Abstract
Background: A variety of clinically important pathogens have developed multidrug resistance (MDR), which threatens global public health. This study aimed to determine the incidence, patterns, and trends of MDR of gram-negative bacterial isolates in clinical specimens in the Tamale Teaching Hospital, Ghana. Methods: This retrospective study analyzed gram-negative bacterial isolates and antimicrobial susceptibility test (AST) results of patients who visited the Tamale Teaching Hospital laboratory between 2017 and 2019. Results: A total of 2,779 gram-negative bacterial isolates and their phenotypic AST results were analyzed. From these, 1,297 gram-negative bacteria (46.7%) were isolated from urine samples, while the rest were isolated from sputum (20.9%), wound (14.3%), and swabs (11.7%) samples, etc. Escherichia coli (23.8%) was the most common gram-negative pathogen found predominantly in the urine samples (33.2%). All gram-negative bacteria isolated between 2017 and 2019 showed high MDR. Klebsiella pneumoniae gradually increased its MDR from 84.0% in 2017, 89.5% in 2018, to 95.1% in 2019. On the other hand, the MDR rates in Pseudomonas aeruginosa were approximately 65.8%, varying from 59.5% in 2017 to 78.7% in 2019. Among tested antimicrobials, amikacin was the most effective. Resistance to amikacin in Enterobacter spp., E. coli, and K. pneumoniae in vitro were 16.2%, 11.8%, and 17.7%, respectively. Conclusion: The study has shown that the high levels of MDR in gram-negative bacteria isolated may be associated with the infections recorded at the Tamale Teaching Hospital. The major gram-negative pathogens isolated have resistance to penicillins, cephalosporins, and fluoroquinolones. Aminoglycosides can offer high antibiotic activity to overcome gramnegative bacterial resistance. Further studies will be needed to decide policy direction on infection prevention and control, and antimicrobial stewardship programs.
Multidrug resistance (MDR) exists within a wide variety of clinically important pathogens and poses a serious and growing global public health threat [1]. In the 2014 global surveillance report on antimicrobial resistance, the World Health Organization (WHO) warned of an apocalyptic post-antimicrobial era, a possible reality in the 21st century. Antimicrobial resistance is a threat to the prevention and treatment of the wide range of diseases caused by infectious pathogens. Viruses, bacteria, parasites, and fungi are increasingly becoming resistant to the currently available antimicrobial agents previously effective at treating medical conditions due to diseases caused by these microorganisms. Currently, MDR is estimated to cause approximately seven hundred thousand deaths globally. If the situation of the trend of resistance persists, MDR could result in the deaths of over 10 million people per year [2].
Infections caused by multidrug-resistant bacteria have been particularly challenging in developing countries and are mostly accompanied by an increased hospital stay and high rates of morbidity and mortality [3]. The threat of antimicrobial resistance in low- and middle-income countries is exacerbated due to several practices pervasive in these countries. A study published in 2017 outlined the causes of antimicrobial resistance in developing countries to include: widespread unnecessary and overuse of antimicrobials due to lack of appropriate regulations in the sales and administration of antimicrobials, counterfeit and lowquality antimicrobials resulting in sub-inhibitory concentration of in vivo, and high rate of self-medication [4]. Ironically, countries in the WHO African region have a problem with the unavailability of data on antimicrobial resistance (AMR) [5]. All the reasons ascribed to be causes of antimicrobial resistance in developing countries are also prevalent in Ghana and studies on AMR have revealed the existence of increasing trends of resistance among several common pathogens [6,7].
Over the years the WHO has developed a list of priorities of organisms deemed as imminent global health threats to guide research and new development of antibiotics. The categorization of these priority pathogens in three levels of severity includes Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacterales, which all fall within the critical priority class of organisms. Other description highlights the threat of these pathogens to positive clinical outcomes. Several studies have espoused the clinical significance of ESKAPE organisms (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) [8,9]. ESKAPE gram-negative bacteria are among some of the most common organisms implicated in healthcare-associated infections. They are documented to be rapidly becoming resistant to commonly used broad-spectrum antibiotics. Not only are these infections prone to negative health outcomes, but they also contribute to adverse socio-economic impacts by putting a burden on health care systems in low- and middle-income countries [10]. However, there are limited data on the rate and burden of multidrug-resistant bacteria, so that it is difficult to accelerate the formulation and implementation of policies to respond to the threat of antimicrobial resistance in Ghana. This study aimed to determine the incidence and antimicrobial pattern of MDR of gram-negative bacterial isolates in clinical specimens cultured at the Tamale Teaching Hospital Laboratory in Ghana from 2017 to 2019.
This retrospective study analyzed gram-negative bacterial isolates and sensitivity test results of patients who visited the Tamale Teaching Hospital laboratory in Ghana, West Africa, from January 2017 to December 2019. This study was conducted with data from the Tamale Teaching Hospital Laboratory. The Tamale Teaching Hospital is the only tertiary health care and referral facility responsible for serving patients from Northern, Upper West, Upper East, Northern Volta, and parts of the Bono Ahafo Regions of Ghana. All isolates were identified by conventional biochemical methods. Only samples that were considered to have clinical significance and were expected to be true pathogens were included and duplicated isolates were excluded from this study.
The antimicrobial susceptibility testing (AST) done at the Tamale Teaching Hospital on each of isolated gram-negative bacteria adopted the disk diffusion method [11]. The diameter of zone inhibition of a panel of antibiotics was determined and interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [12]. MDR in this study was defined as non-susceptibility to at least one agent in three or more antimicrobial categories according to Magiorakos et al. [13].
The antimicrobial susceptibility was expressed as a percentage of isolates that were resistant to a particular antimicrobial agent among the total number of isolates of the same species. This was calculated on an annual basis and trends were assessed from year to year over the entire study period for each bacterial organism. Average annual susceptibility over the entire study period was calculated to provide insight into overall antimicrobial susceptibility patterns.
The sex distribution of clinical samples cultured with the gram-negative bacterial isolates was almost equal proportions between males and females. The sample cultured from females amounted to 1,407, constituting about 51.0% of the total cultures that isolated gram-negative bacteria (Table 1). A total of 1,297 gramnegative bacteria were isolated from urine samples representing 46.7% of all the isolates followed by isolates, from sputum (20.9%), wound (14.3%), and swabs (11.7%).
E. coli (23.8%) was the most common gram-negative bacterial species with predominance from urine samples making up 33.2%. Most gram-negative bacteria were isolated from urine samples. As shown in Table 2, bloodstream infections were dominated by Enterobacter spp. (22.0%) followed by K. pnuemoniae (20.7%). Respiratory isolates were dominated by M. catarrhalis (30.1%) and K. pneumoniae (21.0%).
All gram-negative bacteria isolated from 2017-2019 showed high MDR. Priority gram-negative bacteria were determined to have a resistance rate above 60.0% of all antibiotics tested. A. baumannii (83.3%), Citrobacter spp. (91.4%), Enterobacter spp. (84.2%), E. coli (88.7%), and K. pneumoniae (88.8%) were all determined to have MDR above 80.0%. P. aeruginosa isolated from 2017 to 2019 was determined to have a relatively low MDR of 65.8% (Table 3).
The data showed a progressive increment in the levels of resistance among gram-negative bacteria from 2017 to 2019. Citrobacter spp. showed an increase in MDR from 89.1% in 2017 to 98.0% in 2019. K. pneumoniae similarly showed increased levels of MDR year on year; 2017 (84.0%), 2018 (89.5%), and 95.1% in 2019. P. aeruginosa which showed relatively low levels of MDR (65.8%), was still determined to demonstrate increased resistance from 2017 (59.5%) to 2019 (78.7%). Resistance to ampicillin was the highest among all Enterobacterales tested. Enterobacter spp., E. coli, and K. pneumoniae were demonstrated to have a resistance of above 97.0% to ampicillin (Table 4). Gram-negative bacteria showed the highest susceptibility to antibiotics in the aminoglycoside class, with amikacin the most effective. Enterobacter spp. resistance to amikacin was as low as 16.2%, E. coli and K. pneumoniae were determined to be 11.8%, 17.7%, respectively (Table 4). A. baumannii demonstrated the highest resistance to amikacin with a resistance of 42.8%. All major gram-negative bacteria were shown to have high resistance against tetracycline, trimethoprim-sulfamethoxazole, and nalidixic acid (Table 4). There is varied susceptibility to f luoroquinolones among different gram-negative bacteria. A. baumannii was determined to have a resistance of 28.6% and 30.0% in levofloxacin and ciprofloxacin, respectively. P. aeruginosa was shown to be more resistant to levofloxacin (53.7%) as compared to ciprofloxacin with a resistance rate of 39.6% (Table 4).
Epidemiological and laboratory surveillance of bacterial infection and MDR to antibiotics are essential for informing antimicrobial stewardship and implementation of infection prevention and control measures [14]. This becomes more imperative in low- and middle-income countries where the studies have shown the lack of laboratory diagnostic capacity, poor implementation of infection control measures, and a deficit in the awareness of infections caused by multidrug-resistant bacteria, which have resulted in high mortality and morbidity [15].
The main purpose of this study was to determine the rate of MDR in gram-negative bacteria in a tertiary healthcare facility in Ghana. The study observed a high prevalence of infections among children below the age of 10 and the elderly above 60. This distribution is similar to the finding by Agyepong et al. [16]. The elderly and infants are usually more disposed to infections due to their incompetent immune status. The advancing age is commonly associated with risk factors including reduced immunity, co-morbidity with other diseases such as non-communicable diseases like diabetes, cardiovascular diseases, and renal disorders, while in infants, lack of fully developed immunity, malnutrition, and inadequate hygiene put them at greater risk of infections [17-19].
This study observed a urinary tract infection (58.0%) to be the most common form of gram-negative bacterial infection with a high disease burden on Northern Ghana’s population. Similar studies in Ghana and the Sub-Saharan African region depict similar findings. The finding of this study is comparable to other studies that determined similar trends in gram-negative infection patterns [20-22]. Studies in Korea also came to the finding implicating Enterobacterales such as E. coli, and K. pneumoniae as the major causative organism in urinary tract infections [23]. E. coli (23.8%) and K. pneumoniae (22.6%) were observed to be the most common gram-negative bacterial pathogen responsible for infections among patients in this study. Several similar studies conducted in other healthcare facilities in Ghana observed the same trend that E. coli and K. pneumoniae have been the most predominant pathogens responsible for infections [16,24]. Enterobacter spp., K. pneumoniae, and P. aeruginosa were determined to be the highest causes of bloodstream infections accounting for 22.0%, 20.7%, and 19.5%, respectively. The results agreed with a similar study done in Rwanda and Gabon which observed the major cause of bloodstream infections to be K. pnuemoniae [25,26]. However, a multicenter hospital surveillance study conducted in Korea observed E. coli (47.1%) as the major cause of bacteremia in community-acquired bloodstream infection followed by K. pneumoniae (12.6%) [27].
The major causative organisms for wound infection in this study were P. aeruginosa (27.4%), K. pneumoniae (19.0%), and Proteus spp. (16.3%). These results are comparable to results found in a study of wound infection in Ghana where it was observed that the major gram-negative bacteria responsible for infection in burns patients were E. coli, K. pneumoniae, and P. aeruginosa [28]. The factors associated with wound infections in hospital settings have been suggested to be poorly implemented infection prevention and control, personal hygiene, and a lack of proper wound care. This study observed an increase in the trend of the rate of resistance to all antibiotic groups among major gram-negative bacteria isolated in northern Ghana from 2017 to 2019.
This study determined that the resistance of gram-negative bacteria to commonly prescribed antimicrobial was extensive, indicating MDR rates of between 60.0% in Salmonella spp. and 95.5% in M. catarrhalis. Major clinically important gram-negative bacteria like P. aeruginosa (65.8%), E. coli (88.7%), A. baumannii (83.3%), and K. pneumoniae (88.8%) are reported in Table 3. These results should be interpreted with the backdrop that the study site is a tertiary health care referral facility. That would suggest that majority of the samples received were from severely ill patients who may have received empirical antimicrobial therapy at different levels of the healthcare system. The other factors associated with the observed high levels of MDR are accounted for by widespread unnecessary use and overuse of antimicrobials due to lack of appropriate regulations in the sales and administration of antimicrobials, counterfeit and low-quality antimicrobials resulting in a sub-inhibitory concentration of in vivo, and the high rate of self-medication as stipulated in a 2017 study of the use of antimicrobials in low- and middle-income countries [4]. This result is comparable to studies done in Ghana in a similar setting with the same definition for MDR [20,24]. Agyepong et al. showed MDR rates in a tertiary healthcare facility in Ghana to be 89.9% in E. coli, 94.7% in K. pnuemoniae, 100% in A. baumannii, and 100% in P. aeruginosa [16]. Our study showed similar patterns except MDR rate of P. aeruginosa (65.8%).
The resistance profile of isolated major gram-negative bacteria displayed high-level MDR with high resistance to ampicillin: Enterobacter spp. (98.7%), E. coli (97.9%), and K. pnuemoniae (97.4%). Although the direct comparison was not reliable due to different antimicrobial susceptibility test agents and MDR definitions in each study, the degree of resistance showed in the study was consistent with studies done in Ghana [16,20,24] and also agreed with other studies conducted in the sub-Sahara African countries such as Ethiopia, Zimbabwe, and Rwanda [29-31]. Resistance to fluoroquinolones among major gramnegative bacteria was reported in this study to be range between 28.6% of resistance to levofloxacin in A. baumannii and 65.3% of resistance to ciprofloxacin in E. coli. As shown in Table 4, major gram-negative pathogens exhibited the least resistance to aminoglycosides. Resistance to amikacin among major pathogens isolated was observed to be 42.8% in A. baumannii and 19.4% in P. aeruginosa. Resistance to amikacin in Enterobacterales ranged from 11.8% in E. coli to 17.7% in K. pnuemoniae as shown in Table 4. These f indings were consistent with studies conducted in Ghana [16] and also comparable to studies in other parts of Africa [32,33].
The observation of an increased resistance trend in the African sub-region is indicative of high antibiotic selection pressure mainly due to easy accessibility of counterfeit antimicrobials with suboptimal inhibitory concentrations, high levels of self-medication, and the lack of antimicrobial stewardship programs in both communities and health care settings [4]. Based on the observed high level of, and increasing trend of resistance among gram-negative pathogens, there is an urgent need for the institution of antimicrobial stewardship programs in hospitals and the community. The limitation of this study includes the use of retrospective data. The study site used the disk diffusion method which is qualitative in its interpretation of susceptibility using CLSI guidelines. The samples were cultured, and an antimicrobial susceptibility test was performed in a referral tertiary healthcare facility, and as such the results should be interpreted in that context. Therefore, the prevalence of antimicrobial-resistant pathogens in this study might have been exaggerated and it was not possible to assess whether the infections were hospital-acquired or community-acquired.
In summary, the study has shown high levels of multidrug-resistant gram-negative bacteria commonly isolated as the causative organisms in a range of infections. The predominant gram-negative bacteria implicated in infections in the Tamale Teaching Hospital were E. coli, K. pneumoniae, and P. aeruginosa. There was high resistance to penicillins, cephalosporins, and fluoroquinolones among major gram-negative pathogens. Aminoglycosides exhibited the least levels of resistance to isolated gram-negative bacteria in the Tamale Teaching Hospital. The conduction of nationwide surveillance of MDR is a resource-intensive endeavor and has proven to be difficult to be undertaken in low- and middle-income countries. This study will help decide policy direction on infection prevention and control, and antimicrobial stewardship programs.
Ethics statement
The obtainment of informed consent was waived since it is a retrospective chart review.
Conflicts of interest
Dongeun Yong is currently an editorial board member of the Annals of Clinical Microbiology. However, he was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
REFERENCES
1. WHO. Antimicrobial resistance: global report on surveillance: 2014 summary. https://www. who.int/publications/i/item/WHO-HSE-PED-AIP-2014.2 [Online] (last visited on 14 March 2022).
.
2. Review on antimicrobial resistance. In: O’neill J, ed. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. London; Wellcome trust & HM government, 2014.
.
3. Singh N and Manchanda V. Control of multidrug-resistant gram-negative bacteria in low- and middle-income countries-high impact interventions without much resources. Clin Microbiol Infect 2017;23:216-8.
.
4. Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 2017;6:47.
.
5. Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013;2:31.
.
6. Newman MJ, Frimpong E, Donkor ES, Opintan JA, Asamoah-Adu A. Resistance to antimicrobial drugs in Ghana. Infect Drug Resist 2011;4:215-20.
.
7. Opintan JA, Newman MJ. Prevalence of antimicrobial resistant pathogens from blood cultures: results from a laboratory based nationwide surveillance in Ghana. Antimicrob Resist Infect Control 2017;6:64.
.
8. Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. Resistance among gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013-2015. Braz J Infect Dis 2017;21:343-8.
.
9. Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. Antimicrobial susceptibility of gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Asia–Pacific countries: SMART 2013–2015. J Med Microbiol 2017;66:61-9.
.
10. Zhen X, Lundborg CS, Sun X, Hu X, Dong H. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control 2019;8:137.
.
11. Bauer AW, Kirby W, Sherris J, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1966;45:493-6.
.
12. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: M100-S25. 25th informational supplement. Wayne; PA, 2012.
.
13. Magiorakos AP, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, et al. Multidrugresistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clini Microbiol Infect 2012;18:268-81.
.
14. Tseng SH, Lee CM, Lin TY, Chang SC, Chuang YC, Yen MY, et al. Combating antimicrobial resistance: antimicrobial stewardship program in Taiwan. J Microbiol Immunol Infect. 2012 Apr;45(2):79-89.
.
15. Samuel S, Kayode O, Musa O, Nwigwe G, Aboderin A, Salami T, et al. Nosocomial infections and the challenges of control in developing countries. African J Clin Exp Microbiol 2010;11:2.
.
16. Agyepong N, Govinden U, Owusu-Ofori A, Essack SY. Multidrug-resistant gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control 2018;7:37.
.
17. Hill R, Paulus S, Dey P, Hurley MA, Carter B. Is undernutrition prognostic of infection complications in children undergoing surgery? A systematic review. J Hosp Infect 2016;93:1221.
.
18. Simonetti AF, Viasus D, Garcia-Vidal C, Carratalà J. Management of community-acquired pneumonia in older adults. Ther Adv Infect Dis 2014;2:3-16.
.
19. Chang YJ, Yeh ML, Li YC, Hsu CY, Lin CC, Hsu MS, et al. Predicting hospital-acquired infections by scoring system with simple parameters. PLoS One 2011;6:e23137.
.
20. Gyasi-Sarpong CK, Nkrumah B, Yenli EMT, Appiah AA, Aboah K, Azorliade R, et al. Resistance pattern of uropathogenic bacteria in males with lower urinary tract obstruction in Kumasi, Ghana. African J Microbiol Res 2014;8:3324-9.
.
21. Obirikorang C, Quaye L, Bio F, Amidu N, Acheampong I, Addo K. Asymptomatic bacteriuria among pregnant women attending antenatal clinic at the university hospital, Kumasi, Ghana. J Medical Biomed Sci 2012;1:38-44.
.
22. Gyansa-Lutterodt M, Afriyie D, Asare G, Amponsah S, Abutiate H, Darko D. Antimicrobial use and susceptibility pattern of uropathogens associated with urinary tract infections at the Ghana police hospital. Glob J Pharmacol 2014;8:306-15.
.
23. Kang CI, Kim J, Park DW, Kim BN, Ha U, Lee SJ, et al. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 2018;50:67-100.
.
24. Newman MJ, Frimpong E, Donkor ES, Opintan JA, Asamoah-Adu A. Resistance to antimicrobial drugs in Ghana. Infect Drug Resist 2011;4:215.
.
25. Ntirenganya C, Manzi O, Muvunyi CM, Ogbuagu O. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg 2015;92:865-70.
.
26. Alabi AS, Frielinghaus L, Kaba H, Kösters K, Huson MA, Kahl BC, et al. Retrospective analysis of antimicrobial resistance and bacterial spectrum of infection in Gabon, Central Africa. BMC Infect Dis 2013;13:455.
.
27. Son JS, Song JH, Ko KS, Yeom JS, Ki HK, Kim SW, et al. Bloodstream infections and clinical significance of healthcare-associated bacteremia: a multicenter surveillance study in Korean hospitals. J Korean Med Sci 2010;25:992-8.
.
28. Forson O, Ayanka E, Olu-Taiwo M, Pappoe-Ashong P, Ayeh-Kumi P. Bacterial infections in burn wound patients at a tertiary teaching hospital in Accra, Ghana. Ann Burns Fire Disasters 2017;30:116.
.
29. Carroll M, Rangaiahagari A, Musabeyezu E, Singer D, Ogbuagu O. Five-year antimicrobial susceptibility trends among bacterial isolates from a tertiary health-care facility in Kigali, Rwanda. Am J Trop Med Hyg 2016;95:1277-83.
.
30. Muluye D, Wondimeneh Y, Ferede G, Nega T, Adane K, Biadgo B, et al. Bacterial isolates and their antibiotic susceptibility patterns among patients with pus and/or wound discharge at Gondar university hospital. BMC Res Notes 2014;7:619.
.
31. Mbanga J, Dube S, Munyanduki H. Prevalence and drug resistance in bacteria of the urinary tract infections in Bulawayo province, Zimbabwe. East Afr J Public Health 2010;7:3.
.
32. Ogbolu D, Daini O, Ogunledun A, Alli A, Webber M. High levels of multidrug resistance in clinical isolates of gram-negative pathogens from Nigeria. Int J Antimicrob Agents 2011;37:62-6.
.
33. Kumburu H, Sonda T, Mmbaga BT, Alifrangis M, Lund O, Kibiki G, et al. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Health 2017;22:454–64.
.
Table 1
The number of cultured gram-negative bacterial isolates distributed among age, sex, and specimen
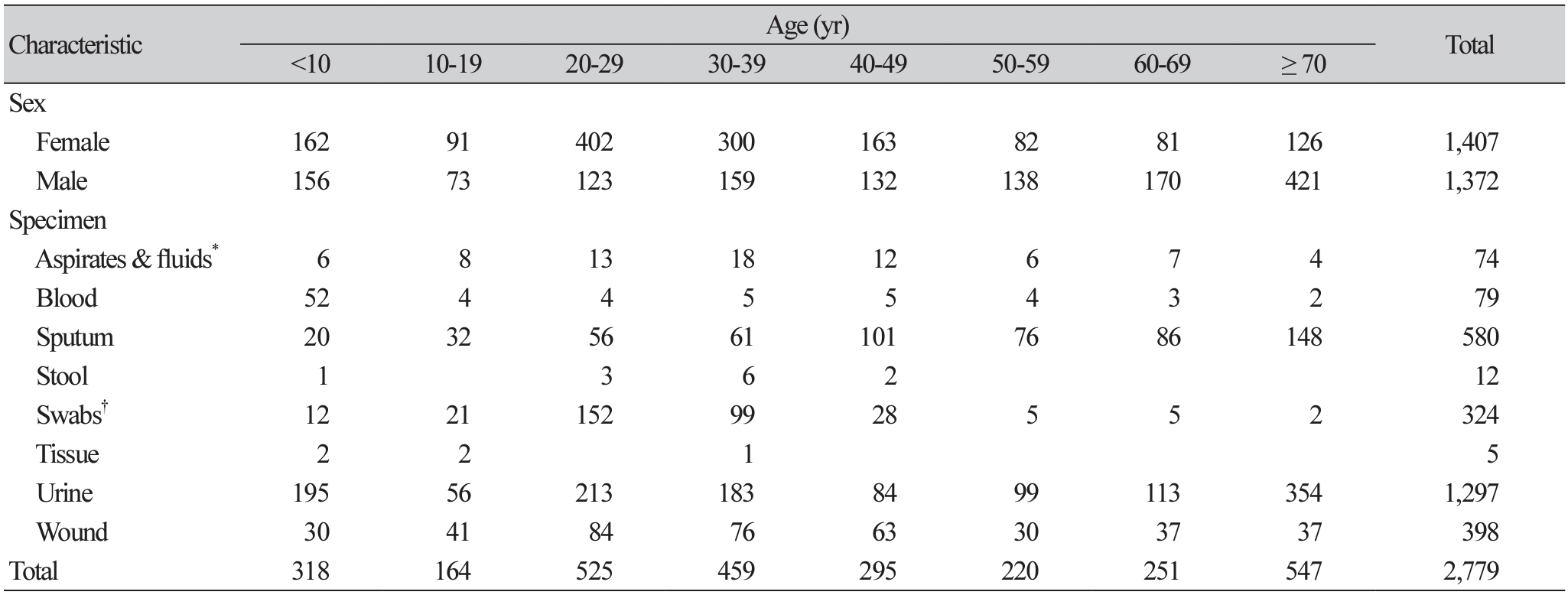
Table 4
Resistance profile of major gram-negative pathogens (2017-2019)
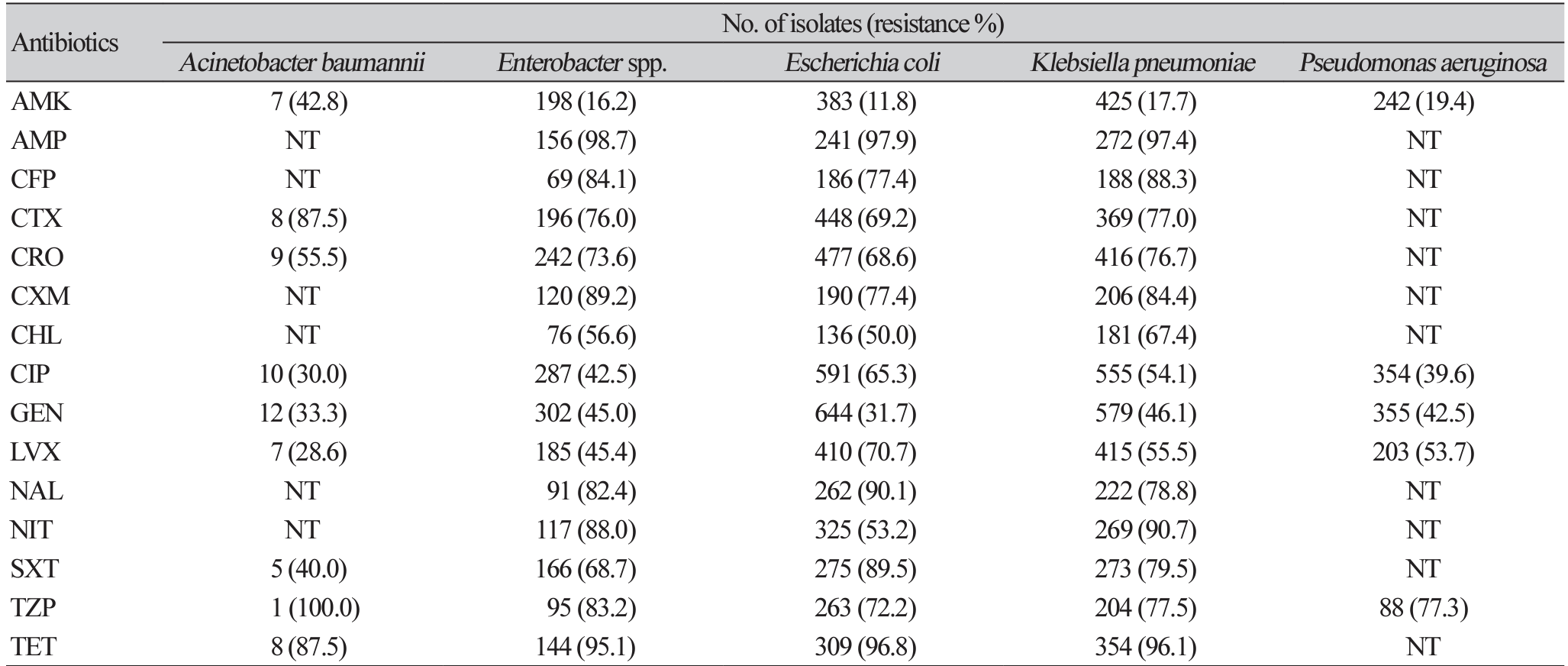
Abbreviations: AMK, amikacin; AMP, ampicillin; NT, not tested; CFP, cefoperazone; CTX, cefotaxime; CRO, ceftriaxone; CXM, cefuroxime; CHL, chloramphenicol; CIP, ciprofloxacin; GEN, gentamicin; LVX, levofloxacin; NAL, nalidixic acid; NIT, nitrofurantoin; SXT, trimethoprim-sulfamethoxazole; TZP, piperacillin-tazobactam; TET, tetracycline.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download