Abstract
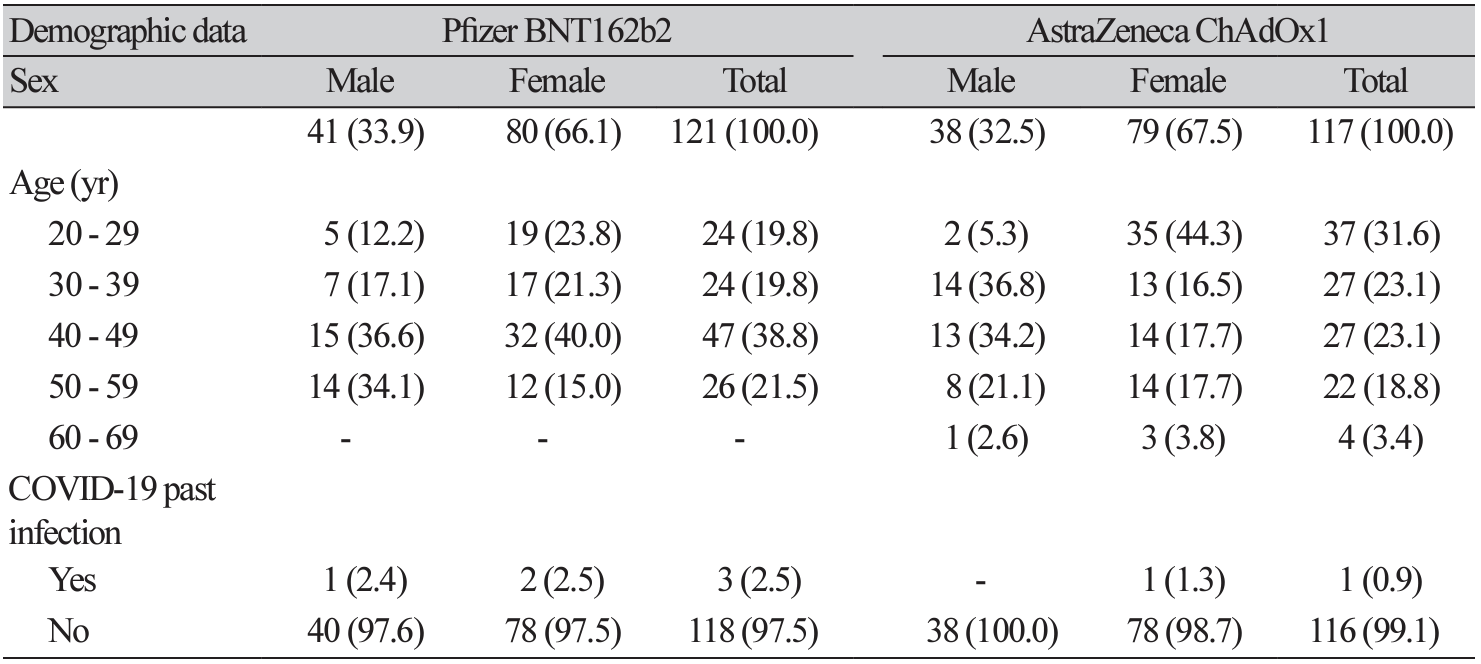
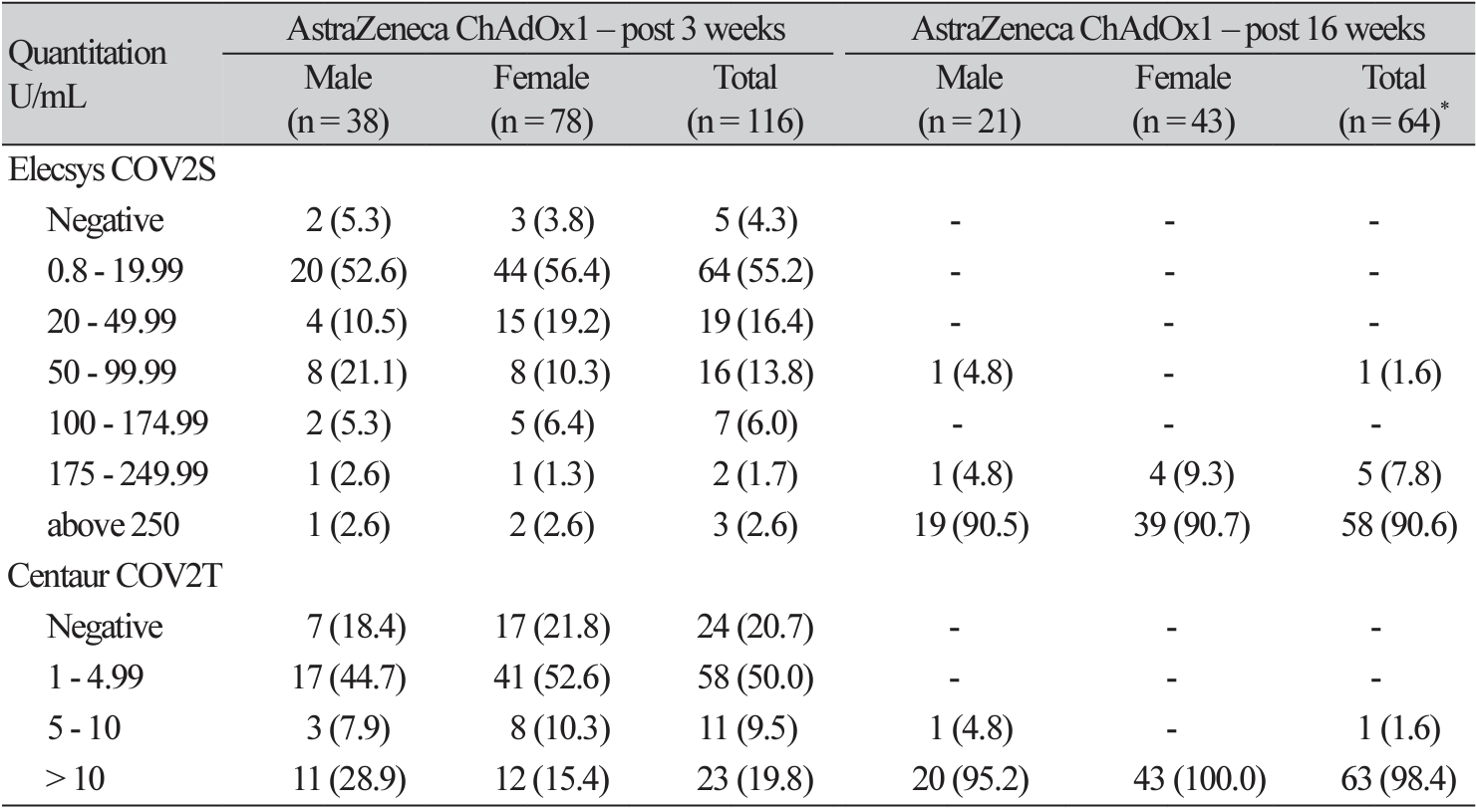
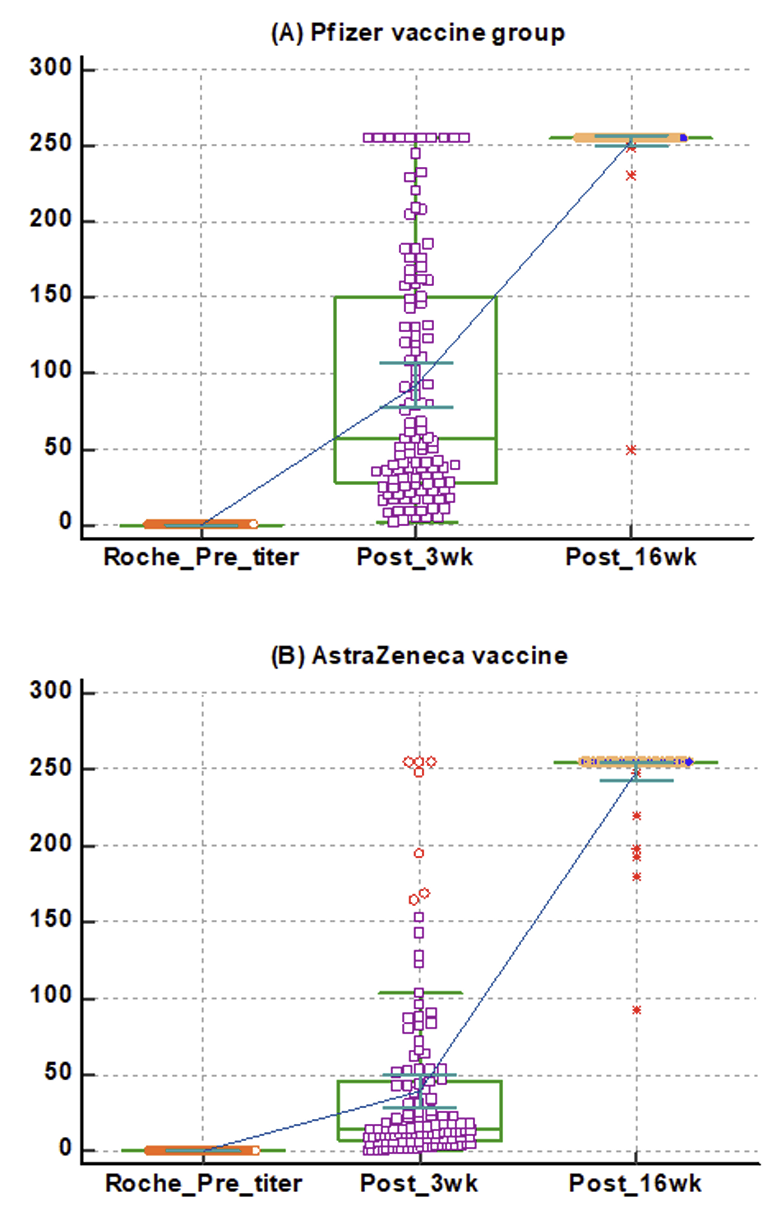
Background: Due to the COVID-19 pandemic, from 2020, many pharmaceutical companies have developed vaccines. To determine the efficacy of AstraZeneca's and Pfizer's vaccines, which were the first and second vaccines to be approved in Korea, respectively, we developed a method to measure their antibody-generating efficacies using immunology analyzers and a rapid antibody test available in Korea. Methods: The antibody-stimulating efficacies of the Pfizer and AstraZeneca vaccines were evaluated using Centaur® XPT SARS-CoV-2 (Siemens Healthineers, Germany), Elecsys® AntiSARS-CoV-2 S (Roche Diagnostics, Germany), and STANDARD F SARS-CoV-2 nAb FIA (SD Biosensor, Korea). Healthcare workers were enrolled in two groups: the Pfizer (121) and AstraZeneca (117) groups. Antibody levels were measured pre-vaccination, three weeks after vaccination, and 16 weeks after vaccination. Results: The Pfizer group comprised 41 males and 80 females, while the AstraZeneca group comprised 38 males and 79 females. Antibody results were analyzed after excluding four individuals who had recovered from COVID-19. Between weeks 3 and 16, there was no significant difference (P= 0.5, 1.0) between the results of the Roche and Siemens antibody tests in the Pfizer vaccine group. However, the SD biosensor results comparing with the Roche and Siemens antibody tests at three weeks after the initial vaccination showed a significant difference (P< 0.0001). Analysis of the Roche antibody test results before, at three weeks, and at 16 weeks after the administration of the Pfizer and AstraZeneca vaccines revealed a statistically significant difference between before and at three weeks after the first injection (P< 0.0001). Conclusion: After two doses of the Pfizer and AstraZeneca vaccines, antibody formation was above the 90th percentile of the measurement range in all subjects.
Go to : 
[in Korean]
Ethics statement
This study was approved by the institutional review board of National Health Insurance Service Ilsan Hospital (IRB No. NHIMC 2021-02-015) and obtained informed consent from participants.
REFERENCES
1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470-3.
.
2. Mahase E. Covid-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ 2020;371:m4552.
.
3. Knoll MD and Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021;397:72-4.
.
4. He X, He C, Hong W, Zhang K, Wei X. The challenges of COVID-19 Delta variant: prevention and vaccine development. MedComm 2021;2:846-54.
.
5. Kang SJ, Kim S, Park KH, Jung SI, Shin MH, Kweon SS, et al. Successful control of COVID-19 outbreak through tracing, testing, and isolation: lessons learned from the outbreak control efforts made in a metropolitan city of South Korea. J Infect Public Health 2021;14:1151-4.
.
6. Sung H, Roh KH, Hong KH, Seong MW, Ryoo N, Kim HS, et al. COVID-19 molecular testing in Korea: practical essentials and answers from experts based on experiences of emergency use authorization assays. Ann Lab Med 2020;40:439-47.
.
7. FDA. COVID-19 Vaccines. https://www.fda.gov/emergency-preparedness-and-response/ coronavirus-disease-2019-covid-19/covid-19-vaccines [Online] (last visited on 15 September 2022).
.
8. MFDS. COVID-19 Vaccines. https://www.mfds.go.kr/vaccine_covid19.jsp# [Online] (last visited on 15 September 2022).
.
9. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, doubleblind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21:181-92.
.
10. Tang MS, Case JB, Franks CE, Chen RE, Anderson NW, Henderson JP, et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin Chem 2020;66:1538-47.
.
11. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-15.
.
12. Emanuel EJ, Persad G, Kern A, Buchanan A, Fabre C, Halliday D, et al. An ethical framework for global vaccine allocation. Science 2020;369:1309-12.
.
13. Grauer J, Lowen H, Liebchen B. Strategic spatiotemporal vaccine distribution increases the survival rate in an infectious disease like Covid-19. Sci Rep 2020;10:21594.
.
14. Padoan A, Bonfante F, Pagliari M, Bortolami A, Negrini D, Zuin S, et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020;62:103101.
.
15. The National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of f ive immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390-400.
.
16. Dimeglio C, Herin F, Martin-Blondel G, Miedouge M, Izopet J. Antibody titers and protection against a SARS-CoV-2 infection. J Infect 2022;84:248-88.
.
Go to : 
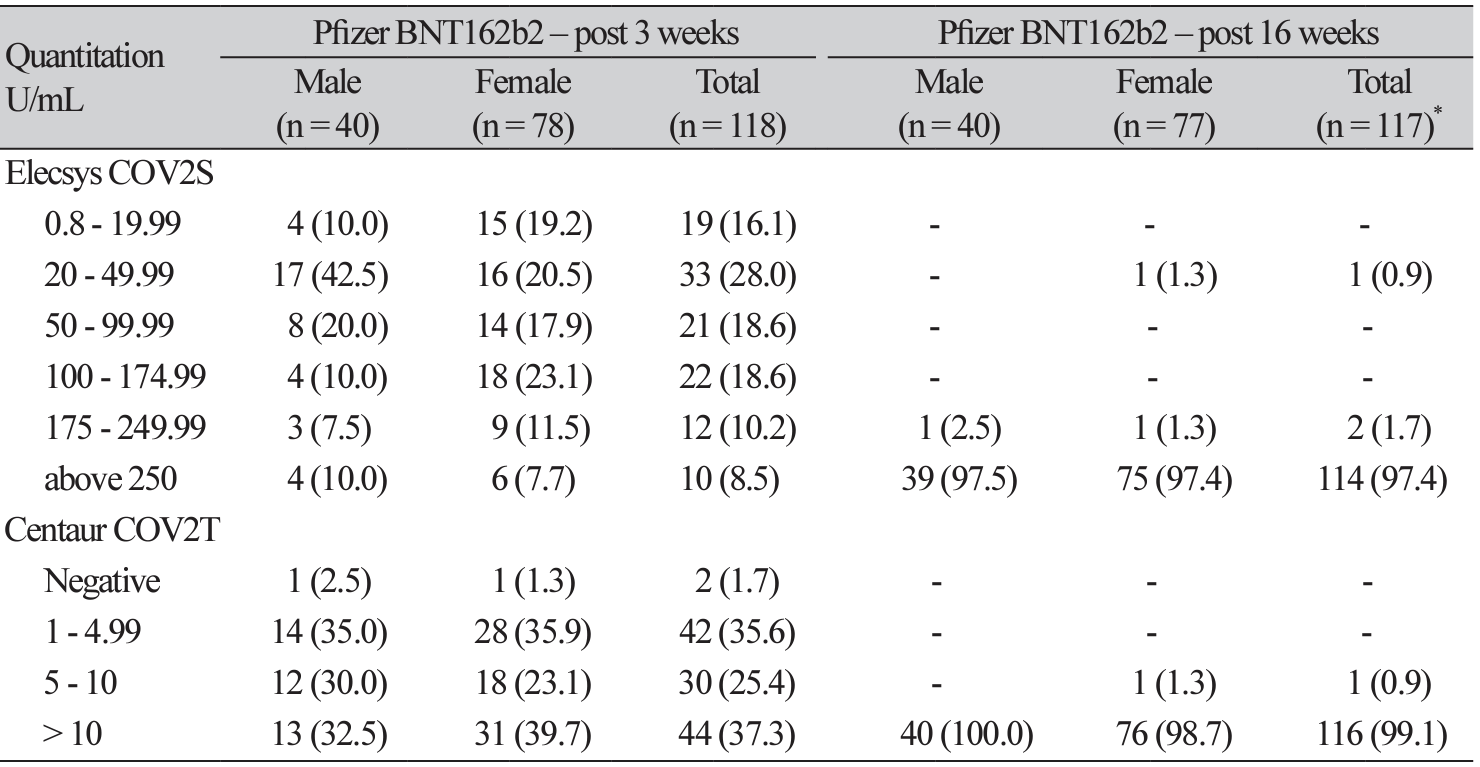
Table 2
Pfizer BNT162b2 seropositive rate and quantitative level of immunoglobin according to Elecsys COV2S and Centaur COV2T




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download