Abstract
Following kidney transplantation, antibody-mediated rejection (AMR) occurs when the antibodies of the immune system attack the transplanted organ, leading to damage of the kidney tissue. De novo human leukocyte antigen donor-specific antibodies (HLA-DSAs) play a key role in AMR. Current therapeutic approaches include intravenous immunoglobulin, anti-CD20 antibodies, and plasmapheresis. In cases resistant to treatment, proteasome inhibitors and C5 inhibitors may be employed. Nevertheless, a pressing need exists for new medications to improve transplant survival and reduce complications. In the context of AMR, interleukin (IL)-6 is instrumental in the development and maturation of B cells into plasma cells, which then produce HLA-DSAs targeting the allograft. IL-6 inhibitors are currently under investigation and show promise due to the essential role of IL-6 in the immune response; however, additional research is necessary.
Kidney transplantation (KT) offers substantial benefits to many patients. While the success rate of the procedure depends on multiple factors, it is generally quite high. The 1-year survival rate for KT exceeds 90%, while the 10-year rate is around 77.6% [1,2]. As such, KT often represents a vital life-extending treatment. However, it necessitates ongoing management and involves a complex regimen of immunosuppressants to prevent rejection, which is a central aspect of care [1,3].
Interleukin (IL)-6 is a key cytokine involved in inflammation and immune responses. Following KT, the immune system may recognize the new kidney as a foreign entity and initiate a rejection reaction, potentially causing the body to reject the transplanted organ. Rejected organs can suffer functional loss. Consequently, IL-6 is crucial in managing rejection after a transplant, and IL-6 inhibitors may be employed to prevent this rejection [4]. IL-6 inhibitors can be used in combination with other immunosuppressants following KT to suppress organ rejection, maintain organ function, and extend patient survival. This review article discusses the use of IL-6 inhibitors in KT, highlighting their importance and the necessity for ongoing research in this area [5].
New human leukocyte antigen donor-specific antibodies (HLA-DSAs) can be produced within the first few months following transplantation, but they more frequently arise later, after an average of 4 to 5 years [6]. The development of these antibodies is linked to several factors, including HLA class II mismatch (particularly HLA-DR/DQ), younger recipient age, history of T cell-mediated rejection (TCMR), and immunosuppressive status. Within the afferent lymphatic vessels, alloantigens are captured by subcapsular macrophages. These antigens are then presented to T cells and B cells, which are subsequently transferred to follicular dendritic cells. This interaction activates the cells at the T-B border. T cells then migrate to the germinal center, where they differentiate into T follicular helper cells. When B cells with high antigen affinity are reactivated by these T follicular helper cells, they transform into antibody-secreting cells, leading to the production of de novo DSAs. By employing single antigen bead technology, most transplant centers can now avoid preformed HLA-DSAs. Consequently, de novo HLA-DSA formation is currently the most prevalent cause of antibody-mediated rejection (AMR), both subclinical and clinical. This condition can progress to chronic active AMR (caAMR), resulting in transplant glomerulopathy and graft loss. Consequently, understanding the natural history of de novo HLA-DSA is valuable for developing new strategies to prevent and treat both AMR and caAMR (Fig. 1) [2,7,8].
When DSAs are generated, the C1 complex is activated; this leads to the production of complement fragments C3a and C5a, followed by C3b. C5 is then cleaved into C5a and C5b. C4d remains bound to the vascular endothelial cells at the site of complement activation. The components C5b through C9 sequentially bind to form the membrane attack complex, which disrupts the membranes of vascular endothelial cells. C3a and C5a function as anaphylatoxins, promoting the migration of inflammatory cells. Natural killer cells and monocytes—which bind to immunoglobulin G through their Fc gamma receptors—produce proinflammatory cytokines, exacerbating endothelial damage [2,3]. This cascade of events begins to harm vascular endothelial cells, peritubular capillaries, and glomeruli, ultimately leading to tissue repair and irreversible graft dysfunction. Higher titers of DSAs have been associated with increased graft loss (Fig. 2) [2,6].
Active AMR is characterized by microvascular inflammation, including features such as moderate transplant glomerulitis and peritubular capillaritis. These findings are indicative of endothelial injury and ischemic damage resulting from DSAs. C4d staining serves as a marker for complement activation in AMR by DSAs, and linear C4d staining on peritubular capillaries is also suggestive of AMR [9].
Currently, no U.S. Food and Drug Administration (FDA)-approved treatment for caAMR is available. However, plasmapheresis, intravenous immunoglobulin (IVIG), steroids, and anti-CD20 antibodies are commonly used to remove circulating DSA. The application of other therapies varies considerably [10,11]. A systematic review published in 2023 [3] indicates that plasmapheresis and IVIG have become the standard of care for the treatment of active AMR. Furthermore, reports from the European Society for Organ Transplantation recommend plasmapheresis, IVIG, steroids, and rituximab as treatment options [2,9,12].
If rejection occurs less than 30 days after transplantation, the treatment regimen includes plasmapheresis, IVIG, and high-dose steroids, and it may also involve rituximab and eculizumab. In cases of rejection occurring more than 30 days after transplant, clinicians should focus on maintaining optimal immunosuppression while addressing any concomitant TCMR. For chronic rejection changes observed more than 30 days posttransplant, therapeutic options such as plasmapheresis, IVIG, high-dose steroids, or rituximab may be considered, particularly in the presence of preexisting DSAs [3,4,13].
Typically, the treatment of caAMR begins with rituximab, IVIG, and plasmapheresis. To eliminate antibodies, recommendations favor administering a low dose of IVIG (0.1 g/kg) after plasmapheresis or a high dose (2 g/kg) following the final plasmapheresis session. Anti-CD20 antibodies, another therapeutic option, work by depleting B cells to inhibit AMR. In cases of AMR that are resistant to standard treatments, clinicians may resort to proteasome inhibitors and C5 inhibitors. Proteasome inhibitors target plasma cells, while C5 inhibitors act on C5, a component of the immune response. However, the therapeutic efficacy of these agents has not been conclusively demonstrated in large-scale studies [12–16].
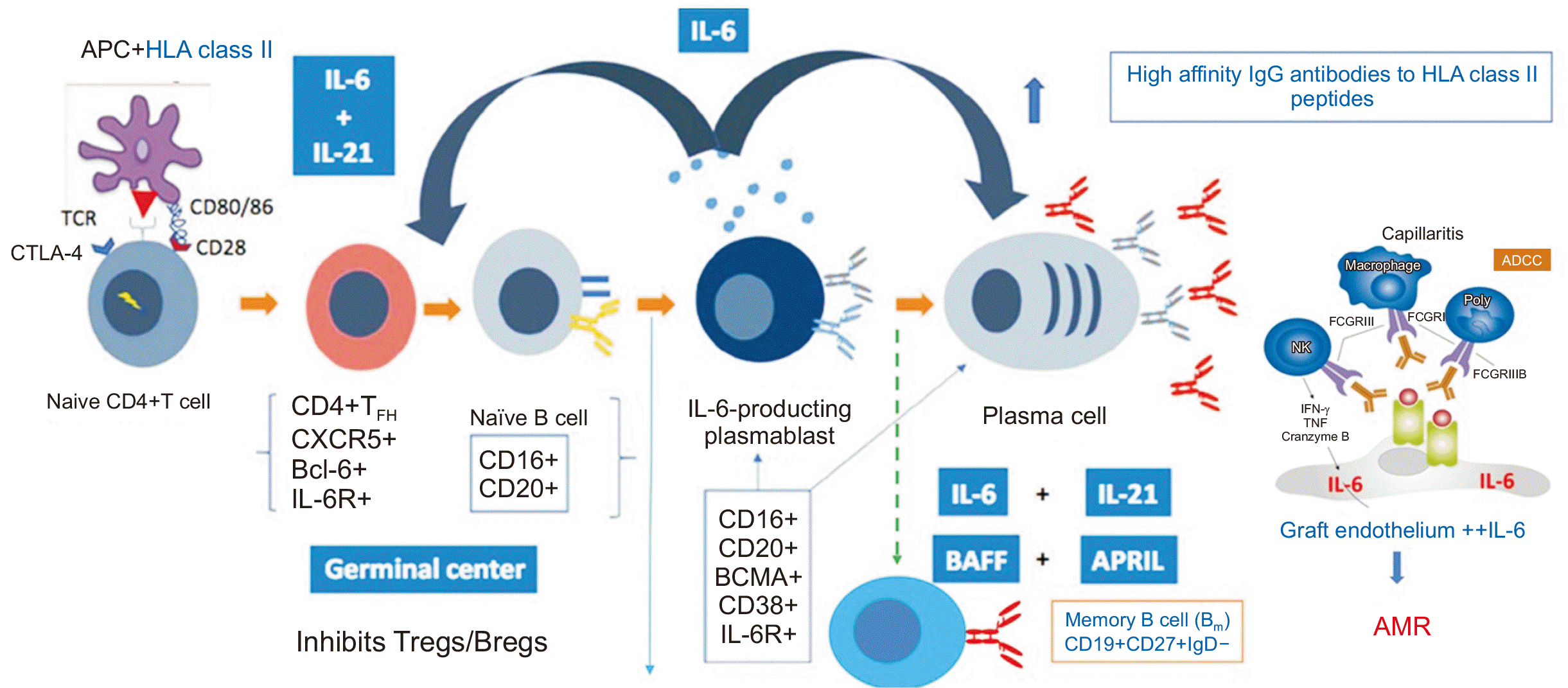
According to a recently published randomized controlled trial, IL-6 inhibitors can be used in the treatment and prevention of caAMR. IL-6 supports the development and maturation of B cells, which can then produce HLA-DSAs targeting the allograft. In addition to B cell maturation, this IL regulates germinal center activation. The production of IL-6 by antigen-presenting cells induces the production of IL-21 by naive T cells. This leads to the maturation of these T cells into T follicular helper cells, characterized by the expression of CXCR5, IL-21, and the transcription factor Bcl-6. Naive B cells migrate to the germinal centers in response to CXCR5+ T follicular helper cells. This stimulates the maturation of B cells into memory B cells and IL-6–producing plasmablasts, which in turn promote the formation of germinal centers and the progression to antibody-producing plasma cells (Fig. 3) [13].
Plasma cells exhibit the highest density of IL-6 receptors among B cell subsets, indicating the key role of IL-6 in pathogenic antibody production and subsequent tissue injury. Furthermore, alloimmune responses can stimulate vascular endothelial cells to express IL-6. This amplifies T effector and B cell responses, leading to vasculitis and fibrotic changes [13,17]. The IL-6 receptor monoclonal antibody inhibits appropriate B cell activation and differentiation while impacting the generation of plasma cells. In principle, this mechanism should regulate caAMR [13].
A survey was conducted regarding caAMR following KT in Europe. The aim of the study was to assess the prevailing European practices for diagnosing and managing caAMR, as well as to understand the protocols for posttransplant surveillance during the first year following KT. Participating European transplant nephrologists, transplant surgeons, and nephrologists completed a 15-minute online survey, which included 58 questions that were either multiple-choice or open-ended. The survey covered various topics, such as patient case scenarios, routine posttransplant examinations, and treatment approaches for caAMR. Notably, fewer than half of patients who develop caAMR were found to receive treatment beyond optimal immunosuppression. The findings suggest that monitoring of clinical indicators of graft function is the mainstay of posttransplant surveillance. However, relying solely on clinical measures to detect rejection may result in delayed diagnosis and progression to an untreatable stage of the condition, due to both the late recognition of the disease and the absence of established effective treatments [18].
IL-6 inhibitors reduce the production of pathogenic antibodies, suppress the action of T effector cells, and enhance the differentiation of regulatory T and B cells. They may also suppress DSA formation and allograft injury [13,17]. Antibodies targeting IL-6, such as clazakizumab, have been utilized and clinically investigated for a variety of diseases. In one study, 10 DSA-positive patients who had developed resistance to standard treatment were treated with a regimen of 25 mg of clazakizumab, administered subcutaneously six times each month. After 6 months of treatment, reductions in DSA, C4d, and g+ptc scores were observed, along with stabilization of the glomerular filtration rate. A phase 2 randomized controlled trial involving 20 DSA-positive patients with confirmed treatment-resistant caAMR demonstrated the efficacy of clazakizumab in reducing DSA levels, while also mitigating rejection as observed on kidney biopsy. However, due to infectious complications impacting some participants, the dosage was reduced to 12.5 mg in later studies. These findings underscore the importance of establishing appropriate entry criteria and the necessity for vigilant monitoring when administering clazakizumab [1,19–21].
In a study examining the effects of subtherapeutic anti-IL-6 antibody administration or cessation of treatment following prolonged cytokine neutralization, an increase in drug-combined IL-6 levels was observed in the IL-6 inhibitor group. The researchers observed no subsequent progression to AMR or significant increases in inflammatory markers, such as C-reactive protein, relative to the control group. These results suggest that IL-6 inhibitors may modulate the IL-6/IL-6R axis [1].
The IMAGINE study was the first phase 3, multicenter, double-blind clinical trial to evaluate KT recipients with caAMR. Participants underwent 1:1 randomization and received either clazakizumab or a placebo. The trial was designed to assess the safety and efficacy of clazakizumab in preventing composite graft loss from all causes and in slowing or preventing the progressive loss of kidney function due to caAMR. The slope of the effective glomerular filtration rate, which the FDA has accepted as a reasonably likely surrogate endpoint for allograft loss, was measured over a 12-month period [3]. The first planned interim analysis of the IMAGINE trial revealed that, as the decline in HLA-DSA did not occur as rapidly as the decline in kidney function, insufficient evidence is available to support the efficacy of the IL-6 inhibitor in treating AMR. Consequently, the trial was deemed unlikely to meet its primary efficacy outcome, leading to the discontinuation of enrollment. Notably, the decision to halt the study was not due to safety concerns. Clearly, a continued need exists to seek effective treatments for transplant recipients at risk of allograft failure, and this must be done in a robust manner that facilitates clear decision-making [20,22].
caAMR is a key contributor to graft failure. While treatments such as plasmapheresis, IVIG, steroids, and rituximab are employed to manage this condition, no therapeutic approach has received formal approval to date. Effective treatments must be developed to extend the survival of transplant recipients and minimize the risk of transplant rejection. Research efforts are focused on more effectively managing AMR by targeting the IL-6 signaling pathway. Within the transplant community, it is essential to establish improved treatments for AMR. Through research on IL-6 inhibitors, we aim to identify the immunosuppressive medications necessary to enhance the success rates of KT and prolong survival following transplantation. The termination of the IMAGINE study further underscores the urgency of continuing our quest for solutions to protect transplant recipients at risk of allograft failure.
REFERENCES
1. Borski A, Eskandary F, Haindl S, Doberer K, Mühlbacher J, Mayer KA, et al. 2023; Anti-interleukin-6 antibody clazakizumab in antibody-mediated renal allograft rejection: accumulation of antibody-neutralized interleukin-6 without signs of proinflammatory rebound phenomena. Transplantation. 107:495–503. DOI: 10.1097/TP.0000000000004285. PMID: 35969004.

2. Sasaki H, Tanabe T, Tsuji T, Hotta K. 2023; Mechanism and treatment for chronic antibody-mediated rejection in kidney transplant recipients. Int J Urol. 30:624–33. DOI: 10.1111/iju.15197. PMID: 37306194.

3. Alasfar S, Kodali L, Schinstock CA. 2023; Current therapies in kidney transplant rejection. J Clin Med. 12:4927. DOI: 10.3390/jcm12154927. PMID: 37568328. PMCID: PMC10419508.

4. The Korean Society of Nephrology. Clinical nephrology. 3rd ed. The Korean Society of Nephrology;2022.
5. Doberer K, Duerr M, Halloran PF, Eskandary F, Budde K, Regele H, et al. 2021; A randomized clinical trial of anti-il-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol. 32:708–22. DOI: 10.1681/ASN.2020071106. PMID: 33443079. PMCID: PMC7920172.

6. López Del Moral C, Wu K, Naik M, Osmanodja B, Akifova A, Lachmann N, et al. 2022; The natural history of de novo donor-specific HLA antibodies after kidney transplantation. Front Med (Lausanne). 9:943502. DOI: 10.3389/fmed.2022.943502. PMID: 36186822. PMCID: PMC9523126.

7. Kim MY, Brennan DC. 2021; Therapies for chronic allograft rejection. Front Pharmacol. 12:651222. DOI: 10.3389/fphar.2021.651222. PMID: 33935762. PMCID: PMC8082459.

8. Sharma R. 2022; Anti-interleukin 6 therapeutics for chronic antibody-mediated rejection in kidney transplant recipients. Exp Clin Transplant. 20:709–16. DOI: 10.6002/ect.2021.0254. PMID: 34981708.

9. Hara S. 2023; The chronology of renal allograft dysfunction: the pathological perspectives. Nephron. 147 Suppl 1:67–73. DOI: 10.1159/000531575. PMID: 37573772.

10. Chancay J, Liu C, Chauhan K, Andersen L, Harris C, Coca S, et al. 2022; Role of time from transplantation to biopsy in histologic ABMR: a single center report. Clin Transplant. 36:e14802. DOI: 10.1111/ctr.14802. PMID: 36069577. PMCID: PMC10211409.

11. Tufan Pekkucuksen N, Sigler KE, Akcan Arikan A, Srivaths P. 2021; Tandem plasmapheresis and continuous kidney replacement treatment in pediatric patients. Pediatr Nephrol. 36:1273–8. DOI: 10.1007/s00467-020-04769-z. PMID: 33108508. PMCID: PMC7588944.

12. Halloran PF, Madill-Thomsen KS, Pon S, Sikosana ML, Böhmig GA, Bromberg J, et al. 2022; Molecular diagnosis of ABMR with or without donor-specific antibody in kidney transplant biopsies: differences in timing and intensity but similar mechanisms and outcomes. Am J Transplant. 22:1976–91. DOI: 10.1111/ajt.17092. PMID: 35575435. PMCID: PMC9540308.

13. Jordan SC, Ammerman N, Huang E, Vo A. 2022; Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts. Am J Transplant. 22 Suppl 4:28–37. DOI: 10.1111/ajt.17207. PMID: 36453709.

14. Miller CL, Madsen JC. 2021; IL-6 directed therapy in transplantation. Curr Transplant Rep. 8:191–204. DOI: 10.1007/s40472-021-00331-4. PMID: 34099967. PMCID: PMC8173333.

15. Boonpheng B, Hansrivijit P, Thongprayoon C, Mao SA, Vaitla PK, Bathini T, et al. 2021; Rituximab or plasmapheresis for prevention of recurrent focal segmental glomerulosclerosis after kidney transplantation: a systematic review and meta-analysis. World J Transplant. 11:303–19. DOI: 10.5500/wjt.v11.i7.303. PMID: 34316454. PMCID: PMC8291000.

16. Ulisses LR, Paixão JO, Agena F, Souza PS, Paula FJ, Bezerra G, et al. 2022; Desensitization using IVIG alone for living-donor kidney transplant: impact on donor-specific antibodies. J Bras Nefrol. 44:527–32. DOI: 10.1590/2175-8239-jbn-2021-0200. PMID: 35438714. PMCID: PMC9838666.

17. Boonpheng B, De Castro IC, Ng YH, Blosser C, Bakthavatsalam R, Gimferrer I, et al. 2023; Tocilizumab for treatment of chronic active antibody-mediated rejection in kidney transplant recipients. Clin Transplant. 37:e14936. DOI: 10.1111/ctr.14936. PMID: 36787372.

18. Rostaing LP, Böhmig GA, Gibbons B, Taqi MM. 2023; Post-transplant surveillance and management of chronic active antibody-mediated rejection in renal transplant patients in Europe. Transpl Int. 36:11381. DOI: 10.3389/ti.2023.11381. PMID: 37529383. PMCID: PMC10389272.

19. Abuazzam F, Dubrawka C, Abdulhadi T, Amurao G, Alrata L, Yaseen Alsabbagh D, et al. 2023; Emerging therapies for antibody-mediated rejection in kidney transplantation. J Clin Med. 12:4916. DOI: 10.3390/jcm12154916. PMID: 37568318. PMCID: PMC10419906.

20. Berger M, Baliker M, Van Gelder T, Böhmig GA, Mannon RB, Kumar D, et al. Chronic active antibody-mediated rejection: opportunity to determine the role of interleukin-6 blockade. Transplantation. 2023; Nov. 9. [Epub]. https://doi.org/10.1097/TP.0000000000004822. DOI: 10.1097/TP.0000000000004822. PMID: 37941113.

21. Degner KR, Wilson NA, Reese SR, Parajuli S, Aziz F, Garg N, et al. 2020; Short-term immunopathological changes associated with pulse steroids/IVIG/rituximab therapy in late kidney allograft antibody mediated rejection. Kidney360. 1:389–98. DOI: 10.34067/KID.0001082019. PMID: 34476406. PMCID: PMC8409258.

22. Nickerson PW, Böhmig GA, Chadban S, Kumar D, Mannon RB, van Gelder T, et al. 2022; Clazakizumab for the treatment of chronic active antibody-mediated rejection (AMR) in kidney transplant recipients: phase 3 IMAGINE study rationale and design. Trials. 23:1042. DOI: 10.1186/s13063-022-06897-3. PMID: 36550562. PMCID: PMC9772593.

Fig. 1
Causal pathways associated with graft failure and death. CNI, calcineurin inhibitor; DGF, delayed graft function; TCMR, T cell-mediated rejection; caTCMR, chronic active TCMR; HLA, human leucocyte antigen; DSA, donor-specific antibody; AMR, antibody-mediated rejection; caAMR, chronic active AMR. Modified from Sasaki et al. [2] according to the Creative Commons License.
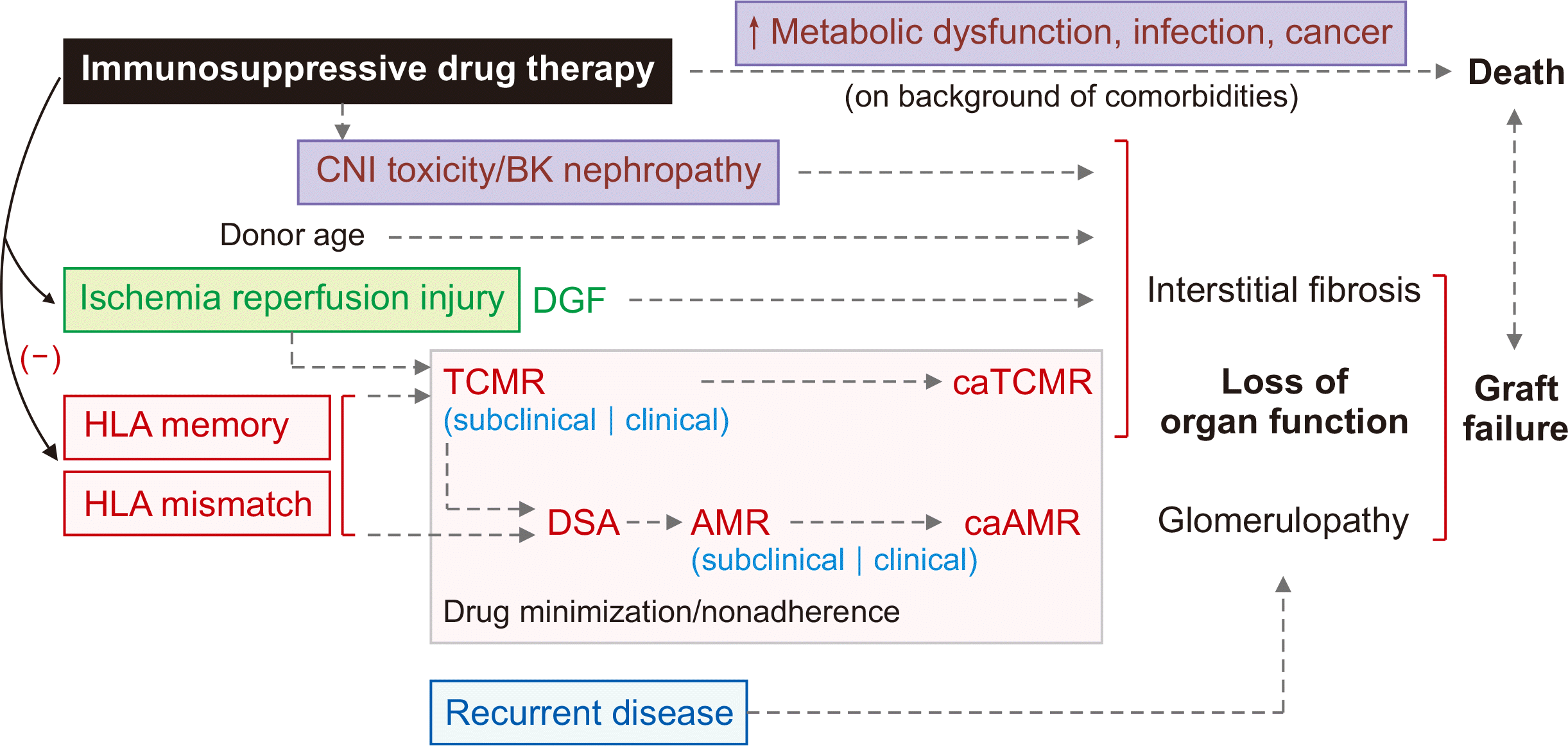
Fig. 2
Initiation of tissue injury by donor-specific antibodies. NK, natural killer; IgG, immunoglobulin G; FCGR, Fc gamma receptor; HLA, human leukocyte antigen; MAC, membrane attack complex. Adapted from Sasaki et al. [2] according to the Creative Commons License.
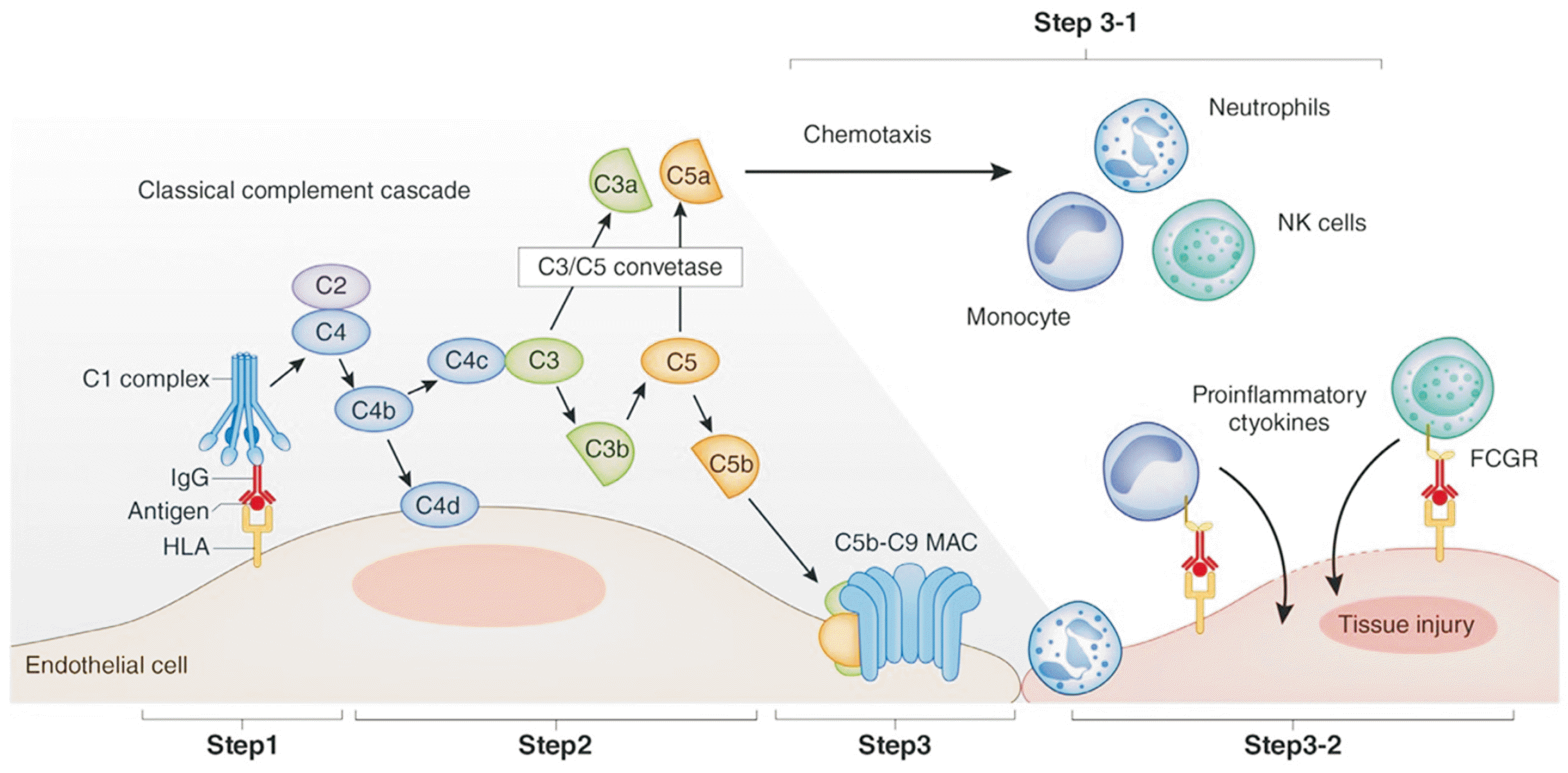
Fig. 3
Regulation of germinal center activation and B cell maturation by interleukin 6. APC, antigen-presenting cell; HLA, human leukocyte antigen; IL, interleukin; IgG, immunoglobulin G; TCR, T cell receptor; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ADCC, antibody dependent cellular cytotoxicity; FCGR, Fc gamma receptor; NK, natural killer; IFN, interferon; TNF, tumor necrosis factor; BAFF, B cell activating factor; APRIL, a proliferation-inducing ligand; Tregs, regulatory T cell; Bregs, regulatory B cell; IgD, immunoglobulin D; AMR, antibody-mediated rejection. Adapted from Jordan et al. [13] with permission from Elsevier.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download