Abstract
Post-liver transplant (LT) hepatic artery vasospasm is a vascular complication that is not well recognized and its incidence is not known. As a possible sequela to vasospasm, hepatic artery thrombosis is the second major cause of allograft failure after primary nonfunction and its reported incidence is 2.9% in adults and 8.3% in pediatric LT. Lacuna in knowledge regarding early hepatic artery vasospasm post-LT makes it a difficult condition to diagnose and treat, as the initial ischemic insult on graft can have devastating consequences. We report a case of pediatric progressive familial intrahepatic cholestasis type 3-related chronic liver disease who underwent cadaveric orthotopic LT and postoperatively developed fever, worsening hypotension, and elevated liver enzymes with an absence of arterial flow in intrahepatic branches on Doppler ultrasound. Suspecting early hepatic artery thrombosis, the patient was re-explored and the graft hepatic artery was found to be in a state of vasospasm. Following the infusion of intra-arterial papaverine, urokinase, and intravenous nicorandil, there was an improvement in blood flow. The patient responded well and was discharged on postoperative day 23 with normal liver enzymes.
Vascular complications post-liver transplant (LT), i.e., hepatic artery thrombosis (HAT), and hepatic artery stenosis (HAS) can result in allograft dysfunction with subsequent serious consequences in a posttransplant patient. HAT is the second major cause of allograft failure after primary nonfunction [1] and its reported incidence is 2.9% in adults and 8.3% in pediatric LT [2]. Although hepatic artery vasospasm has been reported in the literature [3,4], its pathophysiology is not known, nor it can be differentiated readily from HAT, anastomotic stricture, or stenosis early post-transplant. Early diagnosis with Doppler ultrasound (DUS) is critical as management varies according to the time of presentation. Suspected early HAT may need further investigation with computed tomography and angiography to confirm HAT and detect parenchymal ischemic changes [2]. In the absence of raised transaminases and parenchymal ischemia, revascularization should be attempted with or without thrombolysis; however, in the presence of definite parenchymal ischemia, rescue retransplantation may be required [2]. The decision for computed and diagnostic angiography needs careful decision making keeping in mind the time of presentation, clinical and biochemical profile of the patient, and previous intraoperative findings. In our case, surgical revascularization was attempted given the presentation on the immediate postoperative day, worsening hemodynamic instability, clinical, biochemical, and DUS corroboration of suspected early HAT. In the era of organ shortage, urgent revascularization, revision of vascular anastomosis and thrombolytics may practically be more feasible and sometimes the only option available. Herein, we present a case of hepatic artery vasospasm with allograft dysfunction post-deceased donor LT managed successfully with surgical revascularization.
Institutional Review Board approval was waived as our institute does not consider it necessary for the publication of case reports. Written informed consent was obtained from the patient's parents.
A 10-year-old child presented with an 8-month history of abdominal distension and a 1-month history of progressive yellowish discoloration of the eyes, which eventually spread to the skin, accompanied by dark urine. The child had been born from a second-degree consanguineous marriage, with the mother having had six prior fetal losses (Fig. 1). The first two children were born preterm and died within 2 weeks of birth after exhibiting jaundice. The subsequent four pregnancies had ended in miscarriage at 3 months of gestation.
The patient was classified as Child-Pugh C with a pediatric end-stage liver disease score of 18. DNA testing confirmed a diagnosis of progressive familial intrahepatic cholestasis type 3 (PFIC-3), revealing a homozygous missense variation c.2860G>A (p.Gly954Ser) in exon 23 of the ABCB4 gene of chromosome 7. This autosomal recessive mutation entails the substitution of serine for glycine at codon 954.
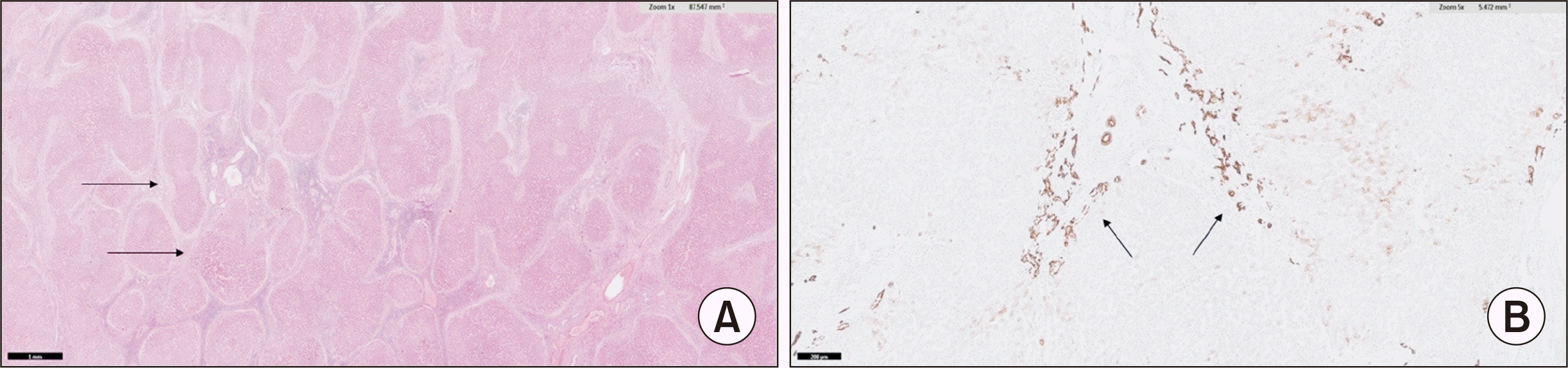
The patient received an orthotopic deceased donor LT. The surgical procedure utilized a piggyback technique for hepatic outflow, and venovenous bypass was not required. The graft liver included a single hepatic artery with right and left arterial branches, while the recipient exhibited normal arterial anatomy with an anomalous nonhepatic artery confluence. The donor and recipient hepatic arteries were anastomosed end-to-end using 8-0 polypropylene interrupted sutures after spatulation. The total cold ischemia time was 300 minutes, and the estimated blood loss was approximately 1.2 L. Intraoperative DUS revealed a hepatic artery peak systolic velocity of 45 cm/sec and a resistive index of 0.6. Postoperatively, the patient was monitored with liver function tests and DUS every 12 hours. On the first postoperative day, the patient presented with high-grade fever, worsening hypotension, elevated liver enzyme levels (Figs. 2 and 3), and absent diastolic flow in the main hepatic artery (MHA) on DUS, with no flow detectable in the intrahepatic branches. Early HAT was suspected, and the patient underwent re-exploration. During surgery, the graft hepatic artery pulsations were found to be feeble, and no kinks or twists were observed at the anastomosis. Upon partial dismantling of the hepatic artery anastomosis, no proximal or distal thrombus was found. The hepatic artery proximal to the anastomosis exhibited good forward flow. To address suspected vasospasm, 30 mg of intra-arterial papaverine, 50,000 IU of urokinase, and 1,000 IU of unfractionated heparin (diluted in 50 mL saline) were administered to the graft hepatic artery. Additionally, an intravenous infusion of nicorandil (48 mg in 50 mL saline over 48 hours) was initiated, and the partially dismantled anastomosis was reconstructed. After the procedure, the graft hepatic artery exhibited strong pulsations, and intraoperative DUS revealed a peak systolic velocity of 35 cm/sec and a resistive index of 0.7. Postoperatively, the nicorandil infusion was continued at a rate of 1 mL/hr for 48 hours, and DUS was repeated every 12 hours. The patient’s clinical status and liver enzyme levels gradually improved, inotropic support was tapered, and extubation occurred on the fourth postoperative day. Liver enzymes normalized within a week (Figs. 2 and 3), and the patient was discharged after 3 weeks on triple immunosuppression, following the established protocol. At the 12-month follow-up appointment, the DUS was normal, and the patient was doing well. The explanted liver confirmed a diagnosis of PFIC-3 (Fig. 4).
Vascular complications following LT are not uncommon, despite advancements in surgical techniques for vascular anastomosis. Early vascular complications, such as HAT and HAS, can lead to allograft dysfunction and may necessitate retransplantation. While hepatic artery vasospasm has been documented in the literature [3,4], its pathophysiology remains unclear, and it is not easily distinguishable from HAT, anastomotic stricture, or stenosis in the early posttransplant period. Poiseuille’s law states that a reduction in vessel diameter results in increased resistance to flow [5]. Vasospasm can cause a reduction in flow or complete cessation, which may lead to thrombosis. Even with a patent anastomosis, tissue perfusion can be compromised in a vessel experiencing vasospasm [6]. Vasospasm has been extensively studied in coronary arteries, both clinically and experimentally, and it may trigger thrombosis and subsequent myocardial infarction [7]. The decrease in arterial flow to the graft due to vasospasm can result in hepatic insufficiency and graft dysfunction and may promote thrombosis. In the present case, timely re-exploration and graft rescue may have occurred prior to the development of arterial thrombosis that would have otherwise led to graft loss and the need for retransplantation. The absence of diastolic flow in the MHA, intrahepatic branches, and high-resistance hepatic arterial flow (resistive index=1) shortly after LT is suggestive of hepatic arterial vasospasm, but management protocols have not yet been standardized [3]. In our case, DUS revealed no diastolic flow in the MHA and no flow in the intrahepatic branches. Factors that may contribute to vasospasm include older donor age (over 50 years) and prolonged cold ischemia time (exceeding 410 minutes) [8]. Hepatic artery vasospasm that leads to HAT suggests the involvement of the causes of early HAT, which include a pediatric recipient, small donor or recipient arteries, a split right liver graft, a neonatal donor liver, a cytomegalovirus-negative recipient, an extended cold ischemia time, a large liver graft (graft-to-recipient body weight ratio over 3%–4%), small-for-size liver syndrome (graft-to-recipient body weight ratio under 0.8%), and ABO incompatibility [2]. Our patient was a pediatric recipient who received a large liver graft (graft-to-recipient body weight ratio, 2.8%) from a 55-year-old donor. Although the cold ischemia time was a favorable 300 minutes, other factors likely contributed to the hepatic artery vasospasm and subsequent graft dysfunction. The University of Wisconsin (UW) solution for organ preservation has been reported to impair the endothelium-dependent relaxation of hepatic arteries [9]. In our case, the UW solution was utilized for organ preservation. Additionally, elevated plasma norepinephrine levels during graft retrieval, due to physiological surgical stress, may also influence arterial vasospasm and the no-flow phenomenon [10]. The reduction in arterial flow to the graft caused by vasospasm can lead to hepatic insufficiency, graft dysfunction, and potentially thrombosis.
The management of vascular complications hinges on early diagnosis and prompt treatment. DUS is considered the gold standard for investigation. For suspected early HAT, the recommended approach is to perform computed tomography with angiography to confirm the presence of HAT and to identify any ischemic changes in the parenchyma [2]. In cases involving no evidence of elevated transaminases or parenchymal ischemia, revascularization should be attempted, with or without thrombolysis. Conversely, the presence of elevated transaminases and clear parenchymal ischemia necessitates rescue retransplantation [2]. The most common clinical manifestation of early HAT is acute fulminant hepatic failure, which can occur in up to 30% of patients [11]. The case presented here, on postoperative day 1, was characterized by high-grade fever, worsening hypotension, elevated liver enzyme levels, and DUS findings consistent with early HAT. Given the immediate postoperative timing, deteriorating hemodynamic stability, and clinical, biochemical, and DUS evidence of suspected early HAT, the decision to proceed with computed or diagnostic angiography was postponed. In the context of organ scarcity, urgent revascularization, the revision of vascular anastomoses, and the use of thrombolytic agents may be the most practical and (at times) the only available option, as was the case with our patient.
Hepatic arterial vasospasm can affect both the MHA and the intraparenchymal branches to varying extents. Segmental MHA vasospasm has been documented in two patients following LT, as detected during re-exploration [12]. In the present case, the graft hepatic artery distal to the anastomotic site was in a state of vasospasm. This vasospasm manifested in our patient as fever, hypotension, and abnormal liver function tests on the first day, accompanied by elevated liver enzymes and bilirubin levels. These symptoms and laboratory findings showed significant improvements after re-exploration.
Papaverine, an opium alkaloid with spasmolytic properties, directly affects vascular smooth muscle by inhibiting oxidative phosphorylation and calcium influx. It has been shown to reverse vasospasm in the internal mammary artery and induce vasodilation following the intra-arterial administration of 30 mg of papaverine hydrochloride over 5 minutes [13]. In the present study, its use in treating hepatic artery vasospasm resulted in vasodilation, which was observed during re-exploration. A retrospective study investigating the impact of hepatic artery vasospasm on DUS in post-LT patients identified nine individuals with suspected vasospasm on DUS. These patients received a single sublingual dose of 10 mg nifedipine. The study noted antegrade diastolic flow in the MHA and its intrahepatic branches, with resistive indices decreasing from an average of 1.0 to 0.76 within 10 to 45 minutes postadministration. The researchers concluded that high-resistance arterial flow shortly after LT suggests vasospasm if it is alleviated by vasodilators [3]. In our case, nicorandil was employed as a vasodilator affecting both venous and arterial beds. It functions as a nitric oxide donor, acting through the cyclic guanosine monophosphate pathway to cause vasodilation in peripheral and coronary vessels. Additionally, as a potassium channel opener, it dilates coronary microvessels and peripheral resistance arteries [14]. Nicorandil is the second-line drug of choice for vasospastic angina after calcium channel blockers. Although no reports have described the use of nicorandil for hepatic artery vasospasm, its effectiveness in preventing coronary artery vasospasm has been demonstrated. A randomized, placebo-controlled, crossover study involving 13 patients with vasospastic angina compared the efficacy of nifedipine (10 mg) and nicorandil (30 mg). The results showed that both drugs were equally effective in eight patients, while nicorandil yielded better outcomes in five patients [14].
Intra-arterial thrombolysis for HAT, which is thought to be most effective in fresh thrombi due to their higher water content and fibrin-deficient matrix, was first described in 1989 [15]. This procedure is considered safe, with bleeding being the most severe and common complication, occurring in up to 20% of cases. Intraoperative intra-arterial thrombolysis offers several advantages, including the requirement of a lower dose of thrombolytic, the high localized concentration, and the limited impact on systemic coagulation [16]. No significant pharmacological difference exists in the efficacy of urokinase and tissue plasminogen activator (t-PA) as thrombolytics [16]. Most of the literature references the use of urokinase for HAT, while some also recommend t-PA as a first option; however, long-term patency with the use of t-PA has not been reported [17]. Additionally, unresolved anatomical issues such as kinks, twists, dissections, or stenoses at the hepatic artery anastomosis can lead to recurrent HAT. Angioplasty with stent placement following intra-arterial thrombolysis has shown more favorable results in the current literature [18]. No standard guidelines exist for the timing of thrombolysis, with recommendations varying from as early as 4 hours to as late as 3 months posttransplantation. However, there is a consensus that thrombolysis should not be attempted more than 3 months after transplantation [19]. The adjunctive use of heparin in thrombolytic treatment is generally not advised due to an increased risk of bleeding [20]. Nevertheless, we used a low dose (1,000 IU unfractionated heparin diluted in 50 mL saline) of intra-arterial heparin flush, and coagulation parameters were strictly monitored postoperatively without bleeding episodes. Although the vasospasm of the graft hepatic artery was relieved by papaverine and sustained by intravenous nicorandil, it is unclear whether urokinase had any effect on the vasospasm or its recurrence. However, its use might be justified in cases of vasospasm with a slow or no-flow state for the thrombolysis of undetectable intrahepatic microthrombi. The management of hepatic artery vasospasm, as reported, involved urgent revascularization in three patients when HAT was suspected—on day 7 in two patients and on day 3 in another. All three patients displayed successful outcomes following revascularization [12]. Another case report described suspected HAT on day 11 posttransplant; this was diagnosed as hepatic artery vasospasm on diagnostic angiography and was successfully managed with transcatheter papaverine, urokinase, and intravenous nicorandil infusion [4].
The reported outcomes following surgical revascularization for HAT indicate a 56% success rate, with the procedure attempted in 75% of adult and 54% of pediatric patients. Patients with early HAT who underwent revascularization had a higher likelihood of a successful outcome. Regular DUS was associated with improved outcomes, with a success rate of 66% compared to 45% without frequent monitoring. Pediatric patients had a higher success rate following early revascularization compared to adults, with 92% of pediatric patients and 61% of adults experiencing successful outcomes. Among those who underwent revascularization attempts, 30% required retransplantation. Retransplantation was the preferred treatment for 53% of these patients, broken down into 50% of adults and 62% of pediatric patients. The overall mortality rate was 50%, with a reported range of 30% to 70% [2].
Hepatic artery vasospasm is not a well-recognized vascular complication following LT due to the scarcity of data and the lack of standardized diagnostic criteria. The intricate interaction between donor and recipient physiology, as well as the surgical technique, is constantly advancing and varies across patients. The potential connection between hepatic artery vasospasm and HAT, as well as its prognosis shortly after transplantation with or without the administration of vasodilators, requires further investigation. Hepatic artery vasospasm may manifest as early HAT, and its management is contingent on the clinical context, necessitating the use of DUS along with diagnostic angiography. Digital subtraction angiography can assist in identifying this rare event and can also be treated with catheter-directed antispasmodics and oral vasodilators, thus averting the need for surgical re-exploration in a transplant recipient. However, surgical re-exploration for early HAT with revascularization also has a favorable prognosis, provided it is performed promptly. In cases of parenchymal ischemic injury, retransplantation remains the treatment of choice.
ARTICLE INFORMATION
Author Contributions
Conceptualization: SS. Data curation: DJ. Investigation: DJ. Methodology: SS. Project administration: SS. Supervision: BB, AB. Validation: BB, CT, AB. Visualization: BB, AB. Writing–original draft: DJ. Writing–review & editing: DJ, CT, AB. All authors read and approved the final manuscript.
REFERENCES
1. Grodzicki M, Anysz-Grodzicka A, Remiszewski P, Cieślak B, Kotulski M, Kalinowski P, et al. 2011; Treatment of early hepatic artery thrombosis after liver transplantation. Transplant Proc. 43:3039–42. DOI: 10.1016/j.transproceed.2011.08.028. PMID: 21996219.

2. Heaton ND. 2013; Hepatic artery thrombosis: conservative management or retransplantation? Liver Transpl. 19 Suppl 2:S14–6. DOI: 10.1002/lt.23739. PMID: 24019107.

3. Chen W, Facciuto ME, Rocca JP, Marvin MR, Sheiner PA, Rachlin S, et al. 2006; Doppler ultrasonographic findings on hepatic arterial vasospasm early after liver transplantation. J Ultrasound Med. 25:631–8. DOI: 10.7863/jum.2006.25.5.631. PMID: 16632787.

4. Behera A, Kaman L, Dahiya D, Tandup C, Kalra N. 2022; Hepatic artery vasospasm masquerading as hepatic artery thrombosis in a case of deceased donor liver transplant. J Clin Exp Hepatol. 12:654–7. DOI: 10.1016/j.jceh.2021.09.004. PMID: 35535101. PMCID: PMC9077215.

5. Carter SA. Zwiebel WJ, editor. Hemodynamic considerations in peripheral and cerebrovascular disease. Introduction to vascular ultrasonography. 4th ed. WB Saunders;2000. p. 3–17.
6. Richards RR, Seaber AV, Urbaniak JR. 1985; Chemically induced vasospasm: the effect of ischemia, vessel occlusion, and adrenergic blockade. Plast Reconstr Surg. 75:238–44. DOI: 10.1097/00006534-198502000-00016. PMID: 3969410.

7. Hellstrom HR. 1979; Evidence in favor of the vasospastic cause of coronary artery thrombosis. Am Heart J. 97:449–52. DOI: 10.1016/0002-8703(79)90391-0. PMID: 425878.

8. García-Criado A, Gilabert R, Salmerón JM, Nicolau C, Vilana R, Bianchi L, et al. 2003; Significance of and contributing factors for a high resistive index on Doppler sonography of the hepatic artery immediately after surgery: prognostic implications for liver transplant recipients. AJR Am J Roentgenol. 181:831–8. DOI: 10.2214/ajr.181.3.1810831. PMID: 12933490.
9. Jeng LB, Lin PJ, Yao PC, Chen MF, Tsai KT, Chang CH. 1997; Impaired endothelium-dependent relaxation of human hepatic arteries after preservation with the University of Wisconsin solution. Arch Surg. 132:7–12. DOI: 10.1001/archsurg.1997.01430250009001. PMID: 9006546.

10. Acosta F, Diaz J, Sansano T, Palenciano CG, Reche M, Beltran R, et al. 2000; Evolution of the plasma concentration of norepinephrine in cirrhotic patients during liver transplantation. Transplant Proc. 32:2659–60. DOI: 10.1016/S0041-1345(00)01829-7. PMID: 11134749.

11. Pareja E, Cortes M, Navarro R, Sanjuan F, López R, Mir J. 2010; Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 42:2970–2. DOI: 10.1016/j.transproceed.2010.07.063. PMID: 20970585.

12. Sakamoto Y, Harihara Y, Nakatsuka T, Kawarasaki H, Takayama T, Kubota K, et al. 1999; Rescue of liver grafts from hepatic artery occlusion in living-related liver transplantation. Br J Surg. 86:886–9. DOI: 10.1046/j.1365-2168.1999.01166.x. PMID: 10417559.

13. Hillier C, Watt PA, Spyt TJ, Thurston H. 1992; Contraction and relaxation of human internal mammary artery after intraluminal administration of papaverine. Ann Thorac Surg. 53:1033–7. DOI: 10.1016/0003-4975(92)90382-E. PMID: 1596124.

14. Lablanche JM, Bauters C, Leroy F, Bertrand ME. 1992; Prevention of coronary spasm by nicorandil: comparison with nifedipine. J Cardiovasc Pharmacol. 20 Suppl 3:S82–5. DOI: 10.1097/00005344-199206203-00014. PMID: 1282182.
15. Hidalgo EG, Abad J, Cantarero JM, Fernández R, Parga G, Jover JM, et al. 1989; High-dose intra-arterial urokinase for the treatment of hepatic artery thrombosis in liver transplantation. Hepatogastroenterology. 36:529–32.
16. Semba CP, Bakal CW, Calis KA, Grubbs GE, Hunter DW, Matalon TA, et al. 2000; Alteplase as an alternative to urokinase. J Vasc Interv Radiol. 11:279–87. DOI: 10.1016/S1051-0443(07)61418-3. PMID: 10735420.

17. Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. 2003; Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 9:612–20. DOI: 10.1053/jlts.2003.50098. PMID: 12783404.

18. Breguet R, Dondero F, Pupulim L, Goossens N, Sepulveda A, Francoz C, et al. 2019; Endovascular treatment of arterial complications after liver transplantation: long-term follow-up evaluated on Doppler ultrasound and magnetic resonance cholangiopancreatography. Cardiovasc Intervent Radiol. 42:381–8. DOI: 10.1007/s00270-018-2108-8. PMID: 30411152.

19. Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. 2010; Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 23:245–56. DOI: 10.1111/j.1432-2277.2009.01037.x. PMID: 20030796.

20. Decrinis M, Pilger E, Stark G, Lafer M, Obernosterer A, Lammer J. 1993; A simplified procedure for intra-arterial thrombolysis with tissue-type plasminogen activator in peripheral arterial occlusive disease: primary and long-term results. Eur Heart J. 14:297–305. DOI: 10.1093/eurheartj/14.3.297. PMID: 8458348.

Fig. 1
Pedigree chart depicting the inheritance of progressive familial intrahepatic cholestasis type 3 in our patient.
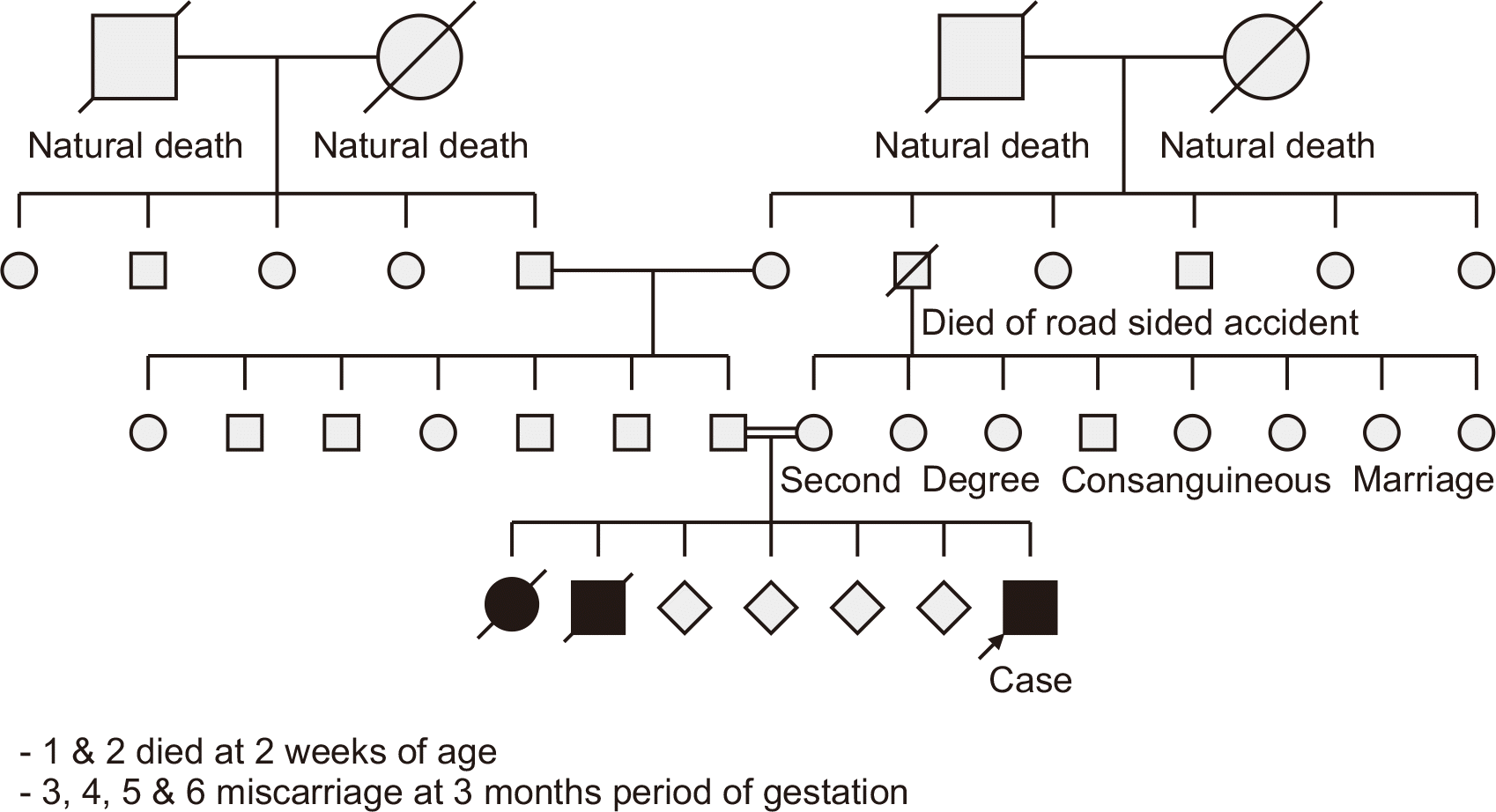
Fig. 2
Postoperative trend of liver enzyme levels. The black arrows indicate re-exploration. AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; PREOP, preoperative; POD, postoperative day.
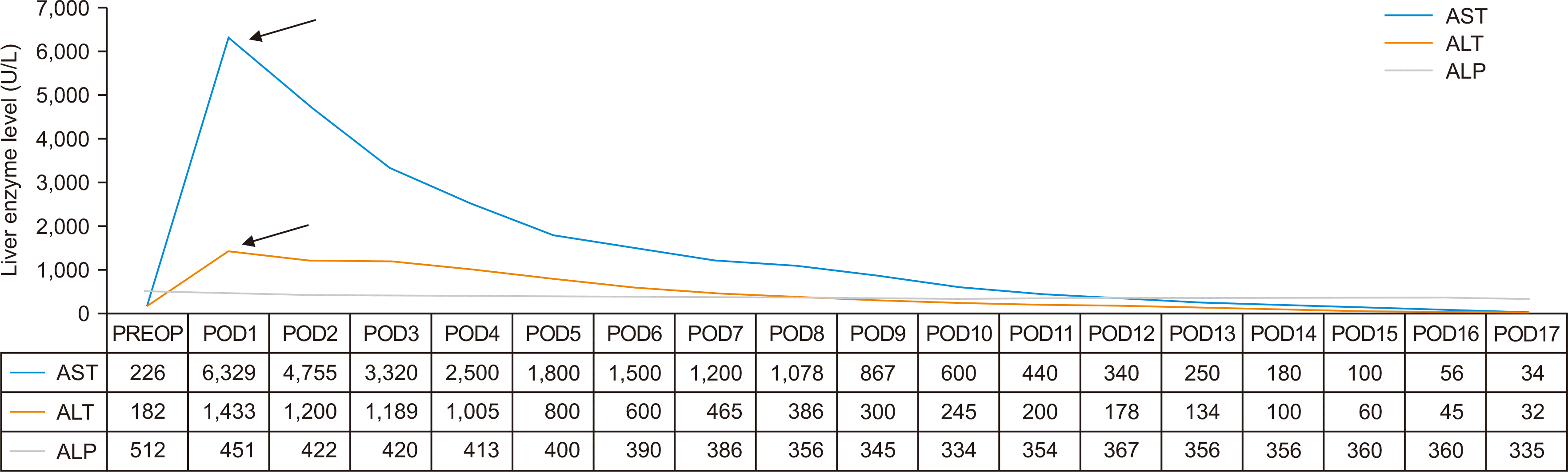
Fig. 3
Postoperative trend of bilirubin. The black arrows indicate re-exploration. PREOP, preoperative; POD, postoperative day.
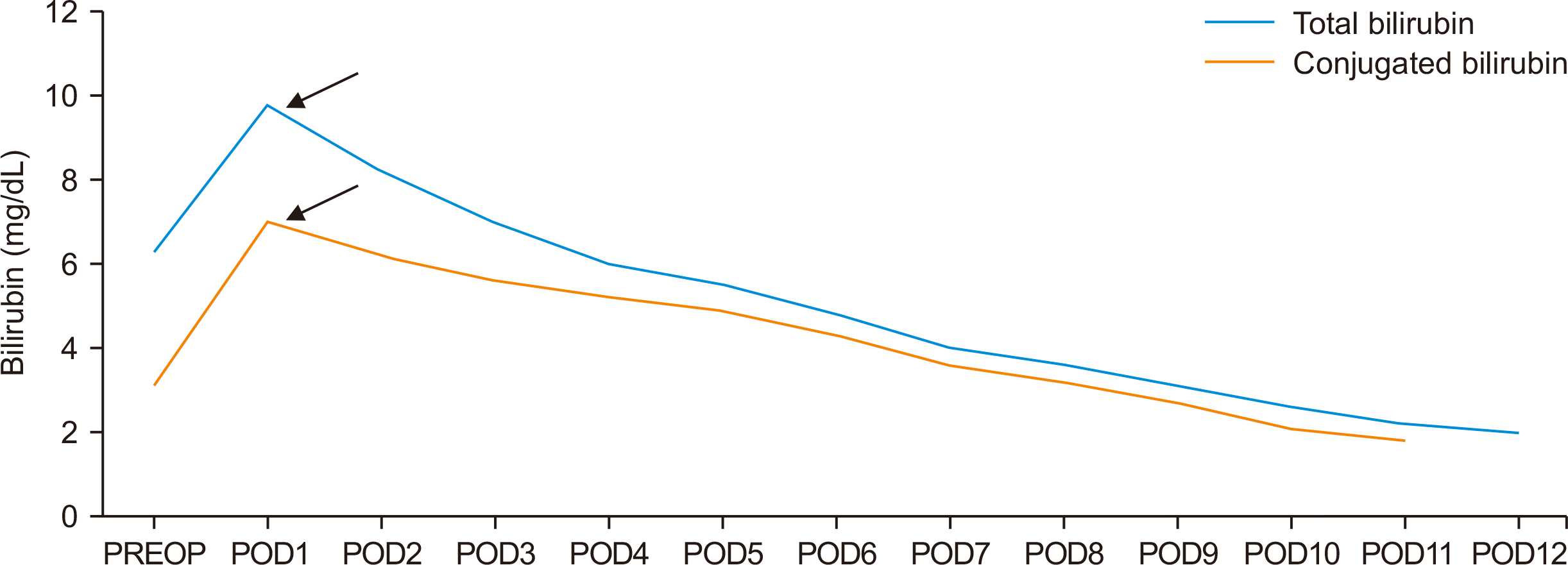
Fig. 4
(A) H&E staining (×400) showing multiple nodules arranged in a typical jigsaw-puzzle-pattern surrounded by fibrosis. The fibrous septa are predominantly porto-portal, have a central area of collagenization and lymphocyte-rich inflammatory infiltrate, and peripheral area of edema giving rise to a "halo" effect (arrows). (B) CK7 immunostaining (×400) showing exuberant ductular reaction without any ductopenia and ductular metaplasia along with copper retention, ductular cholestasis, and maintained reticulin pattern (arrows).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download