This article has been
cited by other articles in ScienceCentral.
Abstract
Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoint tables are commonly used as guidelines for the interpretation of antimicrobial susceptibility testing results. These are updated annually to reflect new and revised antimicrobial susceptibility breakpoints. EUCAST v.12.0, which was published in January 2022, presents updated meropenem-vaborbactam breakpoints for Enterobacterales and Pseudomonas aeruginosa. It also suggests new breakpoints of susceptibility to various antibiotics for Vibrio spp. Flow charts were updated for Streptococcus pneumoniae and Haemophilus influenzae, and the breakpoints for anaerobic bacteria were divided according to each species. Furthermore, recommendations were made for cases without antimicrobial susceptibility testing breakpoints and links to several rationale and guidance documents were provided for technical convenience.
Keywords: Antimicrobial susceptibility testing, Breakpoint, EUCAST v.12.0
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
REFERENCES
1. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. https://www.eucast.org/clinical_ breakpoints/ [Online] (last visited on 31 July 2022).
.
Table 1
New meropenem-vaborbactam breakpoints in Enterobacterales, P.aeruginosa
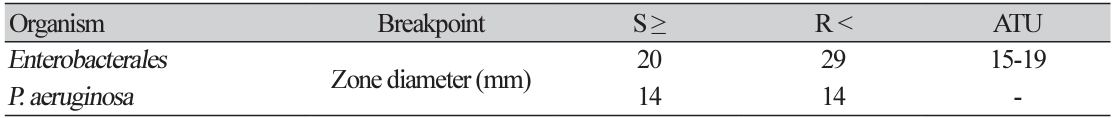
Table 2
New EUCAST v.12.0 clinical breakpoint organism-antimicrobial agent for anaerobic bacteria
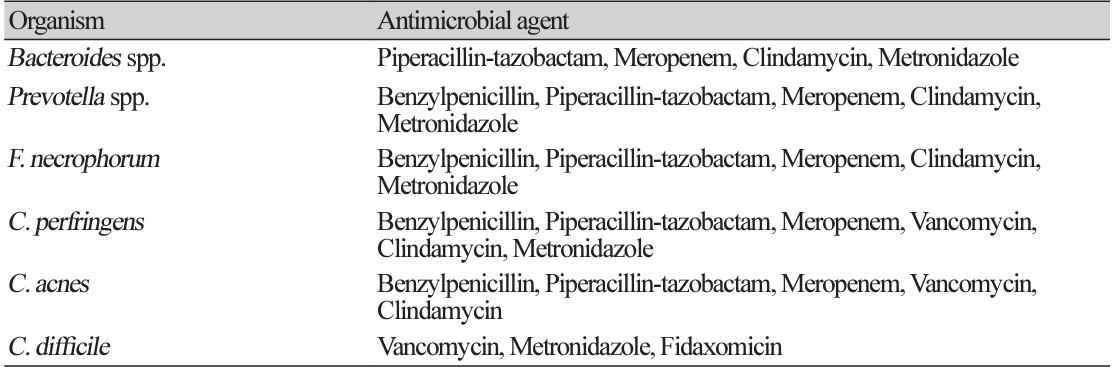
Table 3
Revised EUCAST v.12.0 clinical breakpoints
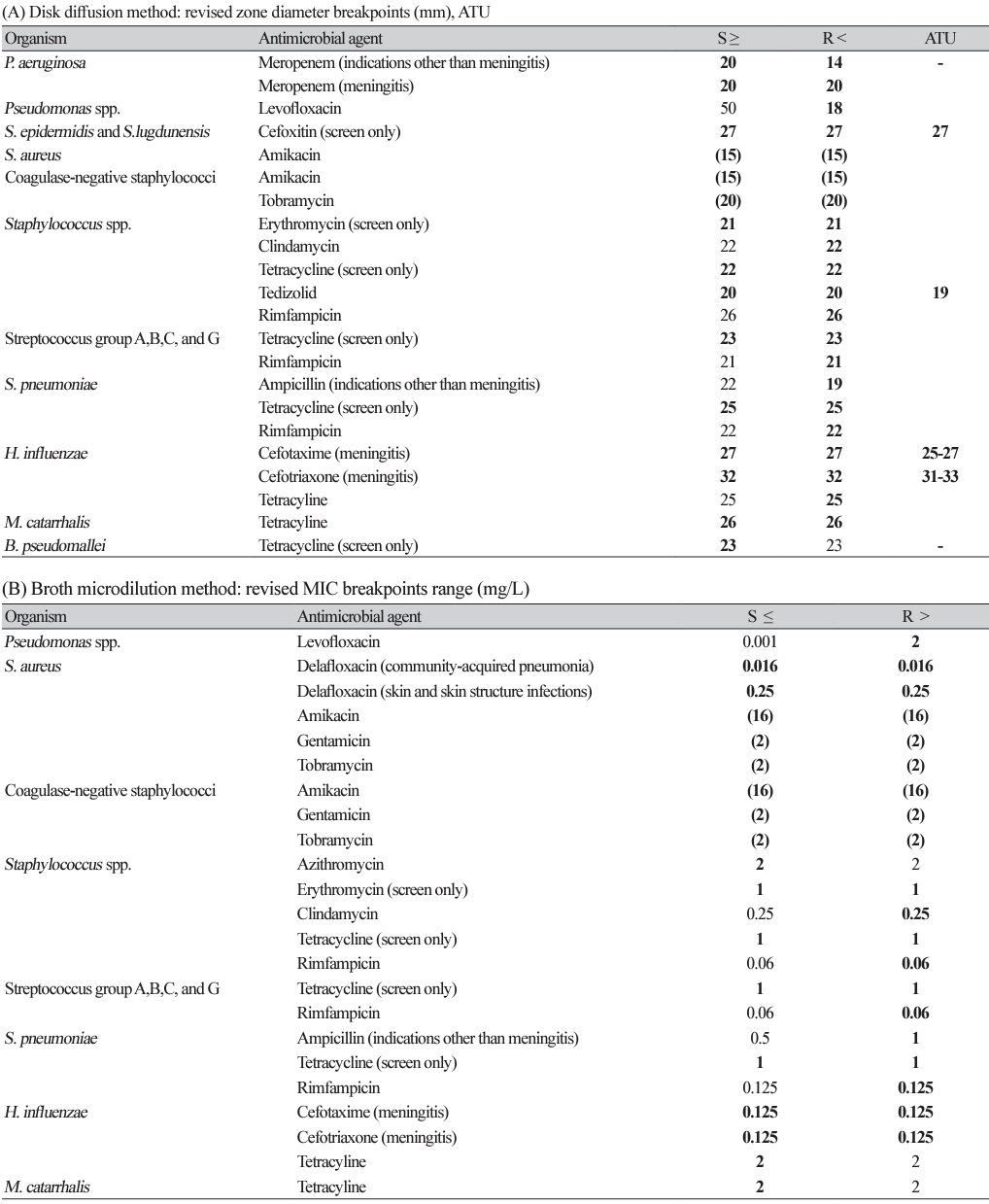
Fig. 1.
Flow chart based on the oxacillin screen test for S.pneumoniae. MIC, minimal inhibitory concentration (This figure has been produced in part under ECDC service contracts and made available by EUCAST at no cost to the user and can be accessed on the EUCAST website www. eucast.org.)
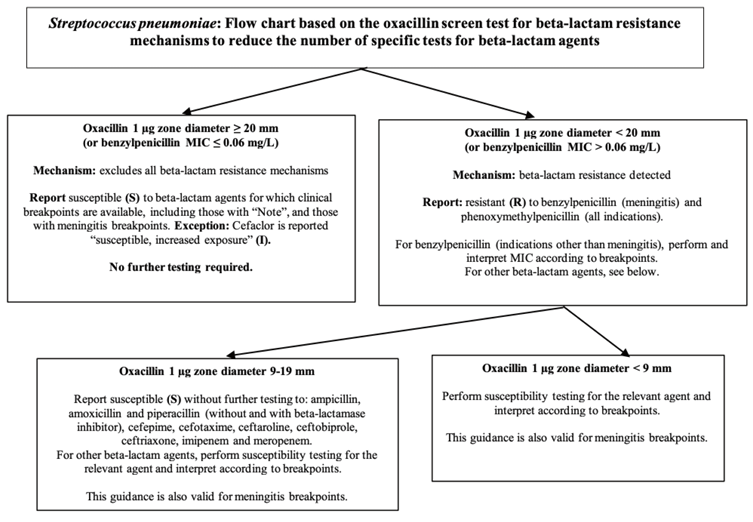
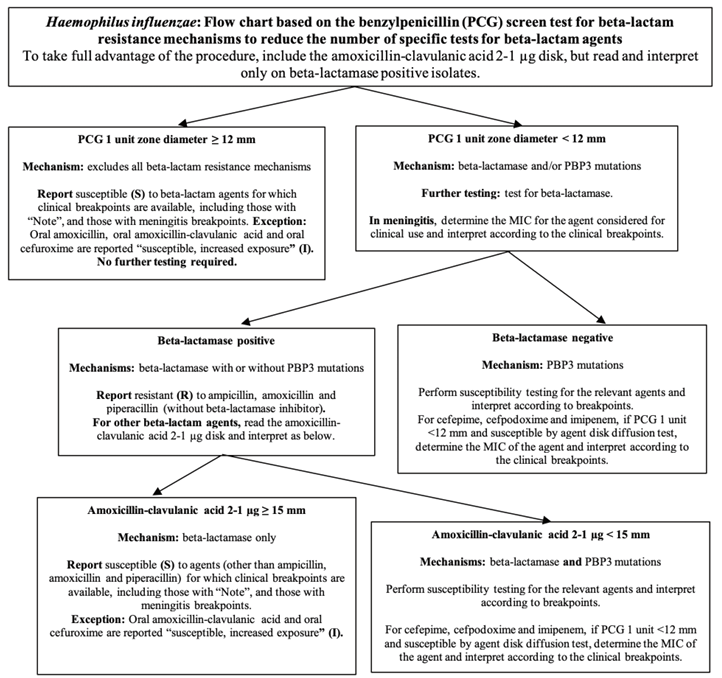
Fig. 2.
Flow chart based on the benzylpenicillin screen test for H. influenzae (This figure has been produced in part under ECDC service contracts and made available by EUCAST at no cost to the user and can be accessed on the EUCAST website www.eucast.org.)




 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download