Abstract
Heart transplantation (HTx) outcomes have improved with careful donor selection and management; nonetheless, donor shortages remain a major challenge. Optimizing donor management is crucial for improving donor utility rates and post-HTx outcomes. Brain death leads to various pathophysiological changes that can affect multiple organs, including the heart. Understanding these alterations and corresponding management strategies is key to optimizing the donor organ condition. This review assesses several aspects of these pathophysiological changes, including hemodynamic and endocrinological considerations, and emphasizes special consideration for potential cardiac donors, including serial echocardiographic evaluations for reversible cardiac dysfunction and coronary assessments for donors with risk factors.
Recently, 180 heart transplant procedures have been performed annually in South Korea [1]. In recent decades, heart transplantation (HTx) outcomes have consistently improved [2], which can be attributed to careful donor selection and advancements in donor management. Donor-related factors significantly influence post-HTx outcomes [3]. Preferred donor criteria include younger age, fewer comorbidities, and minimal size mismatch. These factors are considered nonmodifiable, as opposed to potentially modifiable factors such as reversible cardiac dysfunction and dosage of vasoactive/inotropic agents.
With a waitlist of over 1,000 transplant candidates and an annual transplant volume of around 180 cases, South Korea faces a severe donor shortage, with a utilization rate of 31.4% [4]. Thus, the appropriate management of potential cardiac donors is crucial for improving both the donor utilization rate and posttransplant outcomes. Nevertheless, practical challenges arise in clinical settings due to competing demands for the pretransplant care of various donor organs, potentially limiting the utilization rate of donor hearts. Therefore, this review explores optimal management strategies for potential heart donors to enhance donor utilization rates and post-HTx outcomes.
Brain death (BD) leads to numerous physiological changes that can significantly impact various organs. Understanding these processes during BD is essential for optimizing the condition of organs eligible for donation. In the early stage of BD, brain edema develops, leading to increased intracranial pressure and subsequent brain herniation, resulting in pontine ischemia. Cerebral ischemia triggers an exaggerated sympathetic surge in the brainstem to maintain adequate perfusion pressure, referred to as a catecholamine storm [5]. During this period, the circulating catecholamine level can be increased several-fold, leading to significant increases in the arterial blood pressure and heart rate [6], coronary artery constriction, and subsequent myocardial ischemia [7,8]. Excess catecholamines also can directly affect the myocardium adversely [9]. These responses are generally brief—usually taking place within an hour—after which catecholamine secretion ceases, leading to a prolonged period of hypotension [10]. This hypotension results from brain herniation into the foramen magnum due to increased intracranial pressure [11], along with subsequent impairment of vasomotor responses and loss of sympathetic tone. During this period, organ perfusion pressures may decline, potentially resulting in damage and functional impairment of various organs.
Furthermore, ischemia of the hypothalamic-pituitary axis (HPA) results in reduced secretion of hormones, including cortisol and vasopressin [12,13], which in turn causes fluid loss, impaired vascular responses, and exacerbation of hemodynamic instability. The impairment of thermoregulatory function in the brainstem reduces the metabolic rate of organs, potentially contributing to donor organ dysfunction [14]. This dysfunction can exacerbate vascular relaxation, worsening hypotension. Unregulated hypothermia can also lead to myocardial dysfunction, arrhythmias, and coagulation abnormalities.
During BD, a systemic inflammatory response occurs; this response is primarily due to ischemia-reperfusion injury and leads to organ dysfunction [15,16]. Several studies have reported increased serum levels of various proinflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-1, and IL-8, along with elevated expression of adhesion molecules in organs [17,18]. This sustained systemic inflammatory response has been associated with a functional decline in transplanted organs [19]. Lastly, various hormonal changes can induce hyperglycemia by promoting gluconeogenesis, peripheral insulin resistance, and damage to pancreatic islets in BD donors. Studies have suggested that direct organ dysfunction can be caused by hyperglycemia in individuals experiencing BD, and hyperglycemia-induced glycosuria can contribute to osmotic diuresis, hypovolemia, and subsequent hypotension [20–22]. However, it remains unclear whether maintaining euglycemia with insulin therapy is beneficial for HTx outcomes [23].
The management of potential cardiac donors is crucial for ensuring the success of HTx. This involves a comprehensive approach that addresses the various pathophysiological changes induced by BD. The primary goals are to maintain hemodynamic stability, ensure adequate oxygenation, and manage the systemic inflammatory response and hormonal imbalances (Fig. 1). That is, the aim of management is not only to preserve optimal cardiac function for transplantation, but also to support the viability of other potential donor organs. This section outlines key considerations in the management of potential cardiac donors, including hemodynamic, endocrinological, respiratory, and hematological aspects.
During the initial catecholamine surge in early BD, concomitant hypertension and tachycardia occur, which are typically short-lived and can be managed using short-acting intravenous beta-blockers [24]. Subsequent treatment for sustained hypotensive responses is crucial and begins with an evaluation of the appropriate volume status. Invasive hemodynamic monitoring should be performed to assess the volume status accurately, which requires the insertion of a central venous catheter via the internal jugular or subclavian vein [25]. In South Korea, physicians are often reluctant to insert additional invasive monitoring devices in donors. However, once potential donors are confirmed to be brain-dead, the focus of management shifts from a patient-centered to an organ-centered approach. In this regard, new central and arterial lines should be inserted for the proper monitoring of hemodynamic parameters if they are not already present. This step is essential for optimal fluid resuscitation. Furthermore, it may also be necessary to measure pulmonary artery occlusion pressure and cardiac output through the placement of a pulmonary artery catheter.
Adequate volume replacement can contribute to improved procurement rates and posttransplant outcomes by maintaining perfusion pressure to organs, reducing the required dose of vasoactive agents. However, adequate fluid resuscitation is often hampered by concerns regarding the opposing effects for the utilization of kidney and lung allografts. Therefore, invasive hemodynamic monitoring is essential to maximize organ utilization through an accurate assessment of volume status [26]. An appropriate central venous pressure (CVP) target of 6–10 mmHg is generally recommended (Table 1) [27]. This is supported by previous studies reporting that maintaining the CVP below 6 mmHg did not impact posttransplant outcomes for renal allografts, and the procurement rate of thoracic organs was improved when the CVP was maintained below 10 mmHg [26]. When lung grafts are not utilized for transplantation, a more liberal fluid therapy and a different CVP target may be considered. It has not been established which type of fluid is preferred for fluid resuscitation. Crystalloid solutions, including 0.45% saline and lactated Ringer solution, are generally used and should be tailored to the donor's condition [25]. Hyperchloremic metabolic acidosis precludes the use of 0.9% saline. Colloid solutions, such as 5% albumin, are typically administered in bolus form for the purpose of rapid correction of volume status.
The use of vasoactive agents is often necessary to manage persistent hypotension despite fluid resuscitation. Norepinephrine and dopamine are vasoactive agents that have been traditionally used for this purpose. While maintaining appropriate blood pressure with these agents may be necessary to sustain optimal organ function until procurement, the excessive use of vasoactive agents can increase oxygen consumption and reduce the myocardial energy reserve [10]. A high dose of a vasoactive or inotropic agent may lead to cardiac allograft dysfunction after HTx [28], and, if possible, should be avoided. The concomitant use of vasopressin can be highly effective and often rapidly restores hemodynamic stability. Diabetes insipidus (DI) occurs in up to 80% of donors, and can exacerbate hemodynamic instability through hypovolemia and a vasoplegic response [29]. In donors with polyuria exceeding 3–4 L/day, elevated serum osmolarity, inappropriately diluted urine (urine osmolarity <200 mOsm/kg), and hypernatremia (Na >145 mmol/L), DI should be suspected and vasopressin replacement must be considered [25,30]. Vasopressin replacement has been reported to reduce or even eliminate the need for catecholamines [31], which can increase the potential for heart procurement and improve posttransplant outcomes. Furthermore, the timely replacement of vasopressin has been reported to increase the potential for procuring other organs, making it a preferred agent [32].
Endocrine disturbances are common in BD donors and can significantly impact donor organ quality. One of the most frequent complications is DI, which arises from a deficiency of vasopressin (antidiuretic hormone) due to hypothalamic-pituitary ischemia. Although reports have suggested a potential association between untreated DI and prolonged hypernatremia with posttransplant graft dysfunction [33], these findings have not been consistently replicated [34]. Nevertheless, maintaining a normal sodium level is an important goal in donor management.
Deficits in anterior pituitary hormones are less commonly reported than DI, possibly due to the relative preservation of blood flow [35]. Thyroid dysfunction in BD individuals is most commonly characterized by a form of sick euthyroid syndrome, rather than hypothyroidism due to thyroid-stimulating hormone (TSH) deficiency [12,36]. This typically occurs as a result of the cytokine storm after BD, inhibiting the conversion of free thyroxine (T4) to its active form, triiodothyronine (T3). The evidence supporting thyroid hormone replacement is largely based on the reduction of T3 and T4 levels observed in animal models [5,37]. However, in humans, most studies have not consistently shown significant hemodynamic effects, even when TSH levels are normal or low [38–41]. Nonetheless, some studies have reported hemodynamic improvements, particularly in terms of functional recovery of the donor heart [42].
Corticosteroid deficiency during BD, the reported prevalence rates of which vary in the literature, is caused by HPA dysfunction and relative adrenal insufficiency [43–45]. However, there is insufficient evidence regarding whether corticosteroid supplementation leads to hemodynamic improvement [13]. Nevertheless, relative adrenal insufficiency can occur, especially in proinflammatory conditions that can contribute to graft dysfunction [46], and attenuating inflammation in donor organs with corticosteroids may give potential benefits not only in regard to hemodynamic stability [47], but also an increased procurement rate [48] and improved posttransplant graft function [49,50]. Although definitive evidence is lacking, an intravenous dose of methylprednisolone, typically 1,000 mg or 15 mg/kg, is commonly administered.
Hyperglycemia is also frequently observed and provoked by proinflammatory conditions, insulin resistance, significant hormonal changes, and the use of dextrose-containing solutions [14,25,51]. Although hyperglycemia may affect donor organs by volume depletion through osmotic diuresis and by increasing reactive oxygen species that lead to inflammation [20]. the evidence regarding the benefits in control of hyperglycemia remains insufficient. However, it is generally recommended to maintain glucose levels below 180 mg/dL, similar to the management of general critically ill patients [25].
Pulmonary complications, including neurogenic pulmonary edema, aspiration, and infections, are not uncommon. Especially in a proinflammatory state, the lungs can be vulnerable to damage, potentially disrupting adequate oxygenation and, consequently, adversely affecting various organs. In cases involving lung transplantation, a lung-protective strategy becomes crucial. This involves using a low tidal volume of 6–8 mL/kg and maintaining adequate positive end-expiratory pressure to prevent atelectasis, minimize lung injury, and facilitate bronchoscopy-assisted toileting when indicated. Although there is limited evidence demonstrating benefits for HTx, some reports indicate that dedicated respiratory management may improve lung procurement rates [52].
Hematological disturbances in BD donors are characterized by the release of thromboplastin and coagulopathy induced by systemic inflammation, potentially leading to a bleeding tendency [10]. Moreover, fluid administration can exacerbate coagulopathy by diluting coagulation factors. Transfusion of blood products, when indicated, should be considered if appropriate. Anemia is also common, and unresolved severe anemia can hinder oxygen delivery to organs. Therefore, maintaining hemoglobin levels above 7 g/dL is recommended.
Echocardiographic evaluation of BD donors is essential for assessing the suitability of a donor heart for transplantation, and also allows for the determination of simultaneous surgical correction during the transplant procedure if minor structural abnormalities such as atrial septal defect are identified. Furthermore, because the hemodynamic, endocrinological, and immunological changes in BD donors can lead to myocardial ischemia and injury, dysfunction of the donor heart is not uncommon, especially during the early period of BD [53]. However, most donor cardiac dysfunctions are transient [54]; therefore, myocardial dysfunction alone does not preclude HTx. Therefore, repeated echocardiography should be performed within 24 hours to monitor functional recovery [55]. In Korea hospitals involving BD donor management, it is not uncommon to encounter difficulties in performing repeated echocardiography, often due to resource constraints. Addressing this issue is important for increasing the utility rates of donors with potentially reversible cardiac dysfunction.
While a favorable donor age is generally considered to be 45 years old or younger, the shortage of donors in recent years has led to the acceptance of individuals over 50 years old under extended donor criteria [1]. However, older donors have an increased risk of coronary artery disease (CAD), with a reported prevalence of 6.5% in those in their 40s and 7.3% in those in their 50s [56]. The presence of obstructive or multivessel CAD in a donor heart is associated with early graft failure and the development of cardiac allograft vasculopathy [57–59]. Furthermore, intravascular ultrasound performed one month after HTx revealed detectable coronary plaques in 29% of donor hearts in South Korea [60]. As previously mentioned, when BD donors are identified, the management focus shifts from patient-centered to organ-centered care. From this perspective, coronary angiography should be considered for older donors over 40 years of age or for donors with risk factors for premature CAD [25]. There is a widespread belief that the use of contrast medium before organ donation may adversely affect renal allograft function, which has limited the use of coronary angiography [61]. However, one study reported that performing coronary angiography before procurement had no impact on renal allograft outcomes [62]. Performing coronary angiography represents another critical consideration in enhancing donor utility, particularly in the era of severe donor shortage. This approach is especially relevant for donors meeting expanded criteria, offering a valuable strategy to optimize the use of available donor hearts [63,64].
Given the recent shortage of potential heart donors, it is crucial to optimize the procurement rate and improve posttransplant outcomes through the appropriate management of potential cardiac donors. Standardized protocols for managing BD donors can guide clinicians in providing sophisticated care, ultimately enhancing donor utility and improving outcomes for transplant recipients. Further research and collaboration are necessary to develop and refine these protocols in South Korea.
REFERENCES
1. Kim IC, Youn JC, Kobashigawa JA. 2018; The past, present and future of heart transplantation. Korean Circ J. 48:565–90. DOI: 10.4070/kcj.2018.0189. PMID: 29968430. PMCID: PMC6031715.

2. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, et al. 2019; The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 38:1056–66. DOI: 10.1016/j.healun.2019.08.004. PMID: 31548031. PMCID: PMC6816343.

3. Khush KK, Potena L, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, et al. 2020; The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. J Heart Lung Transplant. 39:1003–15. DOI: 10.1016/j.healun.2020.07.010. PMID: 32811772. PMCID: PMC7737223.

4. Kim IC, Youn JC, Lee SE, Jung SH, Kim JJ. 2020; Donor heart utilization in Korea. Int J Heart Fail. 2:254–63. DOI: 10.36628/ijhf.2020.0011. PMID: 36262172. PMCID: PMC9536728.

5. Chen EP, Bittner HB, Kendall SW, Van Trigt P. 1996; Hormonal and hemodynamic changes in a validated animal model of brain death. Crit Care Med. 24:1352–9. DOI: 10.1097/00003246-199608000-00014. PMID: 8706491.

6. Avlonitis VS, Wigfield CH, Kirby JA, Dark JH. 2005; The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant. 5(4 Pt 1):684–93. DOI: 10.1111/j.1600-6143.2005.00755.x. PMID: 15760391.

7. Novitzky D, Horak A, Cooper DK, Rose AG. 1989; Electrocardiographic and histopathologic changes developing during experimental brain death in the baboon. Transplant Proc. 21(1 Pt 3):2567–9.
8. Kolin A, Norris JW. 1984; Myocardial damage from acute cerebral lesions. Stroke. 15:990–3. DOI: 10.1161/01.STR.15.6.990. PMID: 6506127.

9. Bybee KA, Prasad A. 2008; Stress-related cardiomyopathy syndromes. Circulation. 118:397–409. DOI: 10.1161/CIRCULATIONAHA.106.677625. PMID: 18645066.

10. Ullah S, Zabala L, Watkins B, Schmitz ML. 2006; Cardiac organ donor management. Perfusion. 21:93–8. DOI: 10.1191/0267659106pf851oa. PMID: 16615686.

11. Smith M. 2004; Physiologic changes during brain stem death-lessons for management of the organ donor. J Heart Lung Transplant. 23(9 Suppl):S217–22. DOI: 10.1016/j.healun.2004.06.017. PMID: 15381167.

12. Howlett TA, Keogh AM, Perry L, Touzel R, Rees LH. 1989; Anterior and posterior pituitary function in brain-stem-dead donors. A possible role for hormonal replacement therapy. Transplantation. 47:828–34. DOI: 10.1097/00007890-198905000-00016. PMID: 2718243.

13. Dimopoulou I, Tsagarakis S, Anthi A, Milou E, Ilias I, Stavrakaki K, et al. 2003; High prevalence of decreased cortisol reserve in brain-dead potential organ donors. Crit Care Med. 31:1113–7. DOI: 10.1097/01.CCM.0000059644.54819.67. PMID: 12682481.

14. Anwar AS, Lee JM. 2019; Medical management of brain-dead organ donors. Acute Crit Care. 34:14–29. DOI: 10.4266/acc.2019.00430. PMID: 31723901. PMCID: PMC6849043.

15. Amado JA, López-Espadas F, Vázquez-Barquero A, Salas E, Riancho JA, López-Cordovilla JJ, et al. 1995; Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute-phase reactants. Metabolism. 44:812–6. DOI: 10.1016/0026-0495(95)90198-1. PMID: 7540249.

16. Plenz G, Eschert H, Erren M, Wichter T, Böhm M, Flesch M, et al. 2002; The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am Coll Cardiol. 39:1508–12. DOI: 10.1016/S0735-1097(02)01791-6. PMID: 11985915.
17. Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, et al. 2001; Influence of brain death on cytokine release in organ donors and renal transplants. Transplant Proc. 33:1284–5. DOI: 10.1016/S0041-1345(00)02479-9. PMID: 11267293.

18. Skrabal CA, Thompson LO, Potapov EV, Southard RE, Joyce DL, Youker KA, et al. 2005; Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res. 123:118–25. DOI: 10.1016/j.jss.2004.07.245. PMID: 15652959.

19. Pratschke J, Wilhelm MJ, Kusaka M, Basker M, Cooper DK, Hancock WW, et al. 1999; Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 67:343–8. DOI: 10.1097/00007890-199902150-00001. PMID: 10030276.

20. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. 2000; Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 85:2970–3. DOI: 10.1210/jcem.85.8.6854. PMID: 10946914.

21. Parekh J, Niemann CU, Dang K, Hirose R. 2011; Intraoperative hyperglycemia augments ischemia reperfusion injury in renal transplantation: a prospective study. J Transplant. 2011:652458. DOI: 10.1155/2011/652458. PMID: 21904663. PMCID: PMC3166717.

22. Blasi-Ibanez A, Hirose R, Feiner J, Freise C, Stock PG, Roberts JP, et al. 2009; Predictors associated with terminal renal function in deceased organ donors in the intensive care unit. Anesthesiology. 110:333–41. DOI: 10.1097/ALN.0b013e318194ca8a. PMID: 19194160.

23. Shapey IM, Summers A, Yiannoullou P, Bannard-Smith J, Augustine T, Rutter MK, et al. 2019; Insulin therapy in organ donation and transplantation. Diabetes Obes Metab. 21:1521–8. DOI: 10.1111/dom.13728. PMID: 30924574.

24. Audibert G, Charpentier C, Seguin-Devaux C, Charretier PA, Grégoire H, Devaux Y, et al. 2006; Improvement of donor myocardial function after treatment of autonomic storm during brain death. Transplantation. 82:1031–6. DOI: 10.1097/01.tp.0000235825.97538.d5. PMID: 17060850.

25. Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, et al. 2015; Management of the potential organ donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations consensus statement. Crit Care Med. 43:1291–325. DOI: 10.1097/CCM.0000000000000958. PMID: 25978154.
26. Abdelnour T, Rieke S. 2009; Relationship of hormonal resuscitation therapy and central venous pressure on increasing organs for transplant. J Heart Lung Transplant. 28:480–5. DOI: 10.1016/j.healun.2009.01.018. PMID: 19416777.

27. Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. 2002; Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2:701–11. DOI: 10.1034/j.1600-6143.2002.20804.x. PMID: 12243491.

28. Stoica SC, Satchithananda DK, White PA, Parameshwar J, Redington AN, Large SR. 2004; Noradrenaline use in the human donor and relationship with load-independent right ventricular contractility. Transplantation. 78:1193–7. DOI: 10.1097/01.TP.0000137792.74940.4F. PMID: 15502719.

29. Loh JA, Verbalis JG. 2008; Disorders of water and salt metabolism associated with pituitary disease. Endocrinol Metab Clin North Am. 37:213–34. DOI: 10.1016/j.ecl.2007.10.008. PMID: 18226738.

30. Hwang HP, Kim JM, Shin S, Ahn HJ, Lee S, Joo DJ, et al. 2020; Organ procurement in a deceased donor. Korean J Transplant. 34:134–50. DOI: 10.4285/kjt.2020.34.3.134. PMID: 35769061. PMCID: PMC9186815.

31. Chen JM, Cullinane S, Spanier TB, Artrip JH, John R, Edwards NM, et al. 1999; Vasopressin deficiency and pressor hypersensitivity in hemodynamically unstable organ donors. Circulation. 100(19 Suppl):II244–6. DOI: 10.1161/01.CIR.100.suppl_2.II-244. PMID: 10567311.

32. Plurad DS, Bricker S, Neville A, Bongard F, Putnam B. 2012; Arginine vasopressin significantly increases the rate of successful organ procurement in potential donors. Am J Surg. 204:856–60. DOI: 10.1016/j.amjsurg.2012.05.011. PMID: 23116641.

33. Totsuka E, Fung U, Hakamada K, Tanaka M, Takahashi K, Nakai M, et al. 2004; Analysis of clinical variables of donors and recipients with respect to short-term graft outcome in human liver transplantation. Transplant Proc. 36:2215–8. DOI: 10.1016/j.transproceed.2004.08.052. PMID: 15561195.

34. Kaczmarek I, Groetzner J, Mueller M, Landwehr P, Uberfuhr P, Nollert G, et al. 2005; Impact of donor serum sodium levels on outcome after heart transplantation. J Heart Lung Transplant. 24:928–31. DOI: 10.1016/j.healun.2004.05.026. PMID: 15982624.

35. Tien RD. 1992; Sequence of enhancement of various portions of the pituitary gland on gadolinium-enhanced MR images: correlation with regional blood supply. AJR Am J Roentgenol. 158:651–4. DOI: 10.2214/ajr.158.3.1739013. PMID: 1739013.

36. Masson F, Thicoïpe M, Latapie MJ, Maurette P. 1990; Thyroid function in brain-dead donors. Transpl Int. 3:226–33. DOI: 10.1007/BF00366971. PMID: 2076172.

37. Schwartz I, Bird S, Lotz Z, Innes CR, Hickman R. 1993; The influence of thyroid hormone replacement in a porcine brain death model. Transplantation. 55:474–6. DOI: 10.1097/00007890-199303000-00003. PMID: 8456462.

38. Novitzky D, Cooper DK, Reichart B. 1987; Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 43:852–4. DOI: 10.1097/00007890-198743060-00016. PMID: 3296351.

39. Venkateswaran RV, Steeds RP, Quinn DW, Nightingale P, Wilson IC, Mascaro JG, et al. 2009; The haemodynamic effects of adjunctive hormone therapy in potential heart donors: a prospective randomized double-blind factorially designed controlled trial. Eur Heart J. 30:1771–80. DOI: 10.1093/eurheartj/ehp086. PMID: 19324916.

40. Salim A, Martin M, Brown C, Inaba K, Roth B, Hadjizacharia P, et al. 2007; Using thyroid hormone in brain-dead donors to maximize the number of organs available for transplantation. Clin Transplant. 21:405–9. DOI: 10.1111/j.1399-0012.2007.00659.x. PMID: 17488392.

41. Macdonald PS, Aneman A, Bhonagiri D, Jones D, O'Callaghan G, Silvester W, et al. 2012; A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit Care Med. 40:1635–44. DOI: 10.1097/CCM.0b013e3182416ee7. PMID: 22511141.

42. Novitzky D, Cooper DK, Chaffin JS, Greer AE, DeBault LE, Zuhdi N. 1990; Improved cardiac allograft function following triiodothyronine therapy to both donor and recipient. Transplantation. 49:311–6. DOI: 10.1097/00007890-199002000-00017. PMID: 2305461.

43. Gramm HJ, Meinhold H, Bickel U, Zimmermann J, von Hammerstein B, Keller F, et al. 1992; Acute endocrine failure after brain death? Transplantation. 54:851–7. DOI: 10.1097/00007890-199211000-00016. PMID: 1332223.

44. Powner DJ, Hendrich A, Lagler RG, Ng RH, Madden RL. 1990; Hormonal changes in brain dead patients. Crit Care Med. 18:702–8. DOI: 10.1097/00003246-199007000-00004. PMID: 2194745.

45. Bernard F, Outtrim J, Menon DK, Matta BF. 2006; Incidence of adrenal insufficiency after severe traumatic brain injury varies according to definition used: clinical implications. Br J Anaesth. 96:72–6. DOI: 10.1093/bja/aei277. PMID: 16311283.

46. Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, et al. 2007; Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 7:1584–93. DOI: 10.1111/j.1600-6143.2007.01799.x. PMID: 17430397.

47. Pinsard M, Ragot S, Mertes PM, Bleichner JP, Zitouni S, Cook F, et al. 2014; Interest of low-dose hydrocortisone therapy during brain-dead organ donor resuscitation: the CORTICOME study. Crit Care. 18:R158. DOI: 10.1186/cc13997. PMID: 25056510. PMCID: PMC4220083.

48. Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. 2003; Aggressive pharmacologic donor management results in more transplanted organs. Transplantation. 75:482–7. DOI: 10.1097/01.TP.0000045683.85282.93. PMID: 12605114.
49. Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. 2003; Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 75:1336–41. DOI: 10.1097/01.TP.0000062839.58826.6D. PMID: 12717226.

50. Nagy Á, Szécsi B, Eke C, Szabó A, Mihály S, Fazekas L, et al. 2021; Endocrine management and hormone replacement therapy in cardiac donor management: a retrospective observational study. Transplant Proc. 53:2807–15. DOI: 10.1016/j.transproceed.2021.08.048. PMID: 34756710.

51. Wood KE, Becker BN, McCartney JG, D'Alessandro AM, Coursin DB. 2004; Care of the potential organ donor. N Engl J Med. 351:2730–9. DOI: 10.1056/NEJMra013103. PMID: 15616207.

52. Miñambres E, Pérez-Villares JM, Chico-Fernández M, Zabalegui A, Dueñas-Jurado JM, Misis M, et al. 2015; Lung donor treatment protocol in brain dead-donors: a multicenter study. J Heart Lung Transplant. 34:773–80. DOI: 10.1016/j.healun.2014.09.024. PMID: 25447580.

53. Wheeldon DR, Potter CD, Oduro A, Wallwork J, Large SR. 1995; Transforming the "unacceptable" donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant. 14:734–42.
54. Kono T, Nishina T, Morita H, Hirota Y, Kawamura K, Fujiwara A. 1999; Usefulness of low-dose dobutamine stress echocardiography for evaluating reversibility of brain death-induced myocardial dysfunction. Am J Cardiol. 84:578–82. DOI: 10.1016/S0002-9149(99)00382-3. PMID: 10482159.

55. Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D'Alessandro A, Dec GW, et al. 2002; Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28-29, 2001, Crystal City, VA. Circulation. 106:836–41. DOI: 10.1161/01.CIR.0000025587.40373.75. PMID: 12176957.
56. Grauhan O, Patzurek J, Knosalla C, Musci M, Ewert R, Jonas S, et al. 2001; Coronary angiography in heart donors: a necessity or a luxury? Transplant Proc. 33:3805. DOI: 10.1016/S0041-1345(01)02611-2. PMID: 11750621.

57. Grauhan O, Patzurek J, Hummel M, Lehmkuhl H, Dandel M, Pasic M, et al. 2003; Donor-transmitted coronary atherosclerosis. J Heart Lung Transplant. 22:568–73. DOI: 10.1016/S1053-2498(02)00655-1. PMID: 12742420.

58. Grauhan O, Siniawski H, Dandel M, Lehmkuhl H, Knosalla C, Pasic M, et al. 2007; Coronary atherosclerosis of the donor heart: impact on early graft failure. Eur J Cardiothorac Surg. 32:634–8. DOI: 10.1016/j.ejcts.2007.07.007. PMID: 17702594.
59. Gao SZ, Alderman EL, Schroeder JS, Silverman JF, Hunt SA. 1988; Accelerated coronary vascular disease in the heart transplant patient: coronary arteriographic findings. J Am Coll Cardiol. 12:334–40. DOI: 10.1016/0735-1097(88)90402-0. PMID: 3292629.

60. Park KM, Kim JJ, Hong MK, Lee CW, Kim YH, Park SW, et al. 2007; Characteristics of transplant coronary artery disease after heart transplantation in Koreans: a serial intravascular ultrasound analysis. Korean Circ J. 37:9–15. DOI: 10.4070/kcj.2007.37.1.9.

61. Schweiger M, Klüber J, Bosch A, von Levinski D, Prenner G, Stiegler P, et al. 2014; Low incidence of coronary angiography in the evaluation process of the potential heart donor. Transplant Proc. 46:3339–42. DOI: 10.1016/j.transproceed.2014.06.069. PMID: 25498048.

62. Lesouhaitier M, Legeai C, Savoye E, Cantrelle C, Pipien I, Macher MA, et al. 2018; Impact of donor coronary angiography on kidney transplantation outcomes. Clin Transplant. 32:e13355. DOI: 10.1111/ctr.13355. PMID: 30022530.

63. Ivanes F, Cantrelle C, Genet T, Le Feuvre C, Legeai C, Jasseron C, et al. 2019; Performing diagnostic coronary angiography to evaluate high-risk cardiac donors: a French nationwide cohort study. Int J Cardiol. 277:71–8. DOI: 10.1016/j.ijcard.2018.08.003. PMID: 30089550.

64. Zuckermann A, Auersperg K, Kaider A, De Pauw M, Sutlic Z, Gummert J, et al. 2018; Coronary angiography of potential cardiac donors increases cardiac transplantation rates. J Heart Lung Transplant. 37(4 Suppl):S171. DOI: 10.1016/j.healun.2018.01.416.

Fig. 1
Overall pathophysiologic changes and management of brain-dead donors. CVP, central vein pressure; DI, diabetes insipidus.
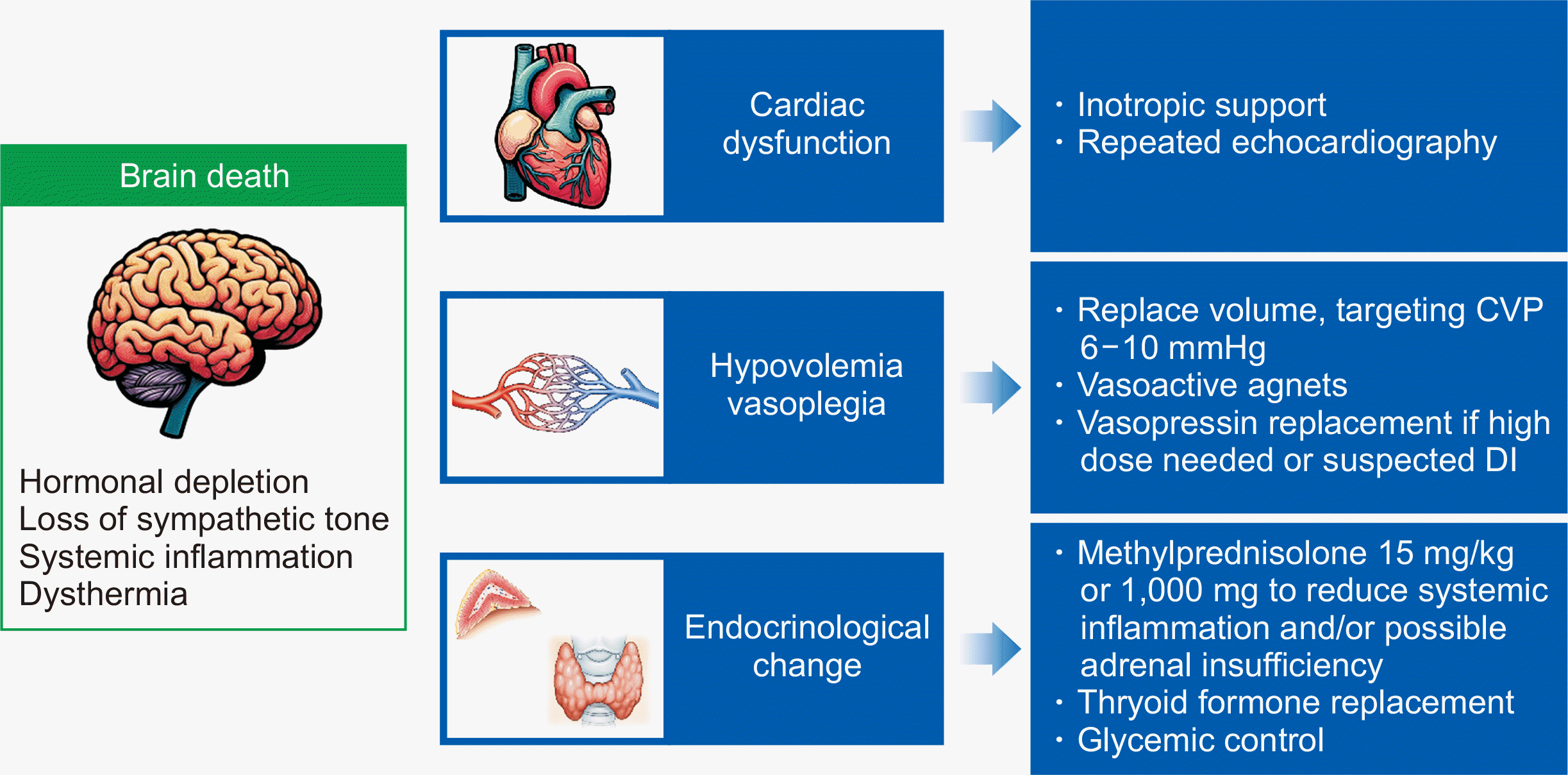
Table 1
Management overview for potential cardiac donors




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download