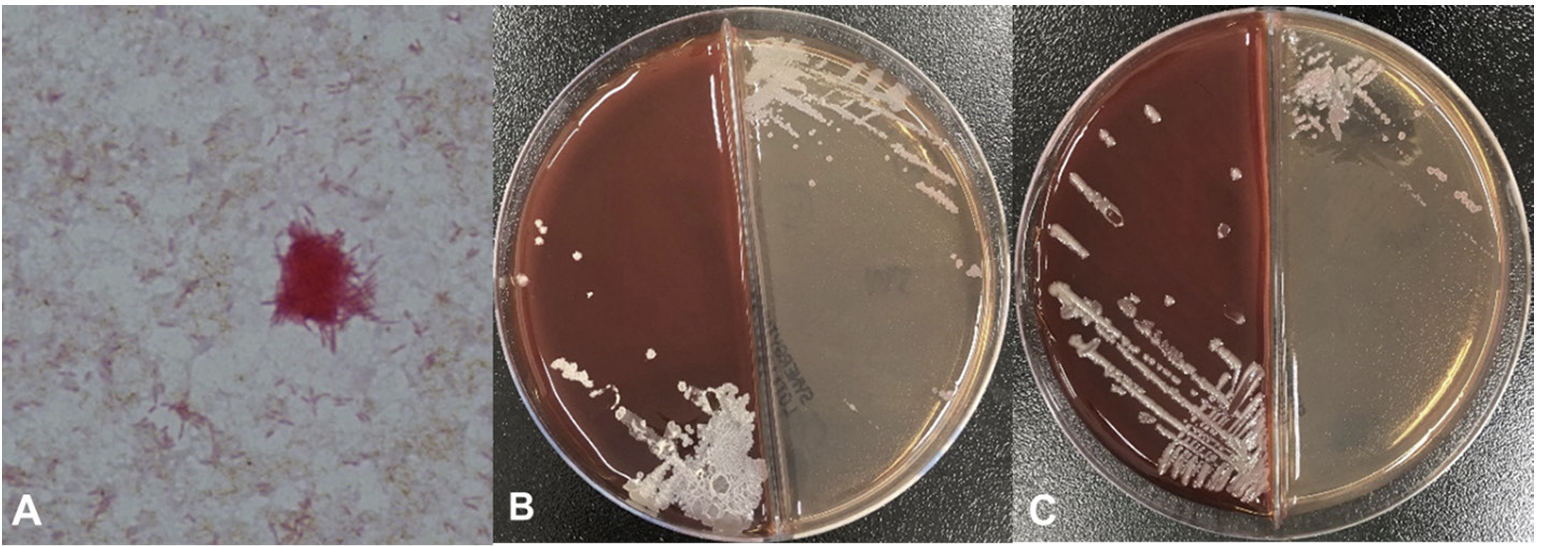
The patient was admitted 2 months later with normal vital signs without any symptoms. Biopsy of the lung mass was taken by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) at the second day of hospitalization (HD). Fever (38.9°C) and chill developed 4 hours later. Leukocyte count was increased to 21,400/μL with 96.1% neutrophils and C-reactive protein (CRP) level was increased to 5.82 mg/dL (Fig. 1). Blood cultures and sputum cultures were performed. Sputum was purulent, but squamous epithelial cells were observed at ≥ 25/low power field on gram-stained smear of sputum samples. The sputum samples showed only heavy growth of normal flora in the culture. Rhinovirus was detected from respiratory virus PCR of a nasopharyngeal swab specimen. All aerobic vials of three sets of blood cultures (BecktonDickenson, Franklin Lakes, NJ, USA) were positive for gram-negative rods with a detection time between 32.4 and 36.0 hours. Two colony types, rough and smooth, were detected on blood agar plate (BAP) and MacConkey plate (Fig. 2). Both colonies were non-hemolytic on the BAP, colorless on the MacConkey plate, and oxidase-positive. The rough colonies were flat, dry, and crinkled, while the smooth colonies were round, raised and glistening. The rough and smooth colonies were biochemically identified as Weeksella virosa and Comamonas testosteronim, respectively using MicroScan NC72 panel (Beckman Coulter, Brea, CA, USA). As matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Bruker, Billerica, MA, USA) was applied, both colony types were first matched to B. hinzii with score values of 2.281 and 2.063, and second to Bordetella avium with score values of 1.614 and 1.533 for the rough and smooth colonies, respectively. Upon a Basic Local Alignment Search Tool (BLAST) similarity search of the GenBank database of 1,327-base-pair sequences of the 16S rRNA gene, it was matched to B. hinzii with similarity of 99.92% (1,326/1,327), followed by Bordetella bronchiseptica (99.25%, 1,317/1,327) and Bordetella avium (99.10%, 1,315/1,327). Minimal inhibitory concentrations of both colonies using MicroScan MSTRP+6 panel (Beckman Coulter) were > 4/2 μg/mL for amoxicillin, > 4 μg/mL for ampicillin, > 2 μg/mL for cefuroxime, cefotaxime, ceftriaxone and cefepime, ≤ 0.25 μg/mL for meropenem, ≤ 0.25/4.7 μg/mL for trimethoprim/ sulfamethoxazole, ≤ 0.5 μg/mL for levofloxacin and minocycline, ≤ 1 μg/mL for tetracycline, and > 8 μg/mL for chloramphenicol.
The lung mass in the left lower lobe was confirmed to be subacute necrosis with no microorganism seen on Ziehl-Neelsen and Gomori’s methenamine silver staining. Intravenous ampicillin/sulbactam was started 8 hours after fever developed. Ampicillin/sulbactam was changed to piperacillin/tazobactam and teicoplanin was added on HD 3. Piperacillin/tazobactam was changed to meropenem on HD 4, and meropenem was continued to HD 8. The follow-up blood cultures were negative on HD 4, and the fever resolved on HD 7. CRP level was decreased to 1.89 mg/dL on HD 6 and normalized to 0.32 mg/dL on HD 10. Antimicrobial treatment was changed to ampicillin/sulbactam on HD 9 (Fig. 1). However, serial chest X-ray showed no change in the mass-like lesion with linear atelectasis in the left lower lobe. The patient was discharged on HD 10 with prescription of oral amoxicillin/clavulanate for 7 days. He denied any previous avian exposure at the follow-up outpatient clinic. There was no significant change in lung mass on chest CT taken 106 days after discharge (Fig. 1).
Discussion
This patient was the first bacteremia case associated with B. hinzii in Korea. It was developed after the EBUS-TBNA procedure. Steinfort et al.[11] reported that bacteremia of Streptococcus and Actinomyces was observed in 3 patients (7%) among 43 patients who underwent EBUS-TBNA. No patients had any signs or symptoms of infection, and transient bacteremia by inoculation of the oropharyngeal bacteria during bronchoscopy was suggested. However, our patient had a fever and elevated CRP which required antimicrobial treatment for 9 days to resolve; therefore, this case was a true bloodstream infection of B. hinzii which developed after EBUS-TBNA. Because prolonged persistence of B. hinzii in the respiratory and digestive tracts has been reported [2,3,8], it is possible that respiratory colonization of B. hinzii prior to the onset of bacteremia occurred in this case. Furthermore, the mass in the left lower lobe was subacute necrosis in this patient. Although AFB or other bacteria were not seen in the biopsy specimen, M. tuberculosis cannot be ruled out, considering that M. tuberculosis is a common causal organism of pulmonary nodules in Korea [12]. Since B. hinzii is a small gram-negative rod, it could be missed on pathologic examination although it is the probable causative organism of necrosis in this case. It may be helpful to perform AFB and bacterial culture of biopsy specimen to differentiate the cause of infection. Because B. hinzii is a rare cause of human disease, unknown exposure to poultry may have occurred in this case, as this is recognized to confer a predisposition to respiratory colonization in humans [3]. Mouse pneumonia model infected by B. hinzii showed interstitial or alveolar pattern of inflammation [13]. Chronic inflammation caused by B. hinzii has not been reported except endocarditis [10]. Therefore, the correlation between mass lesion of lung and B. hinzii require further investigation.
Two distinct colony types of B. hinzii were observed in this case, each of which resulted in the identification of a different species by a biochemical test. Current commercial biochemical identification systems are unable to identify B. hinzii [14]. B. hinzii [1], B. avium [15], and B. bronchiseptica [16] are reported to have both smooth and rough colony types, which could cause a problem in species identification, like in this case. Colony morphology is a phenotypic feature known to be related to virulence in B. bronchiseptica [16] and other species [17]. However, no reports on the virulence of B. hinzii with regard to colony morphology have been published. Biochemical identification was different between the smooth and rough colony types. MALDI-TOF and 16S rRNA gene sequencing successfully identified both colony types as B. hinzii. Colony morphology does not appear to limit the identification performance of MALDITOF and 16s rRNA gene sequencing, which is described as being superior for the species identification of B. hinzii in previous reports [5,18]. Therefore, MALDI-TOF and 16S rRNA gene sequencing are the most reliable methods to identify B. hinzii, regardless of colony morphology.
In this case, the isolate was resistant to ampicillin and cephalosporines, but was not tested for β-lactams/ β-lactamase inhibitors. It was eradicated with 3 days of treatment with ampicillin/sulbactam and piperacillin/ tazobactam plus meropenem. B. hinzii shows variable susceptibility to β-lactams and fluoroquinolones [3,5,6,8,14]. Piperacillin/tazobactam and carbapenems except ertapenem are preferred in B. hinzii infection rather than other β-lactams [3]. Because serious B. hinzii infections are rare, there is a lack of data on antimicrobial susceptibility and standard antimicrobial therapy. The patient in this case received antimicrobial therapy with β-lactams/β-lactamase inhibitors and meropenem for 16 days. A case of septicemia was caused by multidrug-resistant B. hinzii from a lymphoma patient treated with various antimicrobials for several weeks [14]. The initial isolate of B. hinzii was resistant to β-lactams, trimethoprim/sulfamethoxazole and ciprofloxacin, and acquired resistance to meropenem after a 1-week course of it. Therefore, it is noted that B. hinzii can persist in the respiratory tract and acquire antimicrobial resistance with effective β-lactam treatment for 1 week. Antimicrobial susceptibility testing of successive isolates of B. hinzii is required when infection persists with appropriate antimicrobial treatment.
This is the first bacteremia case of B. hinzii in Korea. It was probably associated with bronchoscopic biopsy of lung mass. Colonization of the upper respiratory tract and the contribution of B. hinzii to subacute necrosis of the lung require further investigation.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download