Abstract
Neutralizing antibodies play a critical role in blocking viral infections and in viral clearance during acute infection. The microneutralization assay and enzyme-linked immunosorbent assay (ELISA) targeting the receptor binding domain were performed for 30 patients with mild coronavirus disease (COVID)-19 infections. The elapsed number of days between sample collection and diagnosis was 115 days, and real-time polymerase chain reaction (PCR) cycle threshold (Ct) values at diagnosis were recorded. Clinical characteristics and Ct values were compared between neutralization antibody-positive and -negative patients as measured by the microneutralization assay. Neutralization antibody-positive patients (n = 9) were likely to be older, have low Ct values, have more pneumonia during admission, and have a higher optical density in ELISA than the neutralization antibody-negative patients (n = 21). Elderly people seemed to have a higher viral load causing more pneumonia and to produce more neutralization antibodies. Neutralization antibodies persisted in only 30% of patients as detected by microneutralization test after 100 days of diagnosis.
The coronavirus disease (COVID)-19 antibody test is essential for understanding host response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Additionally, the COVID-19 antibody could be used for measuring the true infection size of the population [1], and for identifying candidates for plasma therapy [2,3]. Neutralizing antibody plays a pivotal role in blocking viral infections as well as viral clearance during acute infection [4-7]. There are limited reports on the serological response, especially for neutralizing antibodies for the recovered patients more than 100 days in Korea.
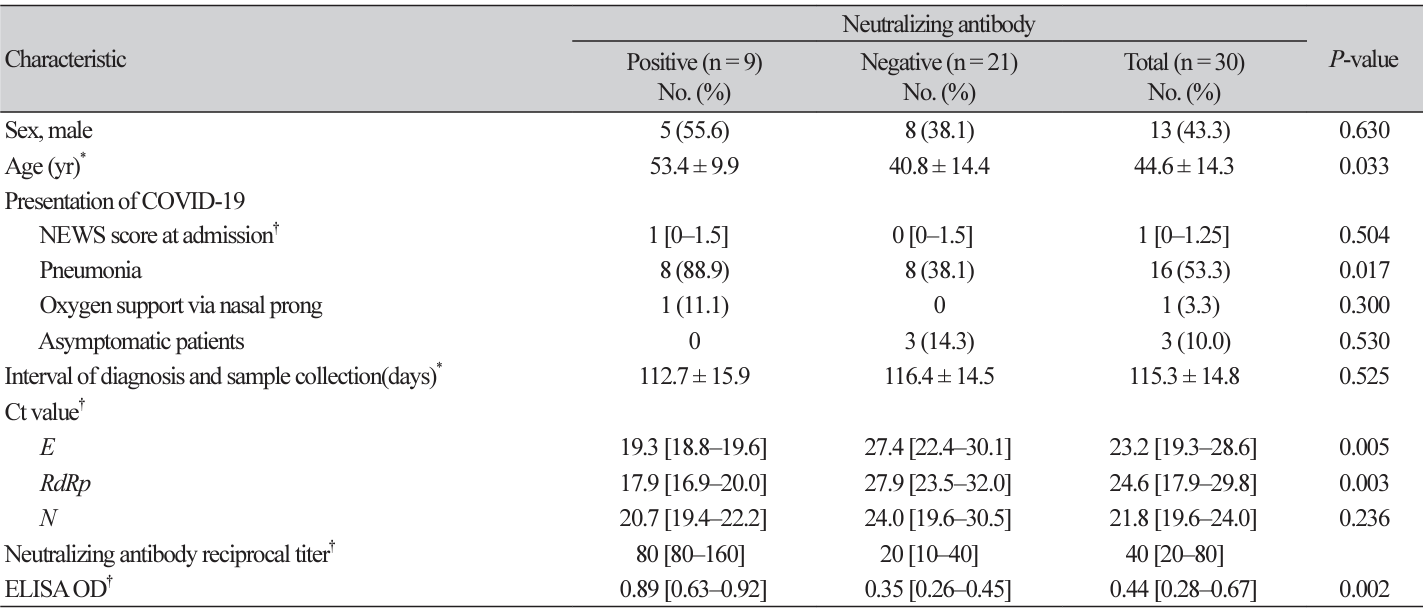
Thirty patients admitted at Masan Medical Center in Gyeongnam Province, Korea who were diagnosed with COVID-19 using real-time polymerase chain reaction (PCR; Allplex 2019-nCoV Assay, Seegene, Seoul, Korea) and who were discharged without any serious sequelae or complications were enrolled in this study. Most patients had mild symptoms, and three patients were asymptomatic at diagnosis of COVID-19 infections. The cycle threshold (Ct) values of E, RdRP, and N genes were recorded to estimate the viral load at diagnosis. The mean age of 30 participants was 44.6 years old (standard deviation [SD], 14.3), with 13 men (43.3%). The average period of sample collection after diagnosis was 115.3 days (SD, 14.8).
A standard micro-neutralization assay was carried out for determining anti-SARS-CoV-2 neutralizing antibodies as previously described [8,9]. Serial two-fold dilutions (1:10–1:1,280) of the test sera were incubated at room temperature for 1 hour with 100 tissue culture infective dose 50 (TCID50 ) SARS-CoV-2 (βCoV/KOR/KCDC03/2020) and were then incubated with Vero E6 cells at 37°C for 1 hour. After 72 hours of incubation, the neutralizing capacity of individual samples was assessed by determining the presence or absence of virus-induced cytopathic effects (CPE) by microscopy. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution of serum that completely inhibited virus-induced CPE in at least 50% of the wells (50% neutralization titer, NT50). A titer of at least 1:80 was considered positive for SARS-CoV-2 neutralizing antibody [10].
The antibody against SARS-CoV-2 receptor binding domain (RBD) was analyzed using in-house enzyme-linked immunosorbent assay (ELISA) [11]. RBD protein of the spike protein was induced using the mammalian cell expression system. The optical density (OD) was measured at 450 nm at a dilution of 1:2,500 for measuring the antibody level to RBD because there is a clear difference in the OD between patients’ sera with a negative control at this dilution.
Descriptive characteristics were computed for variables in all participants. Continuous variables were calculated as mean (± SD) or median (interquartile range) and underwent a normality test (Shapiro-Wilk test) and homogeneity of variance test (Leven's F Test) before statistical analysis. Categorical variables were calculated as numbers and proportions. Depending on the titer of the neutralizing antibody, the variables were divided into two groups: positive (≥1:80) and negative (<1:80). The Mann-Whitney U test was performed for analyzing the differences between the two groups for continuous variables. Fisher's exact test was performed for testing the differences in the proportions between the two groups. The Spearman correlation analysis was performed to confirm the correlation between the titer of the neutralizing antibody and Ct values (E, RdRP, and N genes) and RBD ELISA OD values. All statistical analyses were performed using the R software version 3.6.1(R Foundation for Statistical Computing, Vienna, Austria).
Nine (30.0%) were positive with a neutralizing antibody titer of ≥1:80 measured by microneutralization, and the remaining 21 were negative (Table 1). The antibody tested using ELISA was positive among 20 (66.7%) patients and those nine positive by microneutralization were all ELISA positive. The median age was 11 years older in neutralizing antibody-positive patients than in negative patients (P = 0.033). There were no significant differences in the disease severity calculated using the national early warning score (NEWS) score [12] and oxygen support during admission to the hospital between the two groups. However, more patients of the neutralizing antibody-positive group had radiographically confirmed pneumonia than those of the neutralizing antibody-negative group (88.9% vs. 38.1%, P = 0.017). The difference in Ct values was statistically different for the E gene (P = 0.005) and RdRp gene (P = 0.003) but not for the N gene (P = 0.236) between the two groups. The OD of 0.89 in neutralizing antibody positive patients was significantly higher than the OD of 0.35 in negative patients for ELISA (P = 0.002). There was a close correlation between the Ct values of the E gene (P = 0.031), RdRP gene (P = 0.020), and RBD ELISA (P< 0.001) with the neutralization antibody titer (data not shown).
In a previous study, convalescent plasma with a high titer of neutralization antibodies (>1:640) improved clinical symptoms and laboratory parameters within three days [2]. The four recovered patients who showed high neutralization antibody titer (≥1:160) in this study, might be good candidates for therapeutic plasma donation.
Neutralization antibody-positive patients are more likely to be older, to have more pneumonia during hospitalization, and to have higher viral load at diagnosis. It is plausible that the higher viral load might likely cause pneumonia in the elderly as well as induce a strong host immunological response. Old age and disease severity correlated with neutralizing antibody titers in other studies as well [4,5,13]. Considering that patients recovered a long time ago (more than 100 days) after diagnosis in this study, it is notable to observe low positive rate (30%) of neutralization antibodies. Another study showed a rather higher positive rate of 53.4% (31/58) detected by surrogate virus neutralization test at 8 months after infection in Korea [14]. Difference of positive rate might be either due to detection methods applied, patients’ characteristics, and disease severity. The different positive rate between microneutralization and ELISA is explained by using different numbers or sites of epitopes on RBD as well as different techniques between these two tests [15].
We have several limitations for this study. First, the numbers examined are not enough. Second, more diverse tools to measure neutraliztion antibody, such as surrogate virus neutrlization test are needed. In addtion, there are no serial sera collected to observe the dynamic of antibody according to time span. It is not clear whether high incidence of pneumonia in the elderly was due to SARS-CoV-2 or other underlying illnesses or lower immunity.
As long tracing of persistence of neutralization antibodies measured by microneutrliaztion are rare, our study shows only 30% remained neutralization antibody after 100 days of diagnosis.
Ethics statement
This study protocol was approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (IRB No. 2020-05-025). Written consent was obtained from all participants.
Funding
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT)(2021M3E5E3080382 and 2021R1I1A3044483) and grants from KRIBB Research Initiative Program. The funders had no role in the study design, data collection, and interpretation, or the decision to submit the work for publication.
REFERENCES
1. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA 2020;323:2425-7.
.
2. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci 2020;117:9490-6.
.
3. Espejo AP, Akgun Y, Al Mana AF, Tjendra Y, Millan NC, Gomez-Fernandez C, et al. Review of current advances in serologic testing for COVID-19. Am J Clin Pathol 2020;154:293-304.
.
4. Siracusano G, Pastori C, Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol 2020;11:1049.
.
5. Wu F, Liu M, Wang A, Lu L, Wang Q, Gu C, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med 2020;180:1356-62.
.
6. Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, et al. Isolation of potent SARSCoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020;369:956-63.
.
7. Zost SJ, Gilchuk P, Case JB, Binshtein E, Chen RE, Nkolola JP, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020;584:443-9.
.
8. Amanat F, White KM, Miorin L, Strohmeier S, McMahon M, Meade P, et al. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr Protoc Microbiol 2020;58:e108.
.
9. Manenti A, Maggetti M, Casa E, Martinuzzi D, Torelli A, Trombetta CM, et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus microneutralization assay in human serum samples. J Med Virol 2020;92: 2096-104.
.
10. Lee WT, Girardin RC, Dupuis AP, Kulas KE, Payne AF, Wong SJ, et al. Neutralizing antibody responses in COVID-19 convalescent sera. J Infect Dis 2021;223:47-55.
.
11. Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020;26:1033-6.
.
12. Royal College of Physicians. National Early Warning Score (NEWS) 2. https://www. rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2. [Online] (last visited on 16 March 2022)
.
13. Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibodies responses to SARSCoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis 2020;71:2688-94.
.
14. Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY, et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis 2021;27:928-31.
.
15. Kadkhoda K. COVID-19: are neutralizing antibodies neutralizing enough? Transfusion 2020;60:1602-3.
.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download