Abstract
Robotic surgery is emerging as a feasible minimally invasive approach for donor hepatectomy at specialized centers. The aim of this article is to systematically describe the surgical techniques for robotic parenchymal transection and bile duct division in right donor hepatectomy. The setup of the robotic arms, methods of parenchymal transection using robotic instruments, and right hepatic duct division with the aid of indocyanine green dye are detailed, along with the pearls and pitfalls of these two parts of the operation.
Robotic surgery is emerging as a feasible minimally invasive approach for donor hepatectomy [1-4]. Some of the most important parts of the right donor hepatectomy are hilar dissection, right lobe mobilization, parenchymal transection, and right hepatic duct (RHD) division. The technical paper focuses on parenchymal transection and bile duct division.
Parenchymal transection is arguably the most difficult part of any donor hepatectomy, as the graft is usually divided from the remnant without inflow or outflow control. Compared to open liver parenchymal transection, robotic transection involves challenges specifically related to the robotic approach, including the limitations of currently available robotic energy devices, differences in retraction and hanging maneuvers, and lack of tactile feedback.
Similarly, RHD identification and determination of the location for bile duct division during robotic donor hepatectomy are different from the open technique, as we tend not to perform conventional intraoperative cholangiography or use the probing technique (due to lack of tactile feedback). We instead utilize fluorescence cholangiography using indocyanine green (ICG) dye injection and Firefly (Intuitive Surgical).
We detail the technical aspects of the approach to parenchymal transection and bile duct division, and describe modifications to overcome challenges and take advantage of the technological advances offered by the robotic platform.
Our standard port placements for right-sided liver resection are shown in Fig. 1. The placement of the camera provides adequate visualization of the right side of the liver hilum. The third arm is used for retraction and placed as a right-handed instrument. Two laparoscopic assistant ports are placed in the lower abdomen and the specimen is usually extracted via a Pfannenstiel incision. The location of the camera port is generally in line with the transection plane.
(1) Harmonic scalpel (nonarticulating robotic instrument). (2) Camera. (3) Maryland bipolar forceps (with electrocautery). (4) Cadiere forceps.
We utilize the rubber band retraction technique to provide steady exposure of the parenchymal transection plane without having to use any robotic or laparoscopic arms for this task [1,6]. One end of each rubber band is anchored with stay sutures at the liver edges of the right and left resection margins. Each rubber band is then externalized on the corresponding side of the abdominal wall and secured with a clamp to the drapes (Fig. 2).
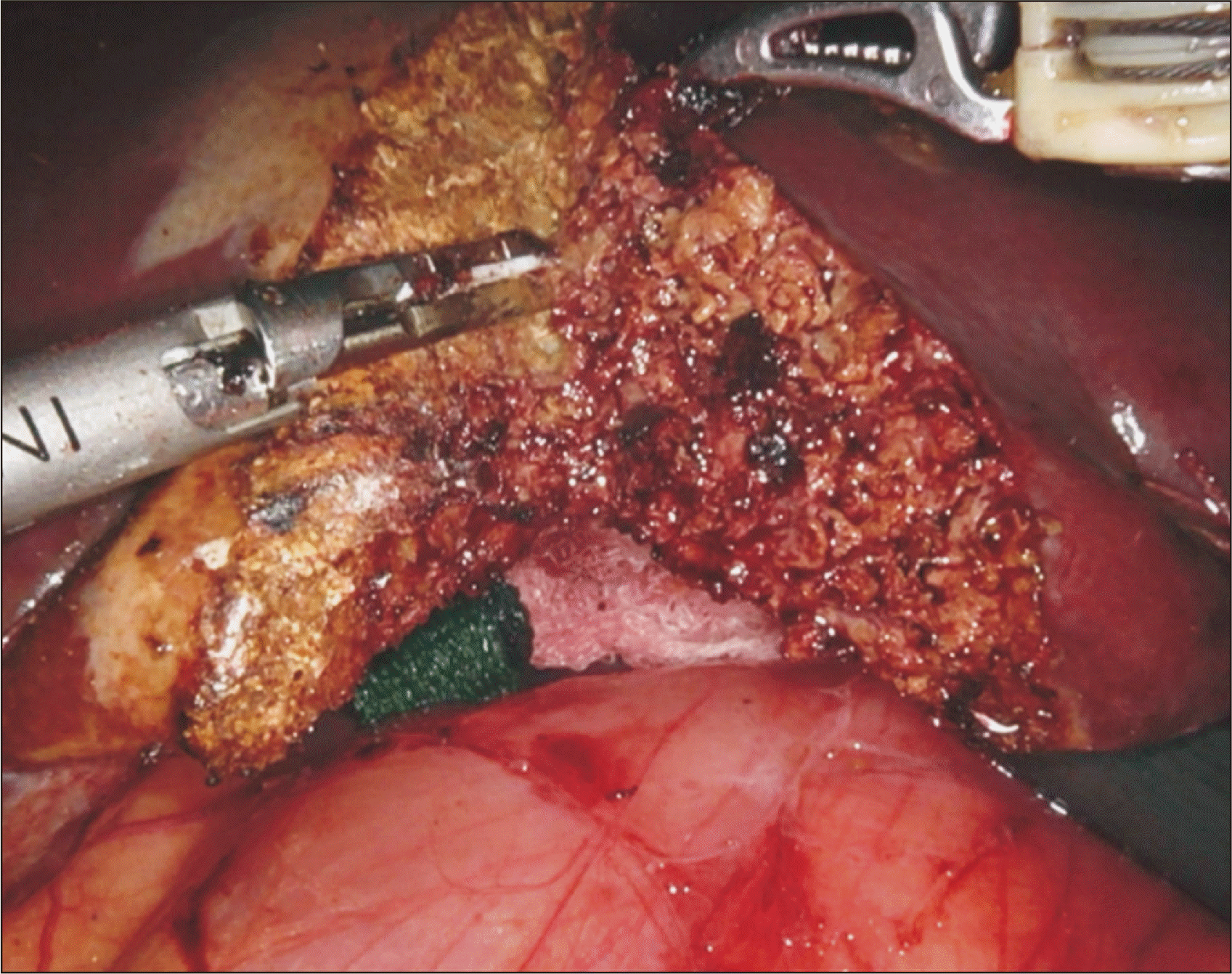
The third arm is used to gently lift the inferior surface of the liver off the hilum. Parenchymal transection is performed with a Harmonic scalpel and Maryland bipolar forceps [1–6] (Fig. 3). The caudate lobe had been previously divided during the mobilization of the right lobe. Small intervening vessels and oozing from the parenchyma are controlled with bipolar electrocautery. As more of the parenchyma is transected, the liver edges are continually retracted due to the elasticity of the rubber bands.
Segment 5 and 8 draining veins are carefully dissected with Maryland bipolar forceps and divided between Hem-o-lock clips (Fig. 4). Smaller branches are ligated with small metal clips. When the majority of the parenchyma has been transected (leaving 1–2 cm of liver anterior to the inferior vena cava [IVC]), we divide the RHD.
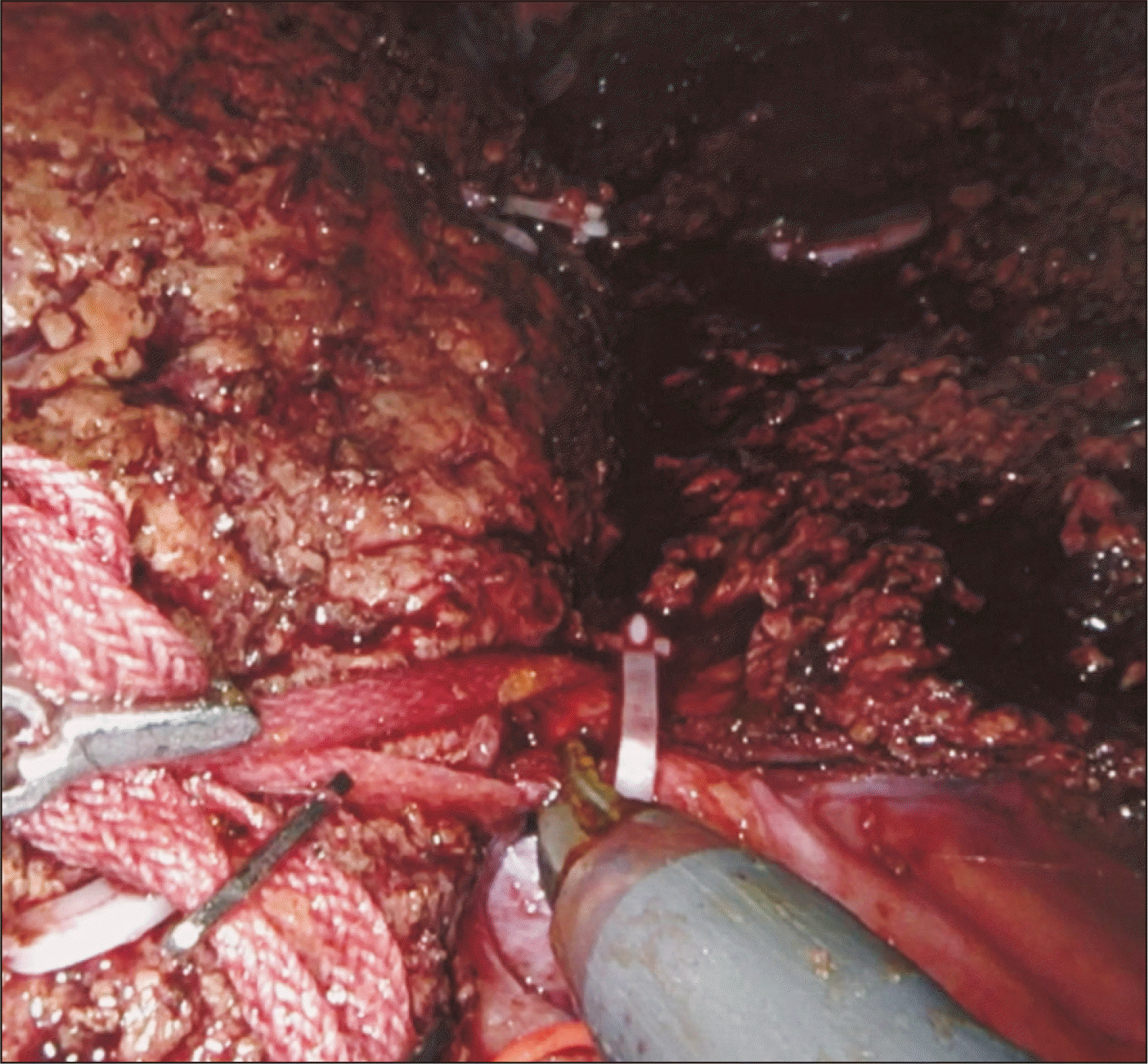
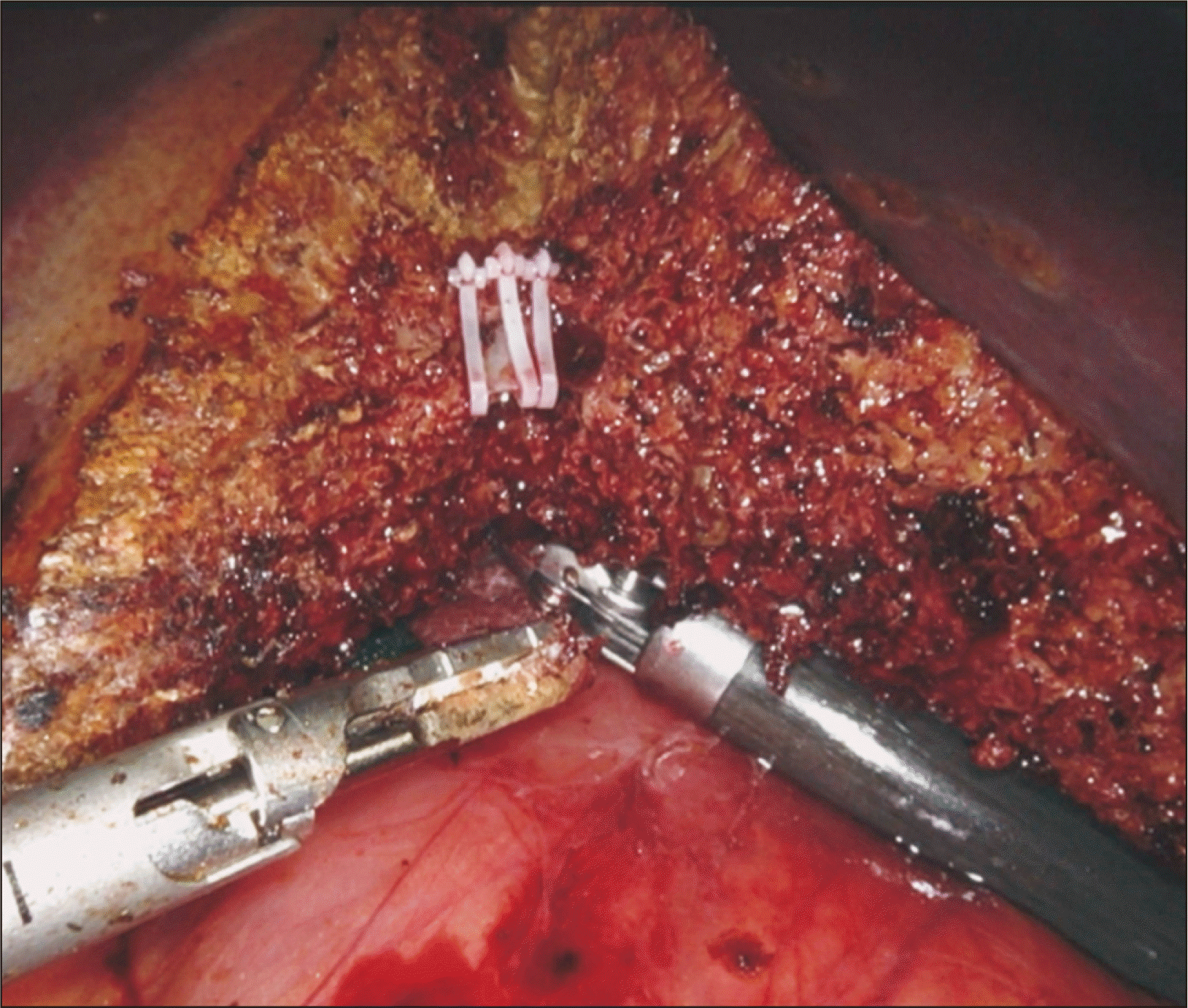
All the parenchyma on a plane anterior to the hepatic duct is divided, exposing most of the circumference of the duct. ICG dye had been previously injected intravenously during hilar dissection, and fluorescence should be visualized in the bile ducts at this stage. Firefly is used to identify the bile duct bifurcation and the RHD (Fig. 5). The location of RHD division is chosen based on the surgeon’s interpretation of the cholangiogram. There are two ways to transect this duct: (1) clip-and-cut; using the Maryland forceps, umbilical tape is circled around the entire hepatic duct with its surrounding hilar plate tissue. A large Hem-o-lock clip is then placed in the distal RHD, ensuring that it does not encroach onto the bifurcation. The proximal RHD on the graft side is then sharply divided (Fig. 6). The stump of the distal RHD on the donor side is suture-ligated with running 5-0 polydioxanone. (2) Cut-and-clip; the RHD is cut at the chosen point without cutting the hilar tissues that are posterior to the duct (there is usually a vessel in this tissue). This hilar plate tissue is divided between two small metal clips. The stump of the distal RHD is suture-ligated with running 5-0 polydioxanone.
The choice of technique is influenced by the length of the RHD, as the Hem-o-lock in the clip-and-cut option tends to take up an additional 0.5–1 mm of RHD length, and the ease of encircling the RHD with its hilar plate tissue. The cut-and-clip option may lead to fewer bile duct openings for recipient anastomosis [7].
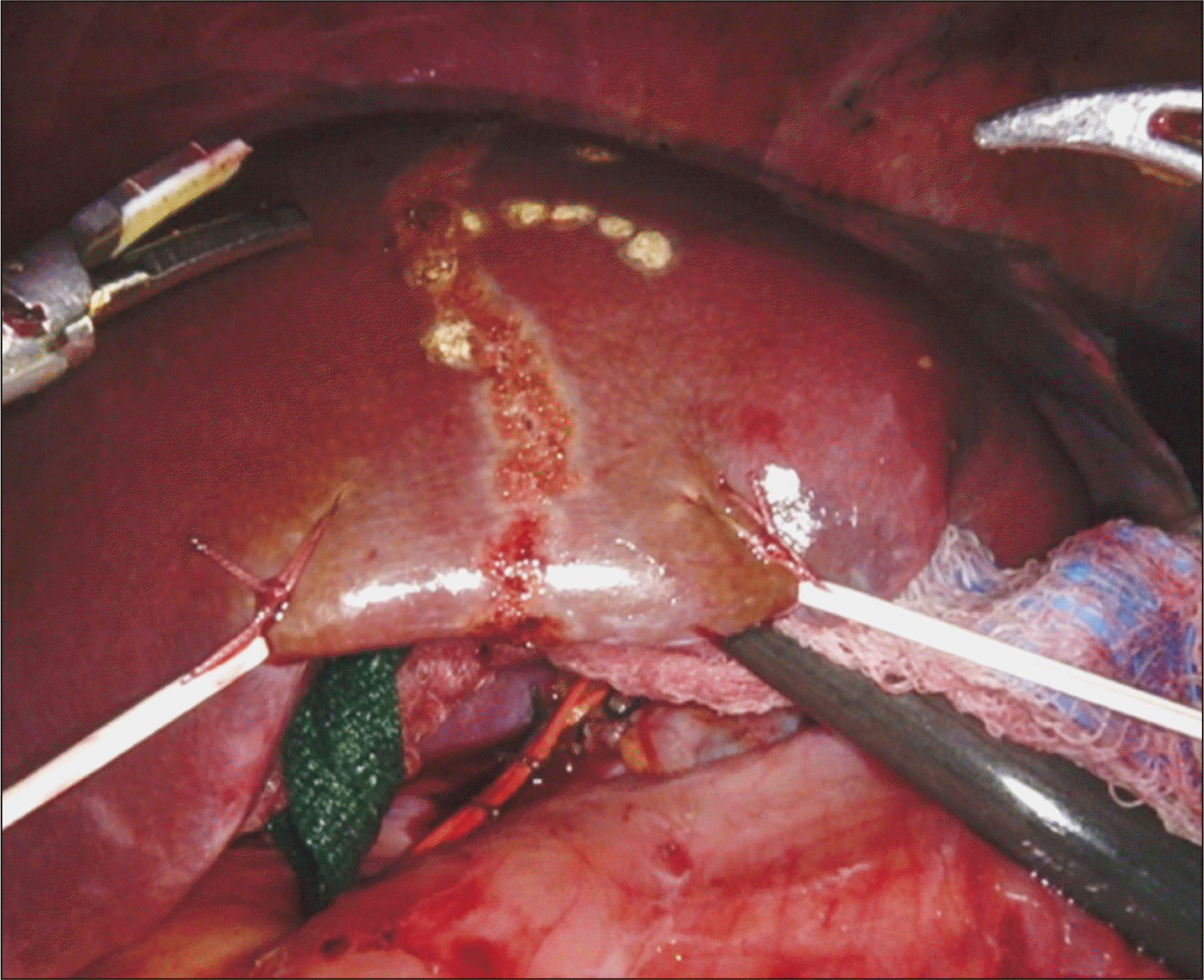
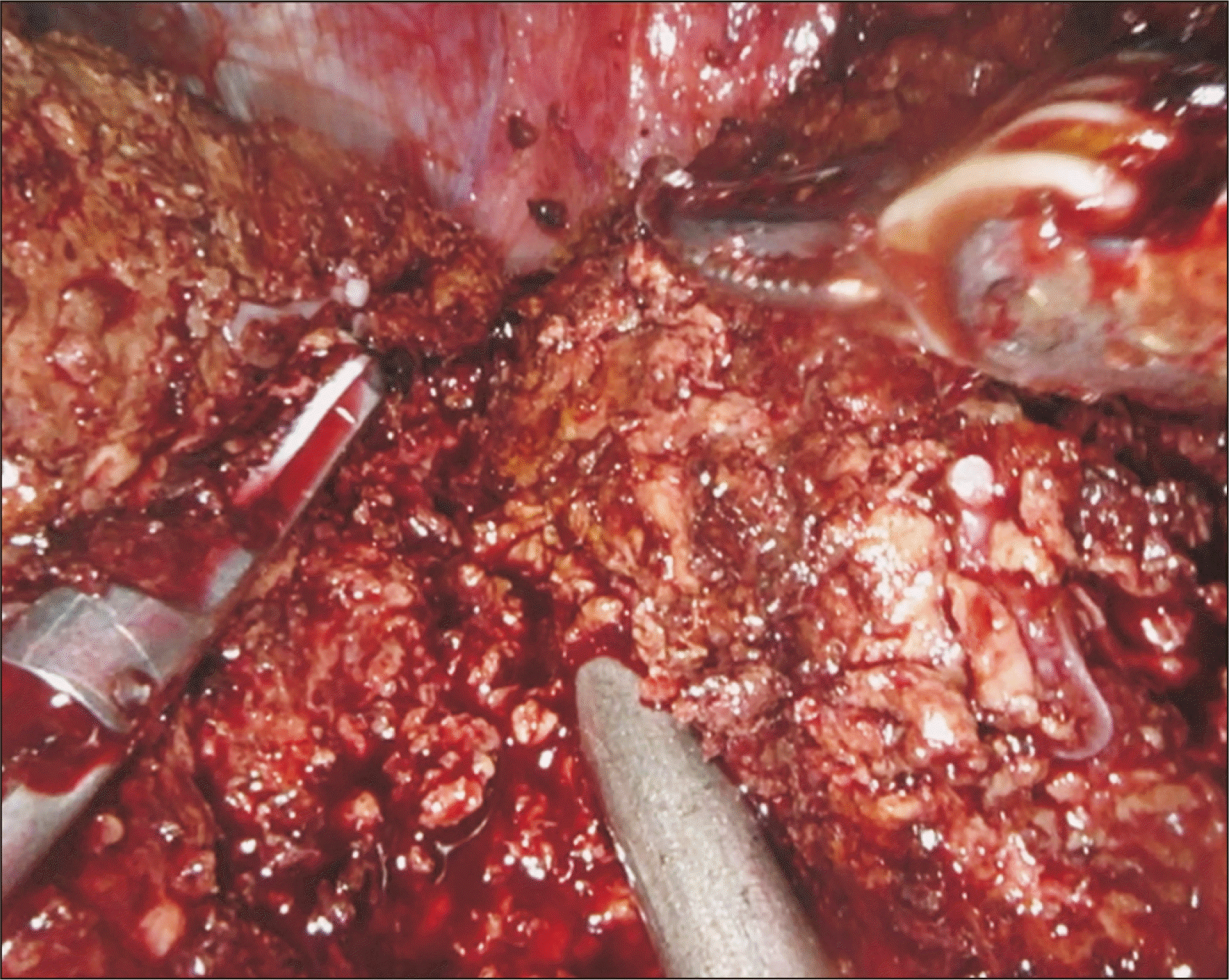
Once the RHD is transected, the laparoscopic assistant inserts a grasper or suction catheter into the space previously created between the posterior liver and retrohepatic IVC. This grasper is used to lift the liver off of the IVC to facilitate transection of the remaining 1–2 cm of parenchyma, starting at the hilum and progressing in a cephalad direction [1-3]. Eventually, the grasper is inserted into the space between the right and middle hepatic vein and the final portion of the parenchymal transection is completed (Fig. 7).
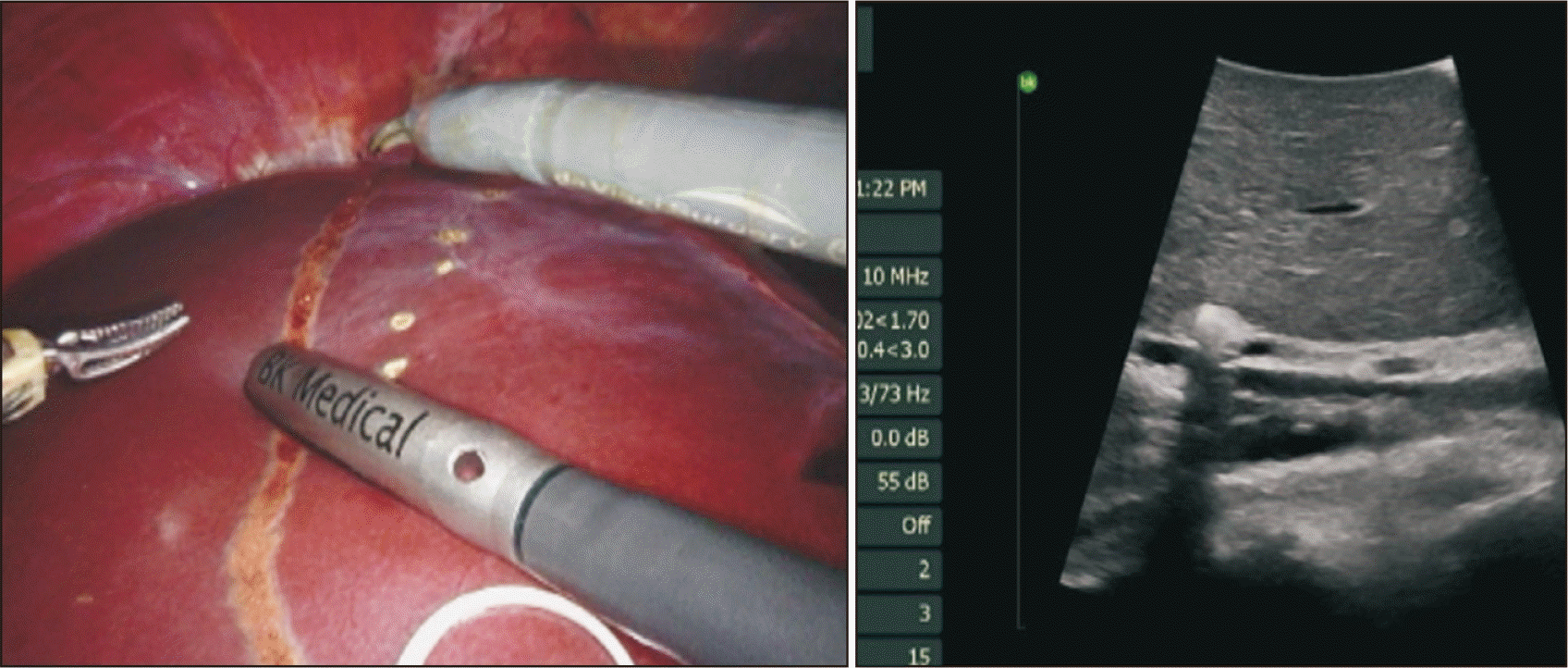
(1) Intraoperative ultrasonography can be performed prior to transection to ensure that the demarcation line previously marked is on the right side of the middle hepatic vein, which is preserved with the donor (Fig. 8). (2) It is important to regularly check that the transection remains along the demarcation lines previously marked on the anterior and inferior surface of the liver. (3) Low central venous pressure should be maintained by the anesthesiology team during parenchymal transection. (4) Inadvertent holes in the hepatic vein branches can lead to bleeding and pose a risk of CO2 embolism; therefore, gentle clamping of the defect using the instrument in either hand during hemostasis is important. Consideration should be given to reducing the pneumoperitoneum pressure during parenchymal transection. (5) We should be prepared to suture-ligate any bleeding during parenchymal transection that could not be controlled with clips or electrocautery; 5-0 and 4-0 Prolene sutures of appropriate lengths must be readily available for this task.
Using the described technique, parenchymal transection can be performed while minimizing blood loss. Since the transection plane is along a relatively straight sagittal plane, the inability of the robotic harmonic scalpel to articulate does not hamper its use for this type of hepatectomy. ICG dye cholangiogram is a useful adjunct to delineate biliary anatomy during the division of the RHD.
REFERENCES
1. Rho SY, Lee JG, Joo DJ, Kim MS, Kim SI, Han DH, et al. 2022; Outcomes of robotic living donor right hepatectomy from 52 consecutive cases: comparison with open and laparoscopy-assisted donor hepatectomy. Ann Surg. 275:e433–42. DOI: 10.1097/SLA.0000000000004067. PMID: 32773621.

2. Broering D, Sturdevant ML, Zidan A. 2022; Robotic donor hepatectomy: a major breakthrough in living donor liver transplantation. Am J Transplant. 22:14–23. DOI: 10.1111/ajt.16889. PMID: 34783439.

3. Chandran B, Varghese CT, Balakrishnan D, Nair K, Mallick S, Mathew JS, et al. 2022; Technique of robotic right donor hepatectomy. J Minim Access Surg. 18:157–60. DOI: 10.4103/jmas.JMAS_35_21. PMID: 35017406. PMCID: PMC8830578.

4. Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT, et al. 2017; Robotic major hepatectomy: is there a learning curve? Surgery. 161:642–9. DOI: 10.1016/j.surg.2016.09.025. PMID: 27884614.
5. Choi GH, Choi SH, Kim SH, Hwang HK, Kang CM, Choi JS, et al. 2012; Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc. 26:2247–58. DOI: 10.1007/s00464-012-2168-9. PMID: 22311301.

6. Choi GH, Chong JU, Han DH, Choi JS, Lee WJ. 2017; Robotic hepatectomy: the Korean experience and perspective. Hepatobiliary Surg Nutr. 6:230–8. DOI: 10.21037/hbsn.2017.01.14. PMID: 28848745. PMCID: PMC5554764.

7. Rhu J, Kim MS, Choi GS, Jeong WK, Kim JM, Joh JW. 2021; A novel technique for bile duct division during laparoscopic living donor hepatectomy to overcome biliary complications in liver transplantation recipients: "cut and clip" rather than "clip and cut". Transplantation. 105:1791–9. DOI: 10.1097/TP.0000000000003423. PMID: 32826797.

Fig. 1
Port placement (blue, robotic; green, laparoscopic assistant ports) for right donor hepatectomy.
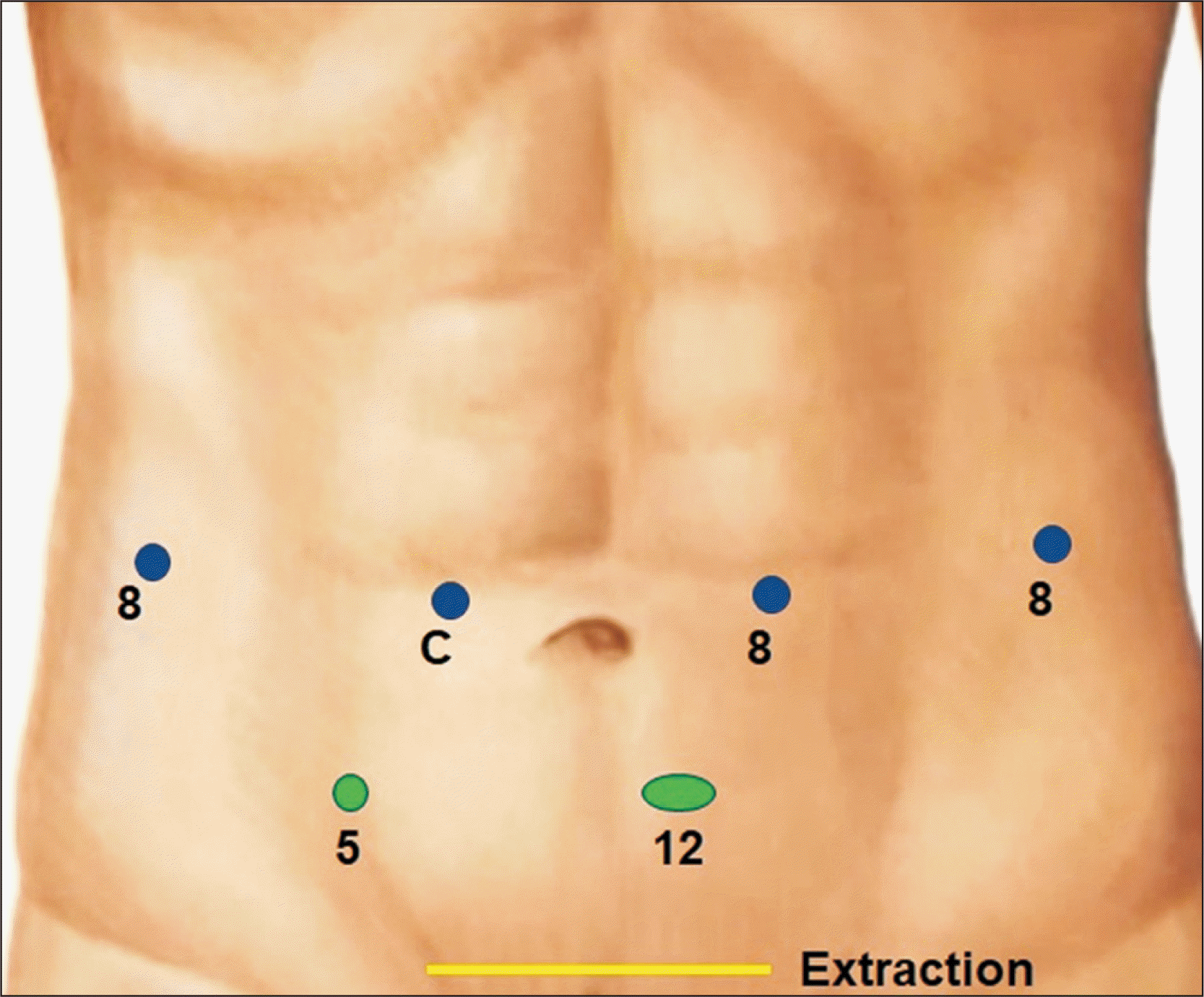
Fig. 5
Visualization of the bile duct bifurcation is enhanced by indocyanine green injection on the Firefly (Intuitive Surgical) view. The arrow points to biliary bifurcation.
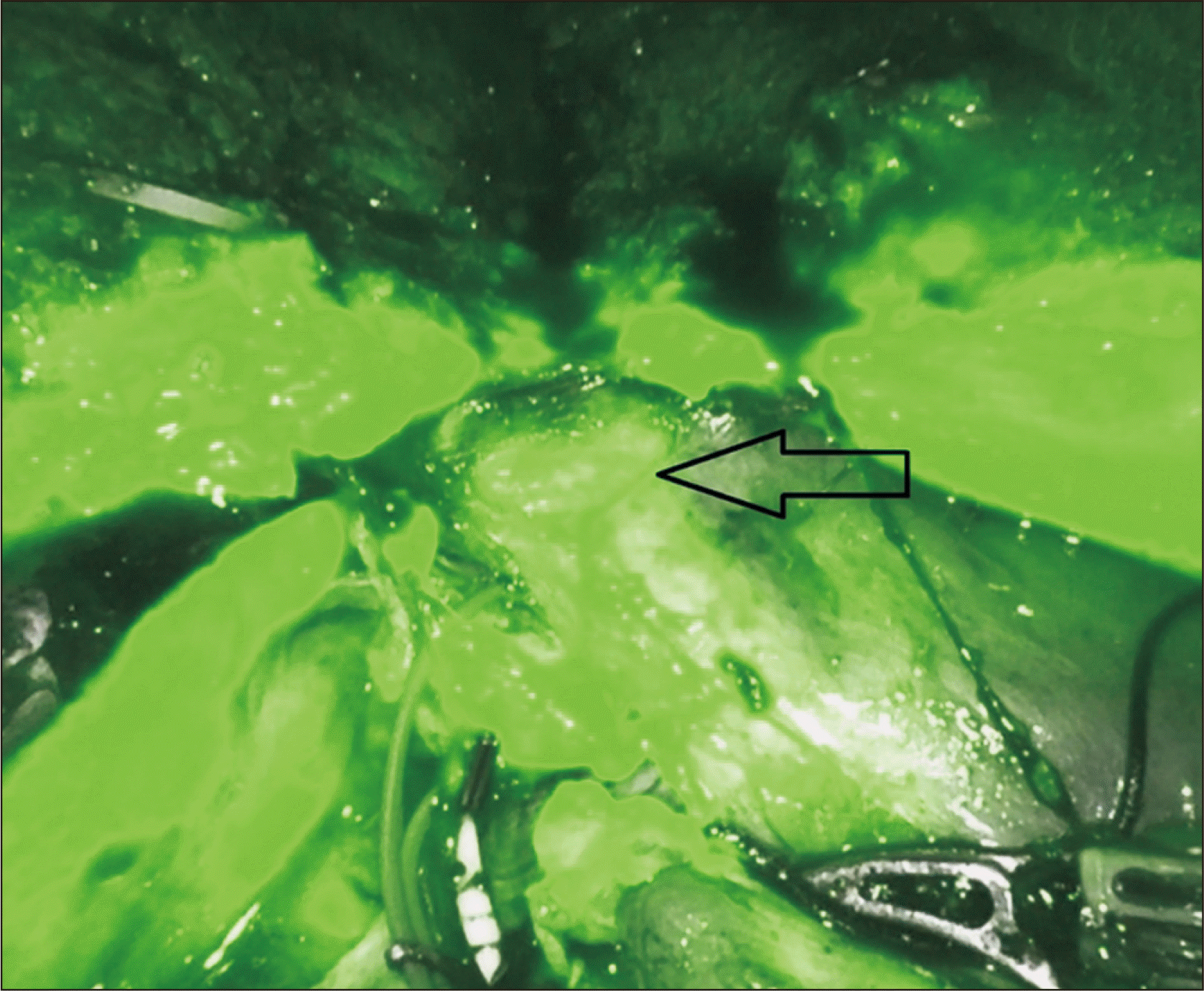
Fig. 6
Clip-and-cut technique; the right hepatic duct is sharply divided proximally after clipping distally.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download