Abstract
Robotic surgery is emerging as a feasible minimally invasive approach for donor hepatectomy at specialized centers. The aim of this article is to systematically describe the surgical techniques for robotic hilar dissection and right lobe mobilization in right donor hepatectomy. The setup of the robotic arms, the dissection of inflow vessels and retrohepatic inferior vena cava, and the pearls and pitfalls of these two parts of the operation are detailed.
Robotic surgery is emerging as a feasible minimally invasive approach for donor hepatectomy [1–4]. Some of the most important parts of right donor hepatectomy are hilar dissection, right lobe mobilization and parenchymal transection. The technical paper focuses on hilar dissection and right lobe mobilization. The purpose of dissecting the liver hilum in right donor hepatectomy is to isolate the inflow blood vessels to the right lobe, namely the right hepatic artery (RHA) and right portal vein (RPV), and to initially identify the biliary bifurcation, in preparation for graft procurement later in the procedure. A preoperative review of cross-sectional imaging scans is useful for delineating the hilar structures and identifying any aberrant anatomy.
Right lobe mobilization involves dividing the attachments of the right lobe to the retroperitoneum and diaphragm, as well as dissecting the posterior right and caudate lobe from the retroperitoneal inferior vena cava (IVC). These maneuvers facilitate transection of the parenchyma immediately anterior to the IVC and between the right and middle hepatic veins.
One of the main advantages of the robotic platform in complex liver surgery is the ability to perform meticulous dissection of the liver hilum and retrohepatic IVC. Comparable to the human wrist, the seven degrees of freedom of robotic instruments facilitate isolation of the various structures in the hilum and short hepatic veins, while the nonworking arms provide stable retraction and magnified three-dimensional (3D) camera visualization.
Herein, we detail the technical aspects of the approach to the liver hilum and right lobe mobilization, and describe dissection of the hepatic artery, portal vein (PV), main bile duct branches, and retrohepatic vein cava. All donors would have completed a live donor evaluation and been approved by a multidisciplinary committee to proceed to donor surgery based on our program’s policy and protocols.
Our standard port placements for right-sided liver resection are shown in Fig. 1. The placement of the camera provides adequate visualization of the right side of the liver hilum. The third arm is used for retraction and placed as a right-handed instrument. Two laparoscopic assistant ports are placed in the lower abdomen, and the specimen is usually extracted via a Pfannenstiel incision.
(1) Maryland bipolar forceps (with electrocautery). (2) Camera. (3) Scissors (with electrocautery). (4) Cadiere forceps.
After the ligamentum teres and falciform ligamentum are divided with robotic scissors using electrocautery up to the level of the hepatic vein-IVC confluence, the inferior segments of the liver can then be retracted superior-anteriorly to expose the hilum. The third arm is used to retract segment 4B and gallbladder; a mini-lap pad is placed between the tip and elbow of the Cadiere forceps to prevent injury to the liver surface. The laparoscopic assistant retracts the duodenum by gently pushing it inferiorly with a blunt-tipped instrument or suction device.
A standard cholecystectomy is performed, and the gallbladder specimen is placed in Morrison’s pouch until later removal. The previously clipped cystic duct stump is now ligated with 6-inch 3/0 Vicryl tie or suture-ligated with 3/0 Vicryl sutures with the tails left long. The tails are then grasped by the Cadiere forceps positioned at segment 4B. Using the forceps as a pulley, the cystic duct is retracted anteriorly and slightly left, exposing the right lateral and posterior aspect of the common hepatic duct (CHD) (Fig. 2). Retraction can be adjusted as needed during the hilar dissection to provide adequate exposure [1,5].
The pulsation of a standard RHA is usually visible behind the CHD at this stage. The lymphatic and nerve tissue lateral and posterior to the CHD are carefully dissected until the lateral wall of the artery is visualized. Care is taken not to injure the CHD and common bile duct (CBD), either via traction or cautery burn. If the artery is not found in this manner, an alternative identification method is to follow the cystic artery to its’ origin, which is usually the RHA or a branch of the RHA.
The anterior aspect of the RHA is dissected away from the posterior wall of the CHD and CBD. The posterior aspect of the standard RHA may be adherent to the anterior surface of the PV, and the artery needs to be gently lifted off of the PV. Once the anterior, lateral, and posterior walls of the artery are cleared, the tissues at the medial aspect of the RHA can be gently pushed away from the arterial wall and the artery encircled with a vessel loop (Fig. 3).
If the preoperative imaging reveals a replaced RHA from the superior mesenteric artery, this artery is usually found lying posterior-laterally along the course of the CBD and CHD, making the dissection of a longer length of this artery easier [6]. In this position, the artery is often covered by a lymph node (station 12b) that commonly stretches the length of the bile duct. This node needs to be excised to gain access to the replaced RHA. The main difference in the dissection of the replaced versus standard RHA is the close association with the PV posterior-medially and CBD antero-medially through the length of the replaced artery (Fig. 4).
The vessel loop around the RHA is now grasped by the third arm (Cadiere forceps) and gently retracted anteriorly, enabling access to the PV bifurcation; this is similar to the retraction of the cystic duct stump ligature. If there is a replaced RHA, retraction of the vessel loop towards the patient’s right side by the laparoscopic assistant instead of anteriorly may help better expose the PV bifurcation (Fig. 5).
The plane between the CHD and anterior surface of the PV is gently developed by pushing the PV posteriorly off the attachments to the duct and RHA. Dissection proceeds cephalad until the bifurcation is encountered. Clear identification of the bifurcation is confirmed by visualizing the right, left, and main PVs. The antero-superior surface of the RPV is then carefully teased off the hilar plate by gently pressing the RPV posteriorly with the Maryland forceps and sweeping the tissues off the vein with the scissors. It is important to mobilize the RHA off any attachments to the RPV to facilitate safe division of the RPV later in the procedure [1–5].
Attention is then turned to the posterior RPV. The peritoneal lining of the posterior aspect of the RPV is divided and retracted posterior-inferiorly by the laparoscopic assistant. The posterior wall of the PV is dissected free from the peritoneal surface until the caudate branches are isolated. The scissors are then switched to a large needle driver and this instrument, in tandem with the Maryland forceps, helps encircle these short branches with 3/0 silk ties (Fig. 6). The PV side of these branches is always ligated with ties to avoid clips on the PV, hampering future clipping or stapling of the RPV (the caudate side may be clipped).
Once all caudate branches are divided, the posterior RPV is then dissected off the hilar plate moving cephalad until we meet our dissection from the anterior side. Once this space is developed, the RPV is encircled with a vessel loop. We mark the PV bifurcation with a 5-0 Prolene stitch on the superficial aspect of the RPV wall to avoid impingement of the bifurcation when we eventually divide the RPV [1,5]. We incise the peritoneal lining at the bile duct bifurcation and place a small piece of Surgicel Fibrillar (Ethicon) at the bifurcation; this guides our transection line near the hilum [1,2].
Demarcation of the right versus left liver lobes is enhanced by indocyanine green (ICG) dye injection and Firefly (Intuitive Surgical; Fig. 7). We use a 2.5-mg dose of ICG given after the inflow to the right lobe is clamped with laparoscopic bulldog vascular clamps. Injecting the dye at this stage also allows enough time for biliary excretion, which assists in identifying the right hepatic duct (RHD) during parenchymal transection. The clamps are then removed, and this completes the hilar dissection [1,2,4–6].
The third arm is moved to the inferior aspect of segments 5 and 6 and used to retract these segments superior-anteriorly, exposing the inferior layer of the right coronary and right triangular ligaments. These ligaments are divided, taking care not to injure the right lobe or the diaphragm. As more of the bare area is exposed, the third arm is moved sequentially superiorly and posteriorly to provide more retraction. The right adrenal gland is mobilized from the posterior surface of the liver. Anteriorly, the superior layer of the right coronary ligament can be accessed by retracting the right lobe medially. Once most of the bare area has been mobilized, we turn our attention medially towards the retrohepatic IVC.
Exposure of the caudate lobe is facilitated by retracting the right lobe anteriorly by the third arm. The peritoneal lining between the caudate and IVC is divided, and the short caudate and hepatic veins draining directly to the IVC are serially divided between clips (Fig. 8). Dissection continues in a cephalad direction on the anterior surface of the retrohepatic IVC, mobilizing the liver from the IVC. Caudate lobe division at this point helps in the cephalad dissection of the IVC. Laterally, the hepatocaval ligament is encountered and is left intact at this stage, as division of this ligament after parenchymal transection is straightforward. The liver is carefully mobilized from the IVC until we reach the area between the right and middle hepatic veins [1,2]. This completes the right lobe mobilization.
(1) There may be a tiny recurrent arterial branch coming off the superior medial aspect of the RHA and coursing toward the posterior CHD, which will need to be ligated and divided to enable complete mobilization of the RHA from the bile duct and PV. (2) We endeavor to avoid grabbing the walls of the RHA with the robotic instruments and instead manipulate this vessel by holding the lymphatic tissue attached to its walls or gentle pushing for retraction. (3) Small branches to the caudate lobe or segment 5 coming off the superior-posterior aspect of the RPV may be difficult to ligate during hilar dissection; these can be approached later once the parenchyma and/or the RHD has been transected. (4) If the length or the angle of the RPV makes encirclement by a Maryland forceps difficult, the RPV can still be clamped with a laparoscopic bulldog for demarcation. Once the parenchyma is transected down to the hilum later in the procedure, there is usually more room to accommodate encirclement. (5) Complete mobilization of bulky right lobes from the diaphragm is not necessary, as the most superior and medial attachments can be divided via a medial approach during graft procurement after dividing the inflow and outflow vessels.
The robotic platform facilitates meticulous dissection of the liver hilum, where important structures are in close proximity to one another. This enables isolation of the vessels at the hilum in preparation for graft procurement. Right lobe mobilization and dissection of the retrohepatic IVC is aided by the excellent 3D visualization and the ability to manipulate robotic instruments in this confined space. ICG dye application is a useful adjunct to delineate biliary anatomy during hilar dissection.
REFERENCES
1. Rho SY, Lee JG, Joo DJ, Kim MS, Kim SI, Han DH, et al. 2022; Outcomes of robotic living donor right hepatectomy from 52 consecutive cases: comparison with open and laparoscopy-assisted donor hepatectomy. Ann Surg. 275:e433–42. DOI: 10.1097/SLA.0000000000004067. PMID: 32773621.

2. Broering D, Sturdevant ML, Zidan A. 2022; Robotic donor hepatectomy: a major breakthrough in living donor liver transplantation. Am J Transplant. 22:14–23. DOI: 10.1111/ajt.16889. PMID: 34783439.

3. Chandran B, Varghese CT, Balakrishnan D, Nair K, Mallick S, Mathew JS, et al. 2022; Technique of robotic right donor hepatectomy. J Minim Access Surg. 18:157–60. DOI: 10.4103/jmas.JMAS_35_21. PMID: 35017406. PMCID: PMC8830578.

4. Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT, et al. 2017; Robotic major hepatectomy: is there a learning curve? Surgery. 161:642–9. DOI: 10.1016/j.surg.2016.09.025. PMID: 27884614.
5. Choi GH, Chong JU, Han DH, Choi JS, Lee WJ. 2017; Robotic hepatectomy: the Korean experience and perspective. Hepatobiliary Surg Nutr. 6:230–8. DOI: 10.21037/hbsn.2017.01.14. PMID: 28848745. PMCID: PMC5554764.

6. Choi TW, Chung JW, Kim HC, Lee M, Choi JW, Jae HJ, et al. 2021; Anatomic variations of the hepatic artery in 5625 patients. Radiol Cardiothorac Imaging. 3:e210007. DOI: 10.1148/ryct.2021210007. PMID: 34498005. PMCID: PMC8415139.

Fig. 1
Port placement (blue, robotic; green, laparoscopic assistant ports) for right donor hepatectomy.
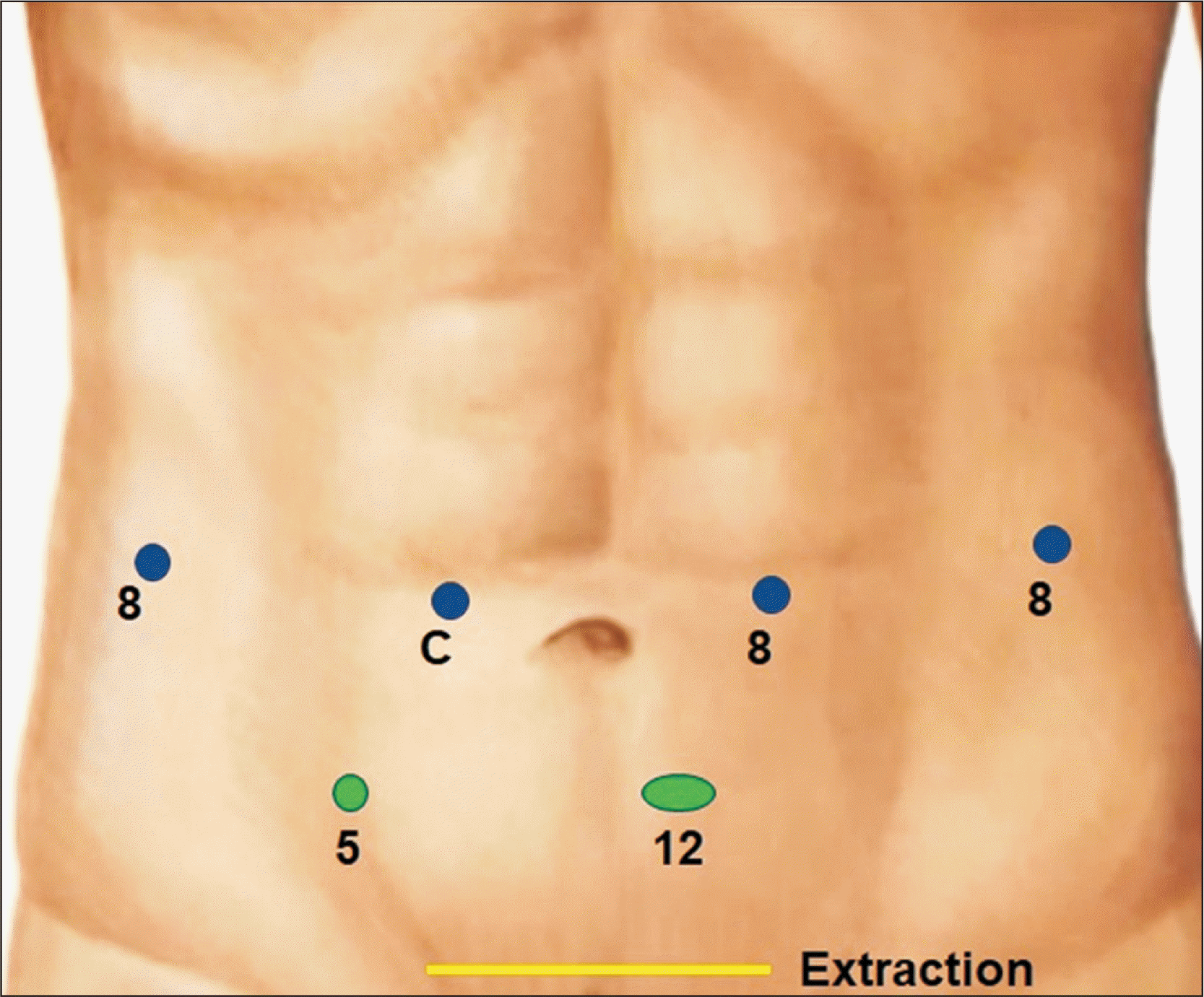
Fig. 2
Usage of the third arm with a gauze as a pulley to retract the ligature placed at the cystic duct stump, which will rotate the common bile duct and common hepatic duct anteriorly and towards the patient’s left, in order to expose the area posterior to the duct where the right hepatic artery courses.
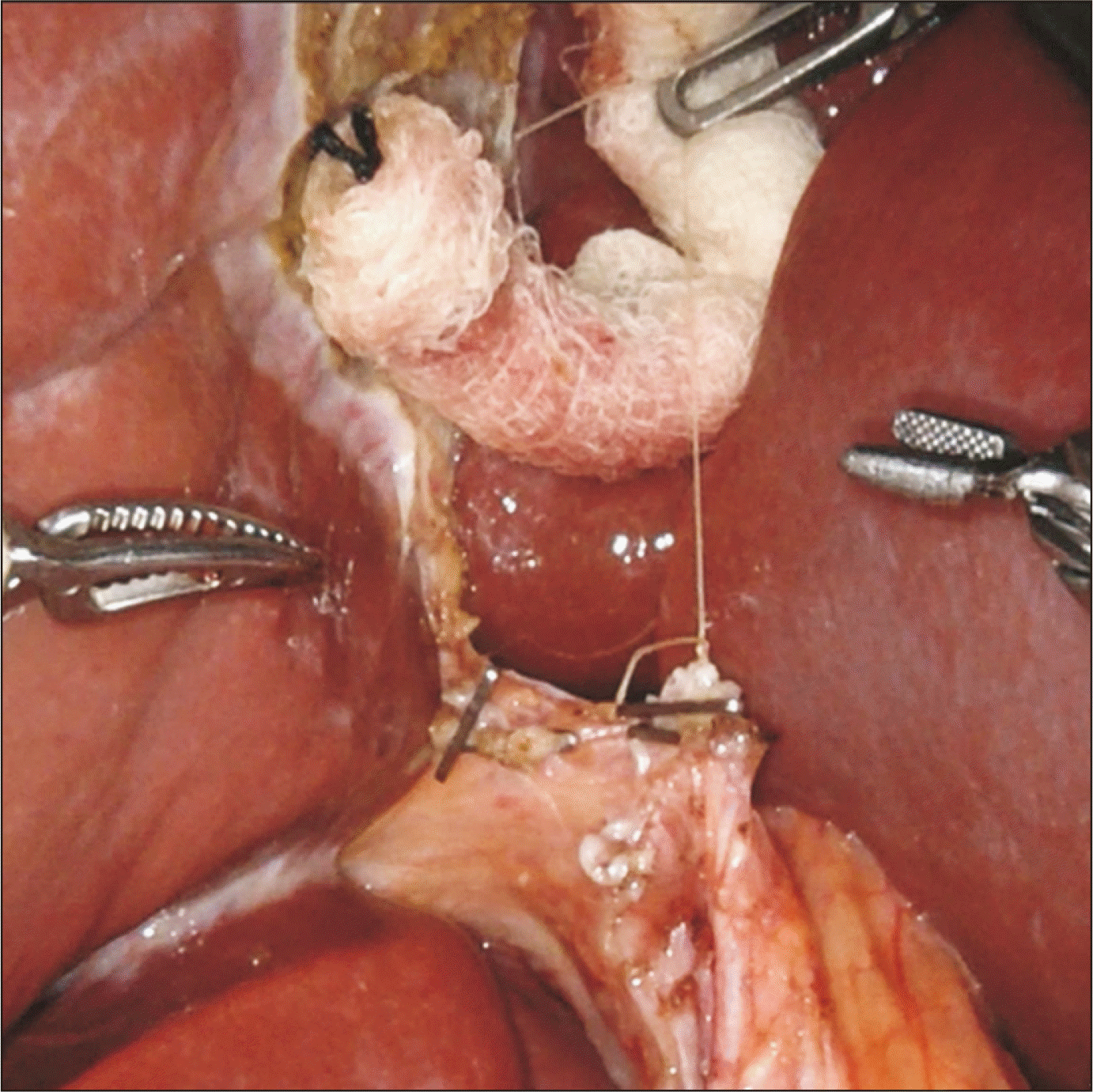
Fig. 3
Standard right hepatic artery coursing posterior to the common hepatic duct encircled with a vessel loop.
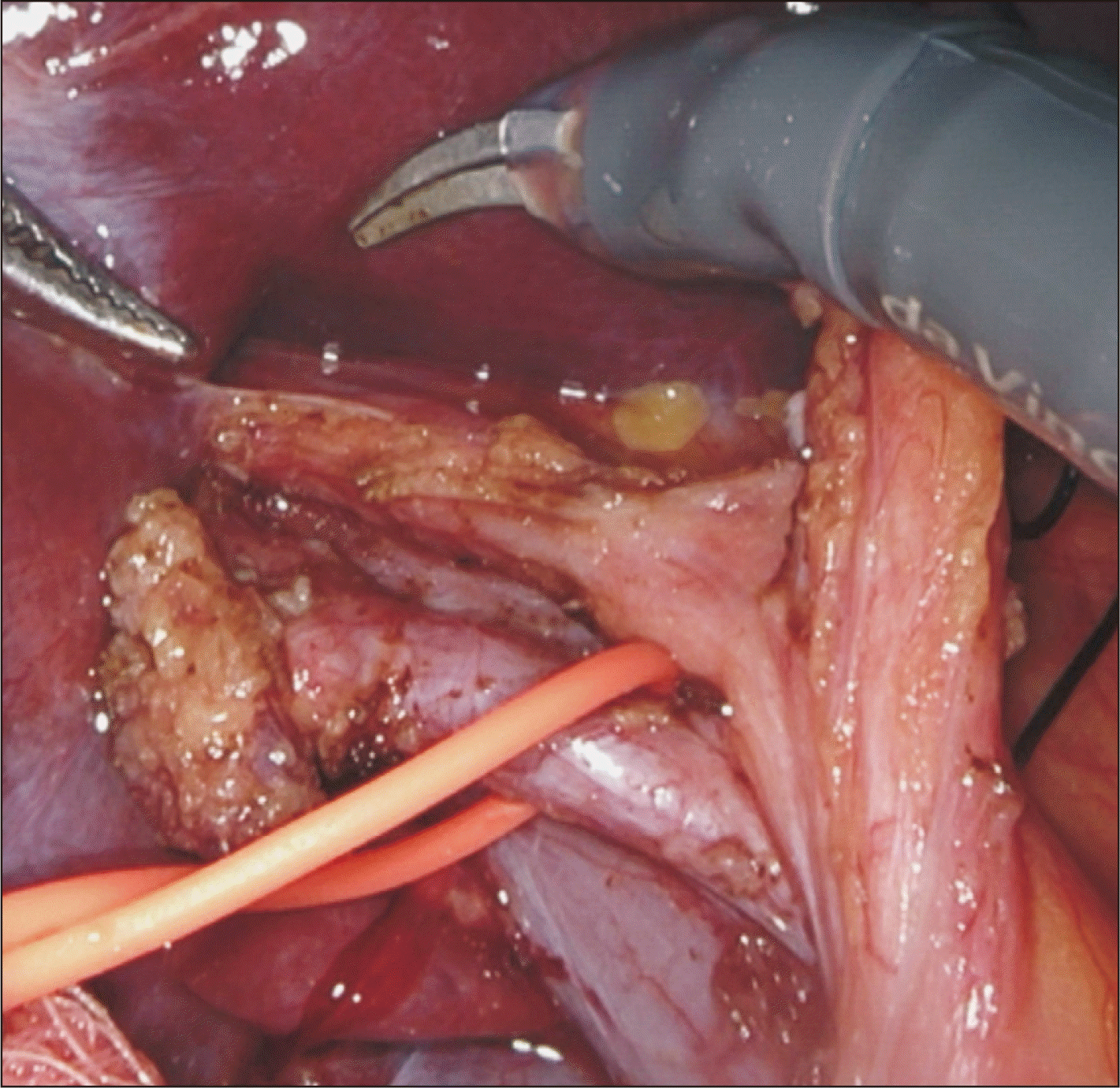
Fig. 4
Replaced right hepatic artery from the superior mesenteric vein, coursing along the lateral-posterior aspect of the common bile duct and common hepatic duct.
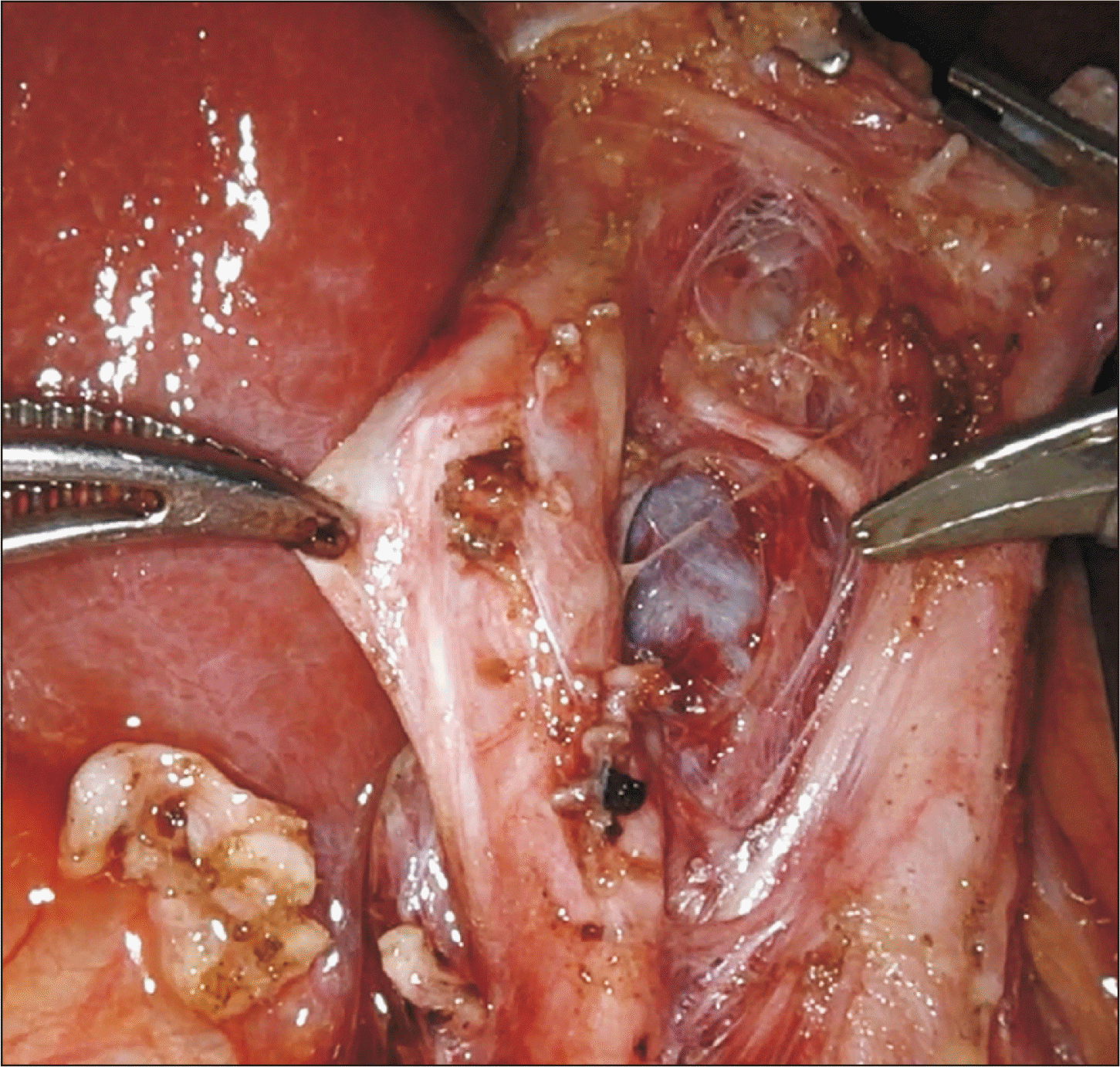
Fig. 5
Approaching the right portal vein; the anterior aspect of the portal vein bifurcation is exposed by retracting the replaced right hepatic artery toward the patient’s right.
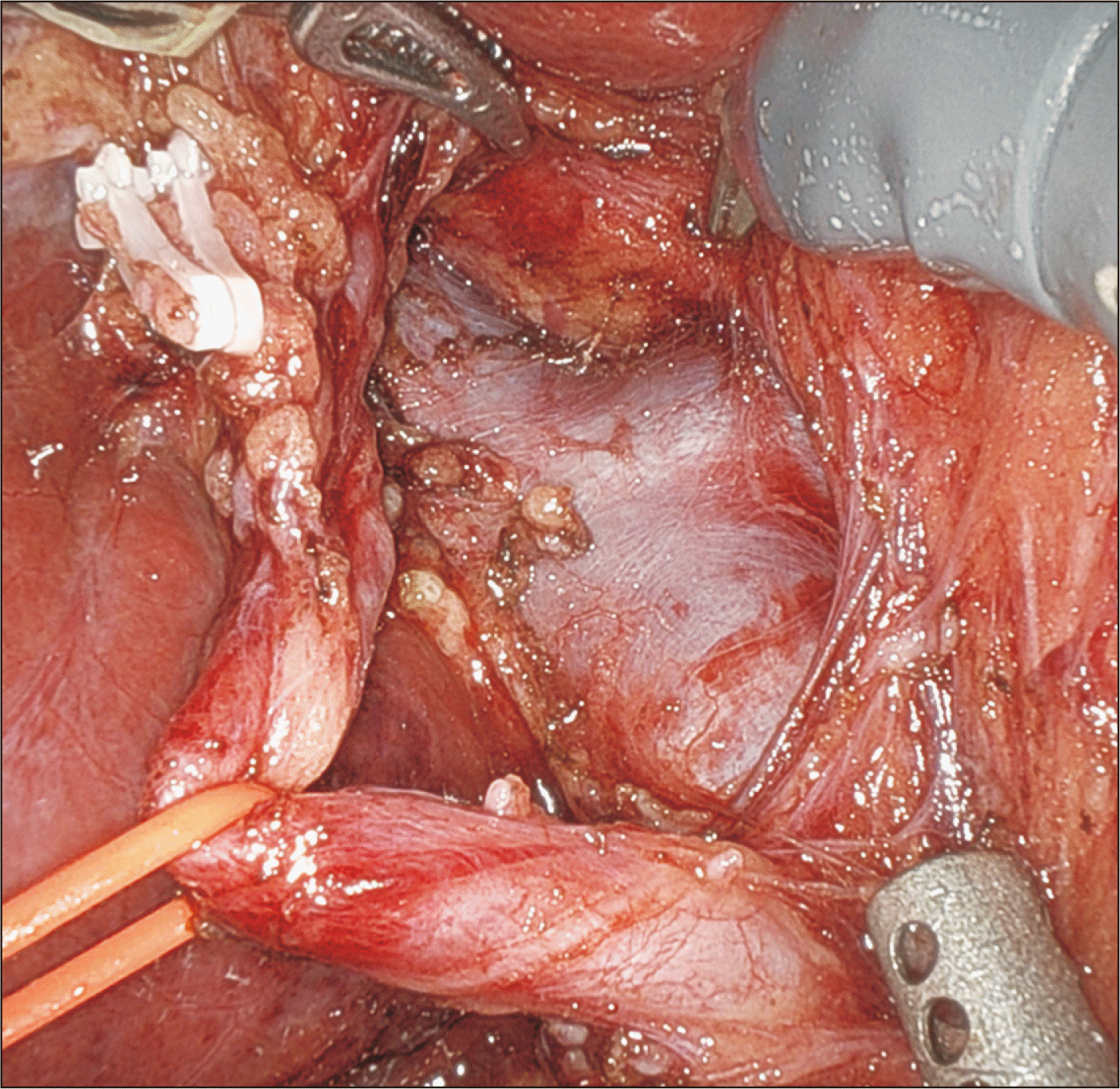
Fig. 6
Caudate branch on the posterior wall of the right portal vein encircled using a large needle driver and Maryland forceps.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download