Abstract
To address a donor kidney shortage, marginal grafts have been applied in deceased donor kidney transplantation (DDKT). These grafts exhibit comparatively unfavorable outcomes, particularly when cold ischemia time (CIT) is prolonged. Hypothermic machine perfusion (HMP) has been investigated to mitigate the effects of prolonged CIT during graft transport. The present case involved successful management of the longest CIT recorded in Korea by employing HMP in DDKT. The donor was a 54-year-old man (Korean Kidney Donor Profile Index, 82%) with diabetes. The recipient, a 51-year-old man on peritoneal dialysis, had end-stage renal disease secondary to diabetic nephropathy. Following procurement, the left kidney was preserved using HMP. Inclement weather delayed graft transportation; consequently, the total CIT was 28 hours and 6 minutes, with the kidney preserved by HMP for 22 hours and 35 minutes. Postoperative graft function gradually recovered, and urine output was satisfactory. Delayed graft function was not observed, and the patient was discharged on postoperative day 13 without significant complications. Five months after surgery, his serum creatinine level was 1.7 mg/dL. Successful DDKT with a marginal donor graft via HMP, despite the longest CIT yet observed in Korea, underscores the usefulness of HMP in enhancing graft quality and preserving function.
Kidney transplantation is the primary treatment for patients with end-stage renal disease, offering improved survival rates and quality of life compared to dialysis. However, a persistent major limitation is the scarcity of donor grafts relative to the number of recipients. To address this challenge, the use of marginal grafts from extended criteria donors and donors after cardiac death (DCD) has become increasingly common [1,2]. Prolonged cold ischemia time (CIT), arising from conditions including distance between donor and recipient, has been associated with a heightened risk of acute tubular necrosis, delayed graft function (DGF), and graft failure, particularly with marginal donors [3].
To address these challenges, researchers have been investigating preservation techniques that may mitigate issues associated with marginal donor grafts and prolonged CIT. Hypothermic machine perfusion (HMP) is a promising preservation method relative to traditional static cold storage (SCS), particularly for marginal grafts subjected to prolonged CIT [4–6]. Consequently, HMP is increasingly being adopted as the standard approach for deceased donor kidney transplantation (DDKT) involving marginal donor grafts with extended CIT [6].
We present a case of successful DDKT despite the longest CIT recorded in Korea, accomplished with the use of HMP.
This study received approval from the Institutional Review Board of Jeju National University Medical Center (IRB No. JEJUNUH 2023-05-009). Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
A 51-year-old man with chronic kidney disease secondary to diabetic nephropathy and underlying coronary artery disease was admitted for DDKT after being on peritoneal dialysis for 11 years. The donor was a 54-year-old man with diabetes and a Korean Kidney Donor Profile Index (K-KDPI) of 82%. We were allocated the left kidney graft, which had a single renal artery and vein. Notably, the left renal vein was shorter than average, approximately 2 cm in length, due to damage sustained during procurement. At the bench following the procurement operation, the renal artery and vein of the donor graft were carefully trimmed; then, the kidney was placed in a hypothermic perfusion machine, as opposed to traditional SCS. This approach was intended to minimize ischemic injury to the kidney. For this process, a portable hypothermic perfusion device (LifePort Kidney Transporter; Organ Recovery Systems) was used (Fig. 1). The sequence for loading the graft kidney into the pump was as follows. (1) Isolate the vascular structure of the kidney. (2) Cannulate the kidney, employing a cannula that best suits the size and shape of the renal artery. When multiple renal arteries are present, cannulation is feasible for those with a diameter of 3 mm or larger. (3) Position the kidney within the kidney cradle. Modify the height of the cannula mount and the cannula’s rotation to ensure the vessel is comfortably situated. Examine the artery for any signs of twisting or occlusion. (4) Place the kidney cradle within the kidney transporter. (5) Prime and initiate perfusion. (6) Monitor the kidney parameters. Pay close attention to the outer display; this conveys key information regarding perfusion status, including pressure, flow, resistance, and temperature.
The delivery of the procured kidney graft to the recipient hospital was delayed by weather-related issues. Consequently, the graft was preserved under HMP for 22 hours and 35 minutes, with a cumulative CIT of 28 hours and 6 minutes.
At the recipient bench, the kidney was removed from the HMP device, and trimming was completed. Induction therapy with antithymocyte globulin was administered to the recipient based on his human leukocyte antigen matching status (which showed an A1B1DR1 mismatch) and the heightened risk associated with the donor graft. The left kidney, weighing 384 g and featuring a single renal artery and vein, was anastomosed to the recipient’s right external iliac artery and vein. All surgical procedures were completed without complications.
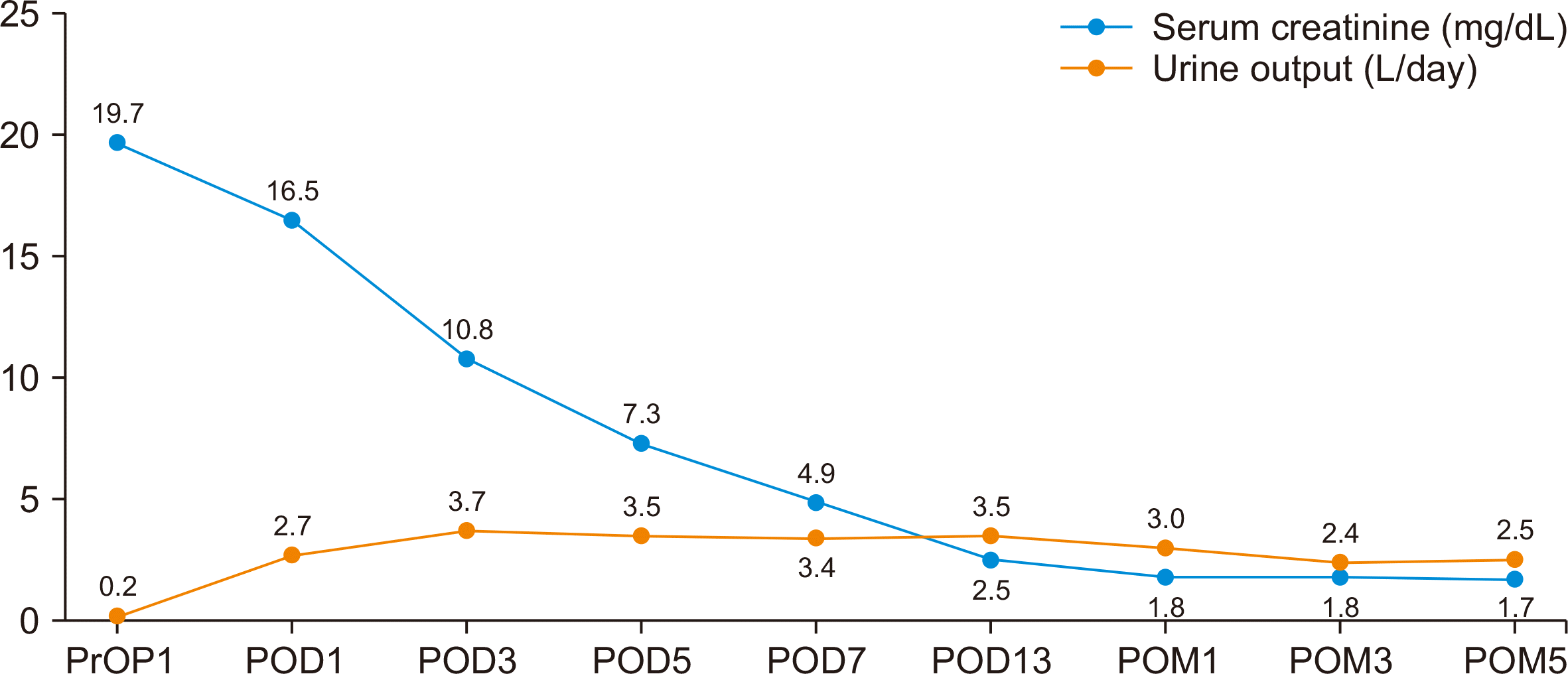
The patient demonstrated good postoperative progress, exhibiting adequate daily urine output beginning on postoperative day (POD) 1. His serum creatinine level showed a gradual decline without evidence of DGF. Doppler ultrasound assessments conducted on POD 1 and POD 12 revealed resistive indices within the normal range (0.65 on POD 1 and 0.67 on POD 12) and displayed satisfactory waveforms (Fig. 2). The patient was discharged on POD 13 without event. During subsequent follow-up examinations at the clinic, his serum creatinine levels were recorded at 1.8 mg/dL and 1.7 mg/dL in postoperative months 3 and 5 (Fig. 3), respectively, with additional laboratory results remaining within normal limits.
As the demand for kidney donor grafts rises, the use of marginal grafts has become increasingly common, necessitating measures to preserve their viability and function. HMP is regarded as a method that may yield superior outcomes. HMP involves a system that delivers cold perfusate, supplying nutrients and oxygen through extracorporeal circulation. This process also prevents the accumulation of toxic metabolic byproducts that are closely associated with organ apoptosis, while improving graft function compared to traditional SCS [7,8]. Technological advancements have led to the development of HMP devices that are compact enough to be carried by a single individual during organ transport [9].
A 2009 randomized controlled study indicated that HMP reduced the incidence of DGF and improved 1-year graft survival rates relative to SCS [5]. Furthermore, a 2022 study from the Netherlands revealed a significantly lower incidence of DGF among patients who received machine perfusion after its introduction, as opposed to a historical cohort that relied on conventional SCS. While no significant difference was noted in graft survival between the cohorts over a 2-year period, the safety and feasibility of HMP were adequately demonstrated. Based on these promising results, HMP was adopted as the national standard for all types of DDKT in the Netherlands starting in 2021 [6].
The island of Jeju, where this report took place, faces several challenging circumstances compared to the Korean mainland. First, the waiting list score for potential recipients is lower because patients registered at Jeju National University Hospital have a shorter average registration period with the Korea Network for Organ Sharing, at 17.5 months. Consequently, kidneys from donors with a relatively high K-KDPI or from extended criteria donors are more frequently allocated, leading to hesitancy in proceeding with kidney transplants. Since the introduction of HMP in Jeju, we have documented the first use of HMP in Korea for patients with marginal donor grafts, demonstrating feasible short-term outcomes [10]. Second, Jeju’s unique geographical location contributes to prolonged CIT due to transportation limitations. It is well-documented that a CIT exceeding 18 hours increases the risk of acute tubular necrosis and DGF, particularly in grafts from extended criteria donors [1,11,12]. A recent study from Italy compared outcomes between HMP (median CIT, 29 hours 57 minutes) and SCS (median CIT, 11 hours 25 minutes). The two groups showed no significant difference in the incidence of DGF (17.0% vs. 12.2%, P=0.756) and displayed similar rates of postoperative complications and 1-year graft survival. These findings suggest that HMP can effectively mitigate the issues associated with prolonged CIT. Moreover, they support broadening the use of HMP to include not only cases involving DCD or extended CIT but also cases with deceased donor grafts in general [13].
In the present study, the graft was harvested from a 54-year-old male donor with underlying diabetes and a K-KDPI of 82%. The left kidney graft was preserved in a portable HMP device for 22 hours and 35 minutes, with a total CIT of 28 hours and 6 minutes. This CIT was markedly longer than the national average due to transportation delays caused by adverse weather conditions. Specifically, this represented the longest CIT recorded in Korea, as verified by the National Institute of Organ, Tissue, and Blood Management. The transplant procedures were uneventful, and the recipient exhibited favorable postoperative progress without DGF.
To validate the impact of HMP on prolonged CIT, it is necessary to examine a greater number of cases from multiple centers. We anticipate that the increasing body of research on machine perfusion will lead to large-scale studies in Korea, specifically focusing on DDKT utilizing HMP. Additionally, future research should include biochemical and pathological investigations to elucidate the metabolic effects of graft protection, ideally through collaboration with specialized laboratories.
The present case, involving the application of HMP during the longest CIT yet seen in Korea, indicates that HMP can safely preserve graft function and may be beneficial in mitigating the detrimental effects of prolonged CIT, as evidenced by the short-term outcomes following kidney transplantation.
REFERENCES
1. Heilman RL, Smith ML, Kurian SM, Huskey J, Batra RK, Chakkera HA, et al. 2015; Transplanting kidneys from deceased donors with severe acute kidney injury. Am J Transplant. 15:2143–51. DOI: 10.1111/ajt.13260. PMID: 25808278.

2. Pascual J, Zamora J, Pirsch JD. 2008; A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 52:553–86. DOI: 10.1053/j.ajkd.2008.06.005. PMID: 18725015.

3. Hernández D, Estupiñán S, Pérez G, Rufino M, González-Posada JM, Luis D, et al. 2008; Impact of cold ischemia time on renal allograft outcome using kidneys from young donors. Transpl Int. 21:955–62. DOI: 10.1111/j.1432-2277.2008.00708.x. PMID: 18564990.

4. Kruszyna T, Richter P. 2021; Hypothermic machine perfusion of kidneys compensates for extended storage time: a single intervention with a significant impact. Transplant Proc. 53:1085–90. DOI: 10.1016/j.transproceed.2021.01.022. PMID: 33579549.

5. Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. 2009; Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 360:7–19. DOI: 10.1056/NEJMoa0802289. PMID: 19118301.

6. Brat A, de Vries KM, van Heurn EW, Huurman VA, de Jongh W, Leuvenink HG, et al. 2022; Hypothermic machine perfusion as a national standard preservation method for deceased donor kidneys. Transplantation. 106:1043–50. DOI: 10.1097/TP.0000000000003845. PMID: 34172648. PMCID: PMC9038234.

7. Lo Faro ML, Akhtar MZ, Boffa C, Ploeg R. 2015; Should pulsatile preservation be the gold standard in kidney transplantation? Curr Transpl Rep. 2:105–12. DOI: 10.1007/s40472-015-0063-8.

8. Henry SD, Guarrera JV. 2012; Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando). 26:163–75. DOI: 10.1016/j.trre.2011.09.001. PMID: 22074785.

9. Yuan X, Theruvath AJ, Ge X, Floerchinger B, Jurisch A, García-Cardeña G, et al. 2010; Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int. 23:561–70. DOI: 10.1111/j.1432-2277.2009.01047.x. PMID: 20074082.

10. Shin YH, Lee T, Chang WB. 2022; The significance of the first living donor kidney transplantation in Jeju: a case report. Korean J Transplant. 36:231–5. DOI: 10.4285/kjt.22.0033. PMID: 36275993. PMCID: PMC9574429.

11. Kayler LK, Srinivas TR, Schold JD. 2011; Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 11:2657–64. DOI: 10.1111/j.1600-6143.2011.03817.x. PMID: 22051325.

12. Lum EL, Homkrailas P, Abdalla B, Danovitch GM, Bunnapradist S. 2022; Cold ischemia time, kidney donor profile index, and kidney transplant outcomes: a cohort study. Kidney Med. 5:100570. DOI: 10.1016/j.xkme.2022.100570. PMID: 36632197. PMCID: PMC9827060.

13. Adani GL, Pravisani R, Tulissi P, Isola M, Calini G, Terrosu G, et al. 2021; Hypothermic machine perfusion can safely prolong cold ischemia time in deceased donor kidney transplantation: a retrospective analysis on postoperative morbidity and graft function. Artif Organs. 45:516–23. DOI: 10.1111/aor.13858. PMID: 33210745.

Fig. 1
Hypothermic machine perfusion of a kidney graft, revealing an increase in flow rate and a decrease in resistance. The parameters displayed, from left to right, include flow pressure (mmHg, high/low), flow rate (mL/min), resistance (mmHg/mL/min), temperature (°C, ice/container), and patient information.
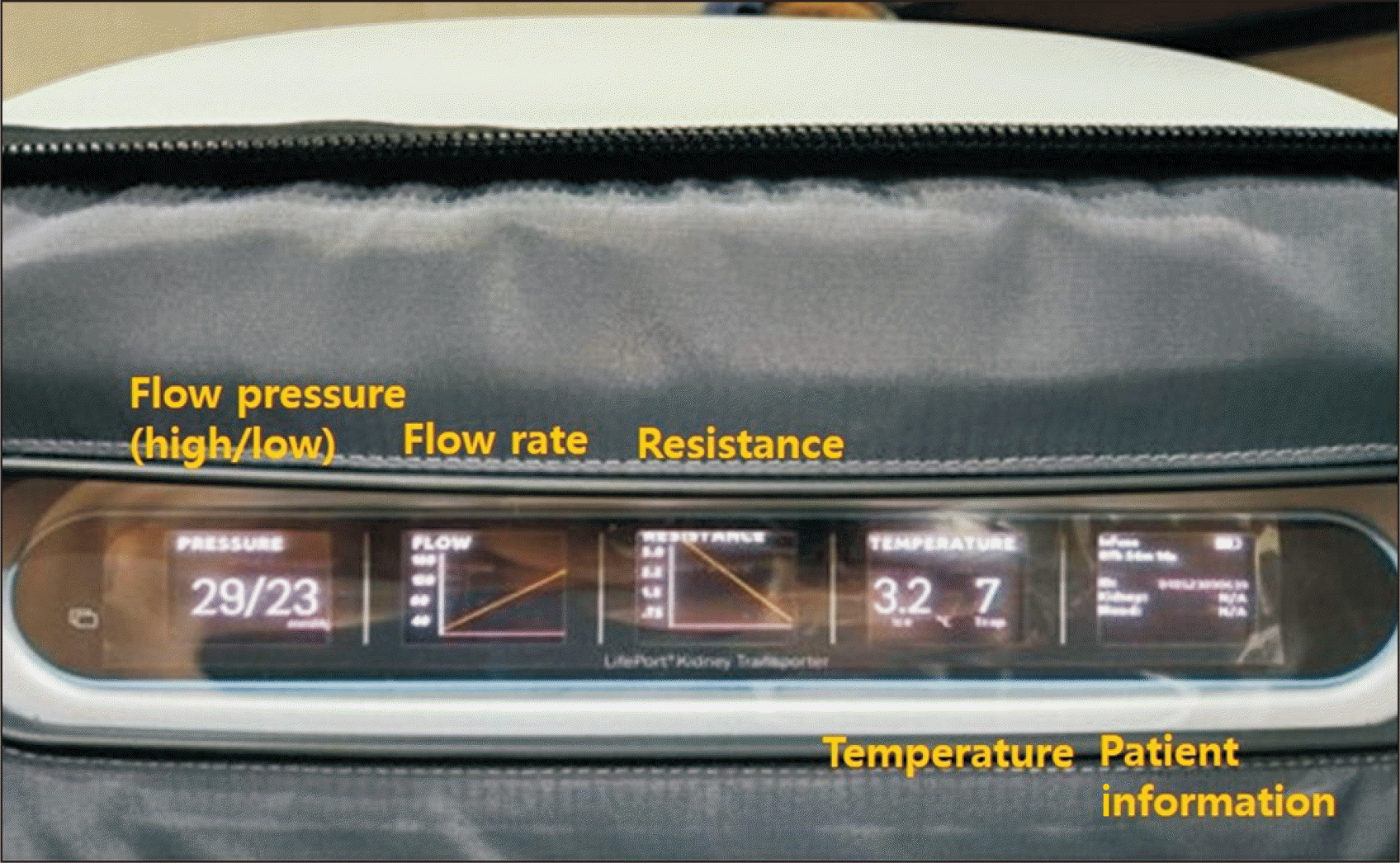
Fig. 2
Kidney Doppler results from (A) postoperative day (POD) 1 and (B) POD 12, which demonstrate satisfactory vascular flow. The resistive index (RI) values fall within the normal range, with an RI of 0.65 on POD 1 and 0.67 on POD 12.
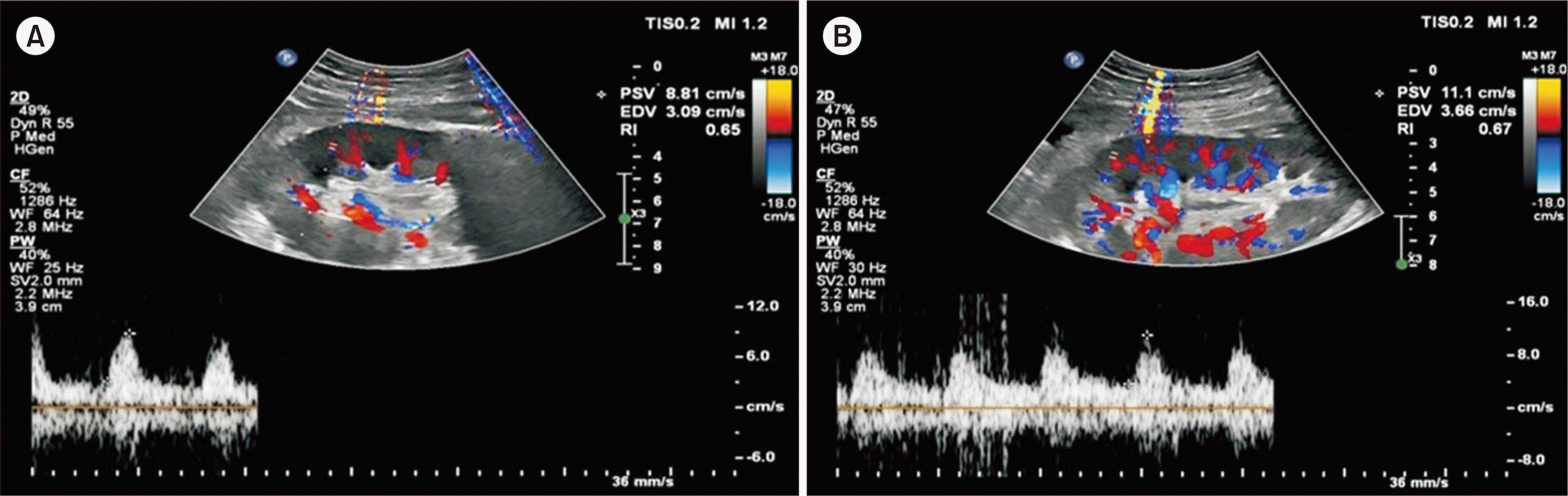
Fig. 3
Preoperative and postoperative urine output and creatinine levels of the recipient. The data indicate adequate daily urine output, and a gradual decrease was observed in serum creatinine levels from preoperative day (PrOP) 1 onward, without delayed graft function. POD, postoperative day; POM, postoperative month.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download