Abstract
Background: The aim of this study was to analyze the inhibitory effects of copper, brass (78% copper, 22% tin), and stainless steel surfaces on multidrug-resistant Acinetobacter baumannii (MRAB), extended-spectrum beta-lactamase (ESBL) Escherichia coli, and carbapenem-resistant Klebsiella pneumoniae (CRKP). Methods: MRAB, ESBL E. coli, and CRKP were isolated at Uijeongbu St. Mary's Hospital in 2020. A. baumannii ATCC BAA-747, E. coli ATCC 25922, and K. pneumoniae ATCC 700603 were used as reference strains. The initial bacterial cell count of each inoculum was adjusted to 8 log CFU/mL using phosphate buffered saline, Copper, brass, and stainless steel plates were inoculated with 9 mL of MRAB, ESBL E. coli, and CRKP inoculum solutions. The bacterial cell count was measured from the beginning to the 20th day in an incubator maintained at 35°C. Results: MRAB, ESBL E. coli, and CRKP isolates were not detected on the copper and brass plates after 4, 5.5, and 6.5 hours, respectively. MRAB, ESBL E. coli, and CRKP isolates were not detected on the stainless steel plate after 15, 20, and 20 days, respectively. The bactericidal effects of copper and brass were much stronger than those of stainless steel. Conclusion: The use of copper and copper alloys should be considered to prevent crossinfection in hospitals.
Antibiotics are trace chemicals that kill or inhibit proliferation of other microorganisms. Since penicillin was first used in the 1940s, more than 5,000 antibiotics have been developed to date [1]. After the use of penicillin and methicillin, penicillin and methicillin-resistant Staphylococcus aureus (MRSA) appeared [2]. With the widespread use of antibiotics, vancomycin-resistant Enterococcus (VRE), carbapenemresistant Escherichia coli, multidrug-resistant Pseudomonas aeruginosa, multidrug-resistant Acinetobacter baumannii (MRAB), and carbapenem-resistant Klebsiella pneumoniae (CRKP) emerged. In particular, these antibiotic-resistant strains have been detected in inpatients and in hospital environments, emerging as a global problem [3]. A. baumannii is a gram-negative, multidrug-resistant pathogen that causes various infections and hospital infections in intensive care units. Common risk factors for the emergence of MRAB are hospitalization period and antibiotic use [4]. The World Health Organization (WHO) has identified A. baumannii as one of the most serious microorganisms that effectively avoid antibiotic effects [5]. Infection by A. baumannii accounts for about 2% of all medical-related infections in the United States and Europe, and about 4% in Asia and the Middle East [6,7]. Extended spectrum beta-lactamases (ESBL) can be defined as a group of enzymes that can provide resistance to penicillin and 1st, 2nd, and 3rd generation cephalosporin and aztreonam without cephamycin and carbapenem. Most ESBL are found in E. coli [8,9]. Of the 1,038 isolated E. coli strains, 44% (457 strains) were resistant to 3rd generation cephalosporin, of which 61.1% (276 strains) were ESBL E. coli. The prevalence of ESBL E. coli has ncreased worldwide and is a major cause of treatment failure in the intensive care unit (ICU) [10]. Carbapenem antibiotics are used widely to treat infections of ESBL-resistant intestinal bacteria. However, CRKP emerged due to misuse of carbapenem antibiotics [11]. CRKP represents a challenge to clinical care due to increased detection rate in the hospital environment [12]. In Korea, newborns were infected with Citrobacter freundii, which shows multidrug resistance to antibiotics, causing major social problems [13]. Therefore, special efforts are needed to prevent infection with antibiotic-resistant strains in hospitals.
Copper was used for drinking water sterilization and patient treatment in ancient Egypt and was found to contribute to food poisoning prevention when used as a material for 19th century tableware [14,15]. However, stainless steel became more widely used and the use of copper has decreased [16]. A study on copper surface sterilization of hospital microorganisms [17,18] and studies using copper as a surface material for equipment in food factories have been conducted [19]. In addition, a study on the use of copper surfaces was conducted to prevent cross-infection caused by facilities and equipment in the hospital [20,21]. Copper has a powerful ability to kill multiple viruses on contact. Copper can kill several infectious viruses, such as bronchitis and single or double-stranded DNA and RNA viruses [22]. Sterilization of food poisoning bacteria on brass (78% copper, 22% tin) and copper surfaces has been reported [23]. The inhibition effect of brass, copper and stainless-steel surfaces against MRSA and VRE also has been reported [13]. However, there is no research on the inhibition effects of brass, copper, and stainless-steel surfaces against MRAB, ESBL E. coli, and CRKP.
Therefore, in this study, the inhibition effects of brass, copper, and stainless steel for MRAB, ESBL E. coli, and CRKP isolated from domestic hospitals were compared to determine the utility of brass and copper surface in preventing cross-infection with antibiotic-resistant strains caused by domestic hospital environments.
Copper, brass, and stainless-steel plates (10×70 mm) were used to estimate the antibacterial effects of metal surfaces. Copper plates (Poongsan corporation, Seoul, Korea) with a copper content of 99% or more were used in this study. Brass plates (78% copper, 22% tin) were purchased from Anseong Bangja (Ansung, Korea), and stainless-steel plates (SUS 304; Fe 74%; Cr 18%, Ni 8%) were purchased from JD Distribution (Daejeon, Korea).
The three strains used in this study, MRAB, ESBL E. coli, and CRKP were isolated and identified from wild strains at Uijeongbu St. Mary’s Hospital in 2020. Acinetobacter baumannii ATCC BAA-747, E. coli ATCC 25922, and K. pneumoniae ATCC 700603 were used as reference strains for studying the inhibition effects of the metal surfaces.
Antibiotic resistance tests of A. baumannii, E. coli, and K. pneumoniae isolates used in this study were conducted according to the Clinical and Laboratory Standard Institute Guideline [24]. Antibiotic resistance tests were performed using the Vitek 2 system (bioMérieux, Hazelwood, MO, USA). Acinetobacter baumannii was tested using the AST–N225 kit, and E. coli and K. pneumoniae were tested using the AST–N224 kit (bioMérieux). Acinetobacter baumannii ATCC BAA-747, E. coli ATCC 29212, and K. pneumoniae`1 ATCC 700603 were used as reference strains for antibiotic resistance tests.
MRAB, ESBL E. coli, and CRKP isolates were inoculated into tryptic soy broth (Oxoid Ltd., Hampshire, England) and cultured at 35°C for 24 hours. After incubation, 1 mL of each culture solution was taken in a sterile tube and centrifuged at 3,000 rpm (Smart R17, Hanil scientific, Gimpo, Korea). After removing the supernatant of the centrifuged culture medium, 1 mL of sterile phosphate-buffered saline (PBS; Oxoid) was added and homogenized, followed by centrifugation at 3,000 rpm. After removing the supernatant, the bacterial cell count in each initial inoculum was adjusted to 8 log CFU/mL with PBS. Copper, brass, and stainless-steel plates were cleaned and disinfected by spraying with 70% ethanol after drying [23].
Each metal plate was placed in a test tube and inoculated with 9 mL of the prepared MRAB, ESBL E. coli, and CRKP inoculum solution, and then the bacterial cell count was measured from the beginning to 0.5 hour, 1 hour, 1.5 hour, 2 hour, 2.5 hour, 3 hour, 3.5 hour, 4 hour, 4.5 hour, 5 hour, 5.5 hour, 6 hour, 6.5 hour, and 7 hour. Also, the bacterial cell count was measured every 24 hours until the 20th day in an incubator at 35°C. To measure the bacterial cell count, 10 µL of the inoculum was spread on a blood agar plate and then incubated at 35°C for 24 hours, and the colonies that formed were counted to measure the bacterial cell counts. All tests were repeated three times and the mean value was calculated.
The antibiotic resistance profiles of A. baumannii, E. coli, and K. pneumoniae used in this study are shown in Table 1. As a result of the antibiotic resistance test, A. baumannii, E. coli, and K. pneumoniae were all found to be multidrug-resistant isolates. A. baumannii, E. coli, and K. pneumoniae used in this study were confirmed as MRAB, ESBL E. coli, and CRKP. The antibiotic resistance of A. baumannii isolates included aztreonam (AZT), cefotaxime (CFT), gentamicin, meropenem, piperacillin, ticarcillin/clavulanic acid, ceftazidime (CAZ), ciprofloxacin, cefepime (CPE), imipenem (IMP), ampicillin/sulbactam, and piperacillin/ tazobactam (TZP). The antibiotic resistance of E. coli isolates included ampicillin (AM), CAZ, CPE, AZT, CFT, cefazolin (CZ), AZT, and cephalosporin. The antibiotic resistance of K. pneumoniae isolates included AM, CAZ, CPE, TZP, amoxicillin/clavulanic acid, AZT, CFT, CZ, ertapenem, cefoxitin, IMP, and carbapenem.
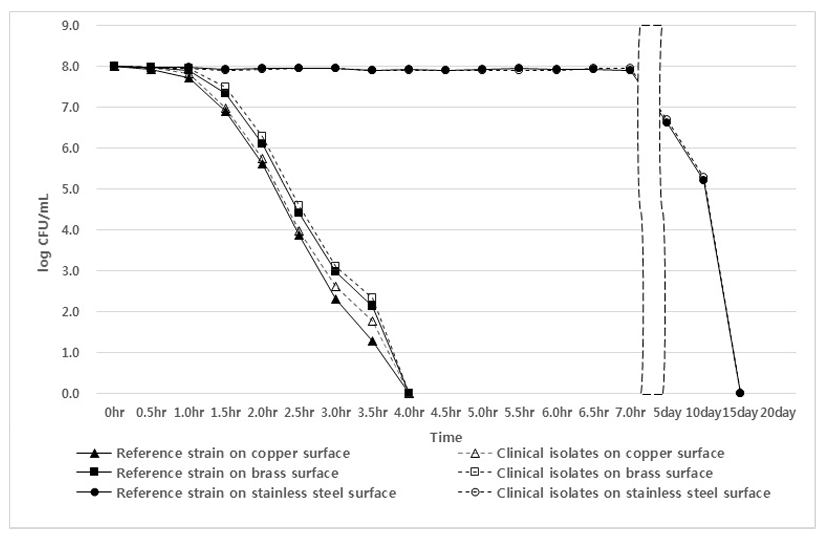
The inhibitory effects of copper, brass, and stainless-steel plates against MRAB, ESBL E. coli, and CRKP isolates and reference strains are shown in Figs. 1, 2, and 3. Testing of a copper plate showed a sterilization effect on MRAB isolates and the A. baumannii reference strain after 1 hour and 30 minutes, and no A. baumannii were detected after 4 hours. In the case of the brass plate, the sterilization effect appeared after 2 hours, and all isolates and the reference strain were not detected after 4 hours. However, in the case of the stainless-steel plate, the sterilization effect appeared from the 5th day, and MRAB isolates and A. baumannii reference strain were not detected on the 15th day (Fig. 1).
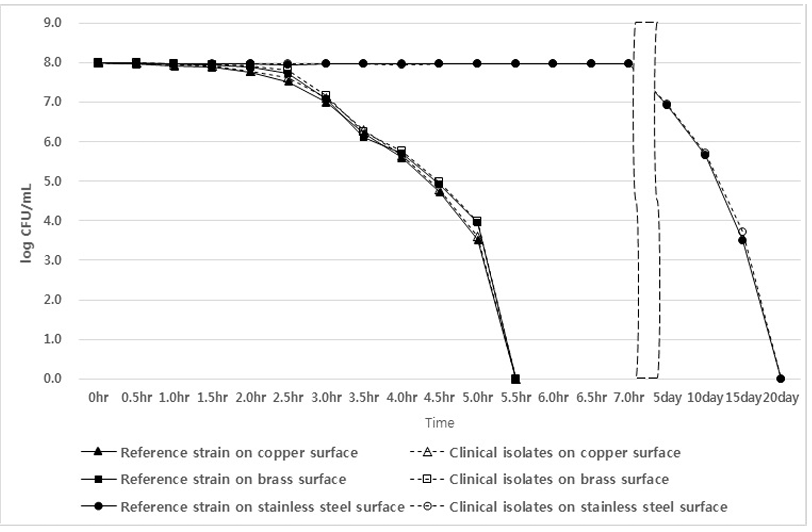
As a result of testing ESBL E. coli isolates and the E. coli reference strain, the copper plate showed a sterilization effect after 2 hours, and no E. coli were detected after 5.5 hours. In the case of the brass plate, the sterilization effect appeared after 2.5 hours, and all isolates and the reference strain were not detected after 5.5 hours. However, in the case of the stainless-steel plate, the sterilization effect appeared from the 5th day, and ESBL E. coli isolates and the E. coli reference strain were not detected on the 20th day (Fig. 2).
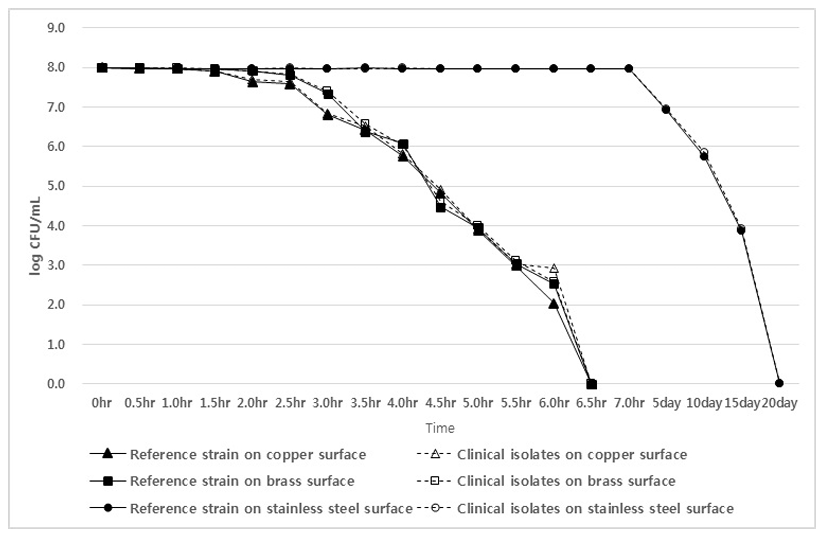
As a result of testing CRKP isolates and the K. pneumoniae reference strain, the copper plate showed a sterilization effect after 2 hours, and no K. pneumoniae were detected after 6.5 hours. In the case of the brass plate, the sterilization effect appeared after 2.5 hours, and all isolates and the reference strain were not detected after 6.5 hours. However, in the case of the stainless-steel plate, the sterilization effect appeared from the 5th day, and CRKP isolates and the K. pneumoniae reference strain were not detected on the 20th day (Fig. 3).
The hands of patients and medical personnel are reported as the main sources of cross-contamination in hospitals [25]; doorknobs, faucets, and bed rails are also reported as major sources of contamination [15]. In order to prevent cross-contamination in hospitals, the inhibition effects of copper, brass and stainless steel were compared and tested. A report describing testing of the sterilization power of copper on VRE found that it was not detected at 1 hour [14]. As a result of this study, MRAB, ESBL E. coli, and CRKP were not detected after 4 hours, 5.5 hours, and 6.5 hours, respectively. The previous report showed a stronger sterilization effect compared to the results of this study, and it is judged that this difference is due to the difference between the report of direct inoculation of VRE on the copper surface and the method of immersing the copper plate in the inoculation solution.
Brass is a Korean-specific tableware made of an alloy of 78% copper and 22% tin [23], and was used in this study to test the sterilization effect of the copper alloy. It was previously reported that MRSA was not detected in an experiment where a bathroom faucet was replaced with brass (Cu 60%-70%) [21]. In this study, MRAB, ESBL E. coli, and CRKP were not detected after 4 hours, 5.5 hours, and 6.5 hours, respectively. These results confirmed the microbial sterilization effect of brass. It was judged that copper alloy could show sterilization power similar to copper when used for door handles, faucets, and bed rails in hospitals.
The mechanism of bactericidal effect of copper is that copper damages the microorganism’s envelop and nucleic acids. It has been suggested that subsequent to the specific binding of copper to DNA, repeated cyclic redox reactions generate several hydroxyl radicals near the binding site causing multiple damage to the nucleic acids [17].
It was previously reported that it took 2 hours for MRSA and VRE to be reduced on a stainless-steel surface [14], and that the S. aureus survived for 4 days on the stainless-steel surface [16]. As a result of this study, MRAB, ESBL E. coli, and CRKP were not detected on the stainless-steel surface after the 15th day, 20th day, and 20th day, respectively. It was judged that the microbial sterilization effect of stainless steel was very weak compared to those of copper and brass. Also, the application of brass will make up for copper’s weakness because copper becomes rusty. Peracetic acid nd hydrogen peroxide may damage the metal surface including brass and copper. But glutaraldehyde and ortho-phthaladehyde may not damage the metal surface including brass and copper.
We demonstrated that there are no differences in resistance to metal surface between multidrug-resistant strain and reference strain, though there are the possibility of lower resistance to metal surface in multidrug- resistant strain than wild type strains because of disappearance of survival ability with acquisition of resistance.
Newborns were infected with multidrug-resistant C. freundii, causing a major public health concern in Korea [13]. Concerted efforts are needed to prevent infection with antibiotic-resistant strains in hospitals. WHO reported A. baumannii as one of the most serious microorganisms [5]. ESBL E. coli is a major cause of treatment failure in the ICU [10]. The detection rate of CRKP has increased in the hospital environment [12]. Therefore, this study confirmed the sterilization effect of copper and brass on multidrug-resistant strains such as MRAB, ESBL E. coli, and MRKP.
Ethics statement
It is not a human population study; therefore, approval by the institutional review board or the obtainment of informed consent is not required.
REFERENCES
1. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol 2006;47:337-49.
.
2. Jevons MP. "Celbenin"-resistant Staphylococci. Br Med J 1961;5219:124-5.
.
3. Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 2011;77:1541-7.
.
4. Vázquez-López R, Solano-Gálvez SG, Vignon-Whaley JJJ, Vaamonde JA, Padró Alonzo LA, Resndiz AR, et al. Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics 2020;9:205.
.
5. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009;48:1–12.
.
6. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 2014;370:1198208.
.
7. Lob SH, Hoban DJ, Sahm DF, Badal RE. Regional dierences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents 2016;47:317–23.
.
8. Mehrgan H, Rahbar M, Arab-Halvaii Z. High prevalence of extended-spectrum betalactamase-producing Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. J Infect Dev Ctries 2010;4:132-8.
.
9. Kassakian SZ and Mermel LA. Changing epidemiology of infections due to extended spectrum beta-lactamase producing bacteria. Antimicrob Resist Infect Control 2014;3:9.
.
10. Singh N, Pattnaik D, Neogi DK, Jena J, Mallick B. Prevalence of ESBL in Escherichia coli isolates among ICU patients in a tertiary care hospital. J Clin Diag Res 2016;10:DC19-22.
.
11. Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 2016;7:895.
.
12. Reyes J, Aguilar AC, Caicedo A. Carbapenem-resistant Klebsiella pneumoniae: microbiology key points for clinical practice. Int J Gen Med 2019;12:437-46.
.
13. Bae JY, Kang CK, Choi SJ, Lee E, Choe PG, Park WB, et al. Sudden deaths of neonates receiving intravenous infusion of lipid emulsion contaminated with Citrobacter freundii. J Korean Med Sci 2018;33 e97.
.
14. Simon WJG, Mark DF, Alison FK, Marina M, Jackie K, Declan PN. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann Microbiol 2009;59:151-6.
.
15. Kuhn PJ. Doorknobs: a source of nosocomial infection? Diagnostic medicine. https://www. antimicrobialcopper.org/sites/default/files/upload/media-library/files/pdfs/ uk/scientific_ literature/kuhn-doorknob.pdf. [Online] (last visited on 28 september 2021).
.
16. Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol 2003;85:227–36.
.
17. Agarwala M, Choudhury B, Yadav RNS. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J Microbiol 2014;54:365-8.
.
18. Carson KC, Bartlett JG, Tan TJ, Riley TV. In vitro susceptibility of methicillin-resistant Staphylococcus aureus and methicillin-susceptibility Staphylococcus aureus to a new antimicrobial copper silicate. Antimicrob Agents Chemother 2007;51:4505-7.
.
19. Lee EJ, Park JH. Inactivation activity of bronze alloy Yugi for reduction of cross-contamination of food-borne pathogen in food processing. J Fd Hyg Safety 2008;23:309-13.
.
20. Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol 2010;87:1875–9.
.
21. Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, et al. Role of copper in reducing hospital environment contamination. J Hosp Infect 2010;74:72–7.
.
22. Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses 2020;142:109814.
.
23. Jung MK, Lee MY, Park JH. Inhibitory effect of cupric ion diffused brass ware on the growth of E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus, and Bacillus cereus. Food Sci Biotechnol 2004;13:680-3.
.
24. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; CLSI M100-S26. Wayne; PA: 2017.
.
25. Weinstein RA. Epidemiology and control of nosocomial infections in adult intensive care units. Am J Med 1991;91:179-84S.
.
Table 1
Antibiotic resistance profiles of Acinetobacter baumanii, ESBL E.coli and Klebsiella pneumonia
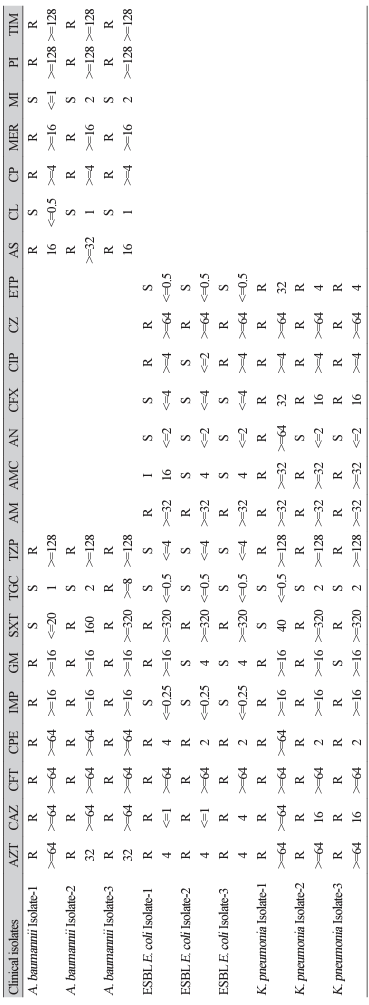
Abbreviations: ESBL, extended spectrum β-lactamases; AZT, aztreonam; CAZ, ceftazidime; CFT, cefotaxime; CPE, cefepime; IMP, imipenem; GM, gentamicin; SXT, trimethoprim/ sulfamethoxazole; TGC, tigycycline; TZP, piperacillin/tazobactam; AM, ampicillin; AMC, amoxicillin/clavulanic acid; AN, amikacin; CFX, cefoxitin; CIP, ciprofloxacin; CZ, cefazolin; ETP, ertapenem; AS, ampicillin/sulbactam; CL, colistin; CP, ciprofloxacin; MER, meropenem; MI, minocycline; PI, piperacillin; TIM, ticarcillin/clavulanic acid; R, resistant; S, sensitive, I, intermediate.
Fig. 1.
Inhibition effects of brass, copper, and stainless-steel surfaces on isolates and reference Acinetobacter baumannii in phosphatebuffered saline.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download