Abstract
The exact mechanism of sialolith formation has yet to be determined. Recurrence of sialolithiasis is rare, affecting only 1%-10% of patients. The current study presents a case of recurrent stones that occurred twice on the right submandibular gland 6 months postoperative and 7 months after reoperation in a 48-year-old female patient. The stones were analyzed using histology, scanning electron microscopy, energy dispersive spectroscopy, and transmission electron microscopy (TEM). The first stone showed a three-layered structure with a poorly mineralized peripheral multilayered zone, highly mineralized middle layer, and the central nidus. The stones were composed of Ca, C, O, Cu, F, N, P, Si, Zn, and Zr. In TEM, compact bi-layered bacterial cell membrane was found on the peripheral layer and the central nidus of the stone as well as exosomes in the central nidus. The results demonstrated the essential components of sialolith formation, including bacteria, inflammatory exosomes, and exfoliated salivary epithelial cells that cooperatively underwent the pathogenetic progresses of central nidus formation, induction of compact zone calcification of the middle layer, and repeated subsequent deposition in the peripheral multilayer zone. The rapid recurrence could have resulted from residual pieces of a sialolith acting as the nidus of bacterial infection.
Sialolithiasis is a disease characterized by mechanical obstruction of the salivary gland caused by partial deposition of calcific materials1,2. Salivary stones occur in approximately 60 cases per million per year and affect approximately 1.2% of the general population. The majority of salivary stones (80%) are located in the submandibular gland, with more than 50% located in the hilar or post hilar aspects and 10% in the intraparenchymal aspect3. This condition is typically associated with pain and swelling of the involved gland caused by obstruction of the food-related surge of salivary secretions4. The higher occurrence in the submandibular gland relative to the parotid gland may be attributed to the anatomy and location of the duct and the alkaline content of saliva that has high mucin and calcium content5.
The exact mechanism of stone formation is not completely understood and several hypotheses have been proposed. The most comprehensive observation describes the occurrence of sialomicroliths in the salivary glands of asymptomatic individuals6. The sialomicroliths accumulate during normal salivary activity and produce atrophic foci that serve as proliferation sites for bacteria to ascend into the duct and lead to inflammation, swelling, and fibrosis. Salivary stones are generally composed of calcium phosphate with small amounts of Mg, NH4+, K, and CO32– with a growth rate of 1-1.5 mm a year ranging from sizes of 0.1-30 mm5,7. Recurrence of sialolithiasis is uncommon and has been documented in only a few studies8 and estimated to occur in 1%-10% of patients.
The primary treatment for salivary stones should be preservation of gland function with low levels of complications and discomfort to the patient. Non-invasive treatments include stimulation of salivary flow and administration of antibiotics and anti-inflammatory drugs in cases where bacterial infection is suspected. In situations where these methods fail, sialendoscopy is a minimally invasive and gland-preserving option7,8.
In the present study, recurrent sialolithiasis in a female patient with two recurrence events after the sialolithotomy was analyzed. The patient was treated with a transoral approach and sialendoscopy and the recurrent stones were analyzed with histopathology, scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and transmission electron microscopy (TEM).
A 48-year-old Korean female was referred to the Department of Oral and Maxillofacial Surgery, Seoul National University with a chief complaint of periodic swelling in the right mandibular region that exhibited pus drainage upon palpation. The Seoul National University Institutional Review Board approved this study (S-D20220023). Upon clinical examination by palpation, a firm swelling was observed with pain in the corresponding area. During the radiographic examination, a 13-mm, round, radiopaque mass was observed in the hilar portion of the right submandibular gland.(Fig. 1, 2. A)
The patient was informed that sialolithiasis was suspected in the right submandibular gland as well as tonsillitis in the left tonsil. After a full explanation of the diagnosis and treatment plan, the patient agreed to undergo the recommended treatment and provided informed consent. A sialolithotomy and tonsillectomy were performed under general and local anesthesia using lidocaine 2% with 1:100,000 epinephrine. A direct linear incision to the stone was made along the longitudinal axis of Wharton’s duct with a No. 15 surgical blade. After gaining access to the stone, an incision was made carefully to avoid the nearby lingual nerve.(Fig. 2. B) Saline irrigation was performed to facilitate visualization of the stone and remove small debris from the surgical field. The first stone, 1.4 cm×0.7 cm×1.1 cm in size, was retrieved.(Fig. 2. C) Sialendoscopy was utilized to explore the duct and irrigate the deeper structure. A drain was placed on the opening duct and the wound was sutured. After the stone was removed, tonsillectomy was performed to remove the mass on the left tonsil.
The clinical and radiographic follow-up appointments were set at 1 week, 1 month, and 6 months postoperatively. At the 1-week follow-up, the patient was instructed to practice tongue movements. At the 1-month follow-up, the patient was prescribed three bottles of artificial saliva. At the 6-month follow-up, radiograph examination revealed recurrent signs of a stone at the previous surgical site.(Fig. 2. D) With the patient’s approval, a sialendoscopy was performed where the duct was irrigated, ductoplasty was completed under local anesthesia, and the stone was removed.(Fig. 2. E, 2. F) A drain was placed upon completion of the surgery. One month after the second surgery, the patient reported spontaneous ejection of a small stone. The patient was followed periodically to monitor healing and observe any signs of recurrence. Radiographic follow-up 7 months after the second surgery showed a larger lesion with greater radiopacity in the same area of the right submandibular gland. The patient also reported painful swelling under her chin. Surgery was scheduled for 1 year later based on the patient’s schedule.
After 1 year, changes in the size and radiopacity of the lesion were not observed.(Fig. 2. G) Under intravenous sedation using midazolam 5 mg and local anesthesia using lidocaine 2% with 1:100,000 epinephrine, surgery was performed. The surgical assistant pushed the submandibular gland extraorally to facilitate exposure of the hilum. A linear incision was made along the duct and tissue dissection was performed to reach the stone. A second stone 7 mm in diameter was identified and removed.(Fig. 2. H, 2. I) A drain was inserted and sutured using 4-0 Polyglactin 910 Vicryl (Johnson & Johnson Co.). Next, ductoplasty-sialendoscopy was performed. The papilla of the submandibular duct was progressively punctured using a probe and dilated and endoscopy was performed. The duct was continuously dilated with saline 0.9% irrigation to avoid collapse of the duct and remove any debris or small stone fragments. Finally, a stent was inserted and sutured and the patient was instructed to massage the surgical site extraorally and perform mouth opening exercises.
The histologic slides of the second stone showed an irregular laminar structure with numerous calcifications. Bacteria was observed scattered throughout the layers.(Fig. 3. A-D) The histologic slides of the third stone showed irregular globular laminar structures and spheroidal bodies. Bacteria was again observed. In a single-occurrence stone, well-defined concentric lamellae were noted with different calcifications interposed by hollow spaces. A small amount of bacteria was observed in the external layer.(Fig. 3. E-H)
The first stone showed a three-layered structure with a poorly mineralized peripheral multilayered zone, highly mineralized middle layer, and the central nidus.(Fig. 4) At the outermost layer of the sialolith, a long rod-shaped bacterial biofilm cave (yellow arrowheads) was observed with calcium nanoparticles at point 01. Points 02, 03, and 04 exhibited dense hydroxyapatite aggregation with empty bacterial casts (blue arrowheads) at 20,000×. Fibrous and irregularly shaped hydroxyapatite crystals were randomly orientated at point 05. Point 06 showed irregularly shaped hydroxyapatite occurring in cluster masses or individual crystals (black arrowheads). Points 07, 09, and 11 revealed coarser hydroxyapatite crystal aggregation. Points 08 and 14 showed a densely aggregated layer of microscopic mineral masses compatible with octacalcium phosphate. Carbonate apatite with different orientations was observed at point 10 (red arrowheads). Calcite-like crystals were noted at point 13 (blue arrows). The irregular structures exhibited platy, rudely hexagonal, and irregularly shaped hydroxyapatite crystals in random orientations (point 13).
The elemental composition of the third stone consistently demonstrated large fractions of Ca, C, and O. Greater traces of chemical elements were observed in the peripheral layer of the sialoliths compared with the middle and core layers. The third stone exhibited an elemental composition of Ca, C, O, Cu, F, N, P, Si, Zn, and Zr.(Fig. 5, Table 1)
Representative TEM images of the first stone revealed bacteria with double membranes on the peripheral layer of the stone (yellow arrowheads).(Fig. 6. A, 6. B) Biological components such as homogenous exosome structure (blue arrowheads) (Fig. 6. C, 6. D), two epithelial cells (black arrows), and a desmosome (white arrow) with a nucleus (asterisk).(Fig. 6. E-H) In the peripheral lamella, a homogenous layer of organic compounds was found on the second stone.(Fig. 7. A) Deposits of large single microcrystalline inorganic compounds were identified.(Fig. 7. B, 7. C) Needle-like filamentary crystals were arranged in clusters and different directions (blue arrowheads).(Fig. 7. A) Exosomes, deposits of inorganic matter in the inner side of the cell, and clusters of prismatic and hexagonal hydroxyapatite crystals were observed at 3,000× and 20,000×.(Fig. 7. C-H)
The presence of sialomicroliths is considered to play a role in stone formation. The accumulation of sialomicroliths during salivary activity facilitates bacterial accumulation, leading to inflammation. This causes duct compression and stagnation and deposition of calcific materials, foreign bodies, microbes, their respective end products, and mucus plugs7. Most of the microbes observed in the saliva of such cases were Streptococcus or Peptostreptococcus species8,9. In the present study, the recurrent sialolith had three main layers: a peripheral multilayered zone, highly mineralized middle layer, and the central nidus.(Fig. 4) A compact bi-layered bacteria cell membrane was found on the outer peripheral layer and the central nidus of the stone. In addition, the prokaryotic nuclear chromatin structure in their cytoplasm indicated the exosomes abundant in the central nidus area were derived from the inflammatory cells recruited for innate immunity against the primary bacterial infection.(Fig. 4, 6, 7) The residual pieces of the sialolith may be the nidus of bacterial infection. The exosomes in the organoid matrix of the central nidus may contain different cytokines and enzymes that can elicit inflammatory reaction and induce the calcification/ossification processes at the outside of the central nidus area.(Fig. 6. C, 6. D, 7. C, 7. D) The results of the present study demonstrated the essential components of sialolith formation, including bacteria (Fig. 4), inflammatory exosomes (Fig. 6. C, 6. D, 7. C, 7. D), and exfoliated salivary epithelial cells (Fig. 6. E-H) that cooperatively underwent the pathogenetic progresses of central nidus formation, induction of compact zone calcification of the middle layer, and repeated subsequent deposition in the peripheral multilayer zone.
The patient reported after the second sialendoscopy surgery that a small fragment of a smaller stone had spontaneously extruded, which may have been a nidus for recurrent stone formation. The second stone was similarly stellate, irregularly shaped, and as large as the first stone. Trujillo et al.7 described the composition and shape differences between the initial stone and recurrent stones. The authors found the recurrent stone was primarily composed of calcium phosphate, whereas the initial stone was 50% brushite and 50% calcium phosphate7. This indicates recurrence of a new stone rather than a remnant of the first stone. In the present study, the recurrent stone contained an organic component dominated by O, C, and Ca, followed by F and Si. Organic domination in the recurrent stone was also noted in the histologic finding based on the large eosinophilic zone and globular structures. In the present study, the structure was irregular, with a laminated structure and clearly defined border of inorganic and organic compounds without a specific core. The stellate and irregularly shaped stones were associated with recurrent inflammation and infection that was likely to induce growth perturbations.
Recurrence of sialolithiasis after minimally invasive surgery is rare, accounting for only 5% of cases. In a previous study, recurrence of sialolithiasis occurred from 3 months after treatment throughout the follow-up period until 46 months after treatment. Galli et al.10 determined that most of the recurrent cases involved the same gland and concluded that salivary gland stones result from pathology of the duct and not the saliva. In the present study, recurrence was observed twice postoperatively: 6 months after the first surgery and 7 months after the second surgery.
Several factors have been proposed for recurrence including severe ductal injury, multiple stone development, chronic infection/inflammation, and altered stone composition and shape7. The location of the stone can also contribute to the recurrence, in which the proximal stone exhibits a higher recurrence rate due to the anatomy of Wharton’s duct complicating the extraction. Furthermore, partial removal of stones due to residual fragments or multiple or large stones showed a higher rate of recurrence compared with complete removal11. Galli et al.10 also explained that during surgery, the surgeon should remove all stones identified on preoperative imaging. However, preoperative imaging is not reliable for actual stone count, especially if several stones are in contact with each other, and whether a small fragment was left in place during the procedure cannot be assessed. In the same manner, a small fragment of a single stone may break off during the procedure and be left in place, especially in the case of an impacted stone. To reduce this risk, controlling the permeability of the duct through retrograde irrigation is important10.
The current approach to treatment for sialolithiasis focuses on organ preservation. Ideally, minimally invasive intervention is recommended as the first line of treatment. Sialoliths that are larger than 3 mm and located proximal to the gland may be retrieved endoscopically or surgically using sialolithotomy12. An intraductal camera and a basket retriever may be used in sialendoscopy. Extracorporeal shock-wave lithotripsy is still effective in combination with intracorporeal shockwave lithotripsy treatment which makes the stone amenable, and the two methods can supplement each other13. If minimally invasive methods fail, the entire gland needs to be surgically removed. Surgical treatment may be intraoral or extraoral and may include the extraction of the sialolith or excision of the gland itself8.
According to Gerni et al.14, a stone larger than 8 mm has a higher incidence of sialolithiasis recurrence due to two hypotheses. In the first hypothesis, large stones can be more friable, hindering complete removal. In the second hypothesis, large stones can increase the diameter of the hilum, which creates saliva flow anomalies such as turbulence and a slower rate of salivary flow, which are favorable for new stone development14. This may explain the recurrence of sialolithiasis in the present case with an initial stone 1.4 cm in size; the sialolith may not have been completely removed and the residual pieces of sialolith might have caused recurrence.
Based on histology, SEM, EDS, and TEM analyses, the recurrent stone exhibited three main layers: a poorly mineralized multilayered peripheral layer, highly mineralized middle layer, and the central nidus. The results of the present study demonstrated the essential components of sialolith formation, including bacteria, inflammatory exosomes, and exfoliated salivary epithelial cells that cooperatively underwent the pathogenetic progresses of central nidus formation, induction of compact zone calcification of the middle layer, and repeated subsequent deposition in the peripheral multilayer zone. The rapid recurrence observed within the 6-month and 7-month postoperative follow-up periods could be explained by residual pieces of the sialolith acting as the nidus of bacterial infection.
Notes
Authors’ Contributions
B.S.-I. analyzed the data and wrote the manuscript. M.Y.E. corrected data and wrote the manuscript. K.R.M. contributed to the manuscript preparation. Y.J.C. prepared the figures. S.M.K. designed the study and coordinated and carefully reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
References
1. Barry R, Schaitkin BM, Walvekar RR. Gillespie MB, Walvekar RR, Schaitkin BM, Eisele DW, editors. 2018. Submandibular stones. Gland-preserving salivary surgery: a problem-based approach. Springer;p. 57–68. DOI: 10.1007/978-3-319-58335-8_6. PMCID: PMC6702475.

2. Lim HK, Kim SM, Kim MJ, Lee JH. 2012; Clinical, statistical and chemical study of sialolithiasis. J Korean Assoc Oral Maxillofac Surg. 38:44–9. https://doi.org/10.5125/jkaoms.2012.38.1.44. DOI: 10.5125/jkaoms.2012.38.1.44.

3. Koch M, Schapher M, Mantsopoulos K, Goncalves M, Iro H. 2019; Intraductal pneumatic lithotripsy after extended transoral duct surgery in submandibular sialolithiasis. Otolaryngol Head Neck Surg. 160:63–9. https://doi.org/10.1177/0194599818802224. DOI: 10.1177/0194599818802224. PMID: 30296893.

4. Nolasco P, Anjos AJ, Marques JM, Cabrita F, da Costa EC, Maurício A, et al. 2013; Structure and growth of sialoliths: computed microtomography and electron microscopy investigation of 30 specimens. Microsc Microanal. 19:1190–203. https://doi.org/10.1017/s1431927613001694. DOI: 10.1017/S1431927613001694. PMID: 24001782.

5. Austin T, Davis J, Chan T. 2004; Sialolithiasis of submandibular gland. J Emerg Med. 26:221–3. https://doi.org/10.1016/j.jemermed.2003.07.007. DOI: 10.1016/j.jemermed.2003.07.007. PMID: 14980352.

6. Schapher M, Koch M, Weidner D, Scholz M, Wirtz S, Mahajan A, et al. 2020; Neutrophil extracellular traps promote the development and growth of human salivary stones. Cells. 9:2139. https://doi.org/10.3390/cells9092139. DOI: 10.3390/cells9092139. PMID: 32971767. PMCID: PMC7564068.

7. Trujillo O, Drusin MA, Rahmati R. 2017; Rapid recurrent sialolithiasis: altered stone composition and potential factors for recurrence. Laryngoscope. 127:1365–8. https://doi.org/10.1002/lary.26357. DOI: 10.1002/lary.26357. PMID: 27753112.

8. Kraaij S, Karagozoglu KH, Forouzanfar T, Veerman EC, Brand HS. 2014; Salivary stones: symptoms, aetiology, biochemical composition and treatment. Br Dent J. 217:E23. https://doi.org/10.1038/sj.bdj.2014.1054. DOI: 10.1038/sj.bdj.2014.1054. PMID: 25476659.

9. Duong LT, Kakiche T, Ferré F, Nawrocki L, Bouattour A. 2019; Management of anterior submandibular sialolithiasis. J Oral Med Oral Surg. 25:16. https://doi.org/10.1051/mbcb/2018039. DOI: 10.1051/mbcb/2018039.

10. Galli P, Ceva A, Foletti JM, Iline N, Giorgi R, Chossegros C, et al. 2021; Salivary gland lithiasis recurrence after minimally-invasive surgery: incidence, risk factors and prevention. Laryngoscope. 131:794–9. https://doi.org/10.1002/lary.28991. DOI: 10.1002/lary.28991. PMID: 32786079.

11. Kim JK, Shin SM, Lee H, Lee S. 2016; Factors affecting long-term outcome of transoral surgery for submandibular stones: a follow-up study of 125 patients. Clin Otolaryngol. 41:365–70. https://doi.org/10.1111/coa.12523. DOI: 10.1111/coa.12523. PMID: 26292653.

12. Avishai G, Ben-Zvi Y, Ghanaiem O, Chaushu G, Gilat H. 2020; Sialolithiasis-do early diagnosis and removal minimize post-operative morbidity? Medicina (Kaunas). 56:332. https://doi.org/10.3390/medicina56070332. DOI: 10.3390/medicina56070332. PMID: 32630773. PMCID: PMC7404452.

13. Koch M, Mantsopoulos K, Müller S, Sievert M, Iro H. 2021; Treatment of sialolithiasis: what has changed? An update of the treatment algorithms and a review of the literature. J Clin Med. 11:231. https://doi.org/10.3390/jcm11010231. DOI: 10.3390/jcm11010231. PMID: 35011971. PMCID: PMC8746135.

14. Gerni M, Foletti JM, Collet C, Chossegros C. 2017; Evaluation of the prevalence of residual sialolith fragments after transoral approach of Wharton's duct. J Craniomaxillofac Surg. 45:167–70. https://doi.org/10.1016/j.jcms.2016.04.011. DOI: 10.1016/j.jcms.2016.04.011. PMID: 28040303.

Fig. 1
Preoperative computed tomography images showing a 13-mm round radiopaque mass (arrowheads) on the hilar portion of the right submandibular glands in the axial (A), coronal (B), and sagittal view (C).
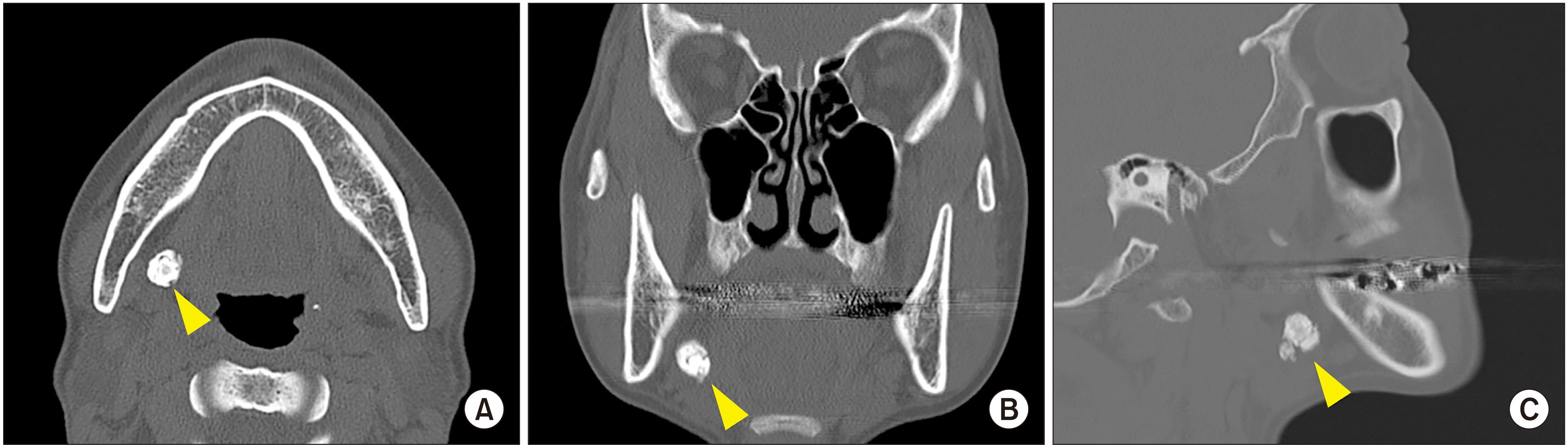
Fig. 2
A. Panoramic view revealed the presence of a stone in the submandibular gland area (arrowheads). B. During the first sialolithotomy operation, the stone was identified after access opening through a transoral approach. C. A 1.4 cm×0.7 cm×1.1 cm stone was retrieved. D. The panoramic radiograph showed signs of recurrence (arrowheads). E. The second surgery consisting of ductoplasty-sialendoscopy was performed 6 months later after signs of recurrence were observed. F. The retrieved stone. G. The panoramic radiograph showed the presence of a recurrent stone in the same area of the submandibular gland with a higher radiopacity compared with the previous radiograph (arrowheads). H. For the third sialolithotomy, the stone was identified in the same site as in the previous surgery. I. A 0.6 cm×0.4 cm×0.3 cm stone was retrieved.

Fig. 3
A. Histological findings of the second stone showing no distinct core (H&E staining, 4×; scale bar=500 μm). B. Highly mineralized globular structures found in the center of the sialolith (black arrows; H&E staining, 20×; scale bar=50 μm). C. Details of alternating layers of mineralized globular structures (black arrows; H&E staining, 20×; scale bar=50 μm). D. Amorphous basophilic materials with mineralized nodules (black arrowhead; H&E staining, 20×; scale bar=50 μm). E. Histological findings of the third stone showing no distinct core (H&E staining, 4×; scale bar=500 μm). F. A nodule with a highly mineralized outer layer (black asterisk; H&E staining, 20×; scale bar=50 μm). G. Alternating layers of mineralized and less mineralized globular structures (H&E staining, 20×; scale bar=50 μm). H. Globular structures in the outer layer (black arrows; H&E staining, 20×; scale bar=50 μm).
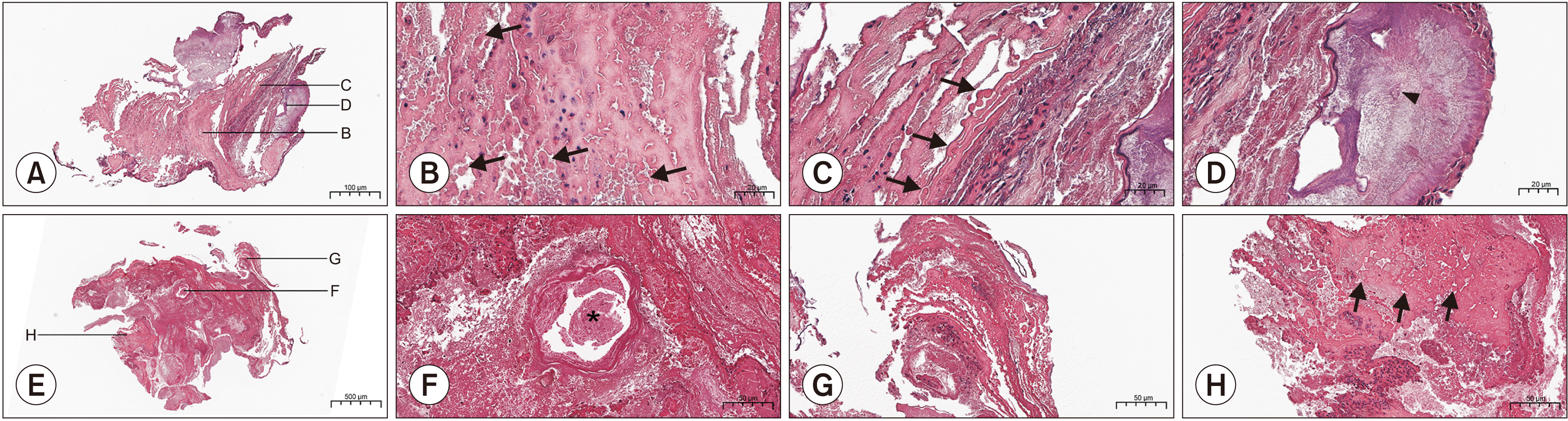
Fig. 4
Combined scanning electron microscopy images of the third stone with 14 points of interest. A long rod-shaped bacterial biofilm cave (yellow arrowheads) was observed with calcium nanoparticles at point 01. Points 02, 03, and 04 exhibited dense hydroxyapatite aggregation with empty bacterial casts (blue arrowheads). Fibrous and irregularly shaped hydroxyapatite crystals were randomly orientated at point 05. Point 06 showed irregularly shaped hydroxyapatite occurring in cluster masses or individual crystals (black arrowheads). Points 07, 09, and 11 revealed coarser hydroxyapatite crystal aggregation. Points 08 and 14 showed a densely aggregated layer of microscopic mineral masses compatible with octacalcium phosphate. Carbonate apatite with different orientations was observed at point 10 (red arrowheads). Calcite-like crystals were noted at point 13 (blue arrows).

Fig. 5
Energy dispersive spectroscopy (EDS) results of the third stone with five representative points of interest on the peripheral (P), middle (M), and core (C) layers. (SEM: scanning electron microscopy)

Fig. 6
A, B. Representative transmission electron microscopy images of the first sialolith. Bacteria with double membranes are observed on the peripheral layer of the stone (yellow arrowheads; A: 2,000×, B: 10,000×). C, D. A homogenous structure of exosome (blue arrowheads; C: 2,000×, D: 1,000×). E, F. Two epithelial cells (marked with black arrows) and a desmosome (white arrow; E: 2,000×, F: 10,000×). G. The nucleus and the endoplasmic reticulum of the epithelial cell (asterisk; 6,000×). H. The desmosome of the cell at higher magnification (20,000×).
Fig. 7
A, B. In the peripheral lamella of the second stone, exosomes (blue arrowheads; 3,000×) and a homogenous layer of organic compounds was found (yellow arrowheads; 20,000×). C, D. Exosomes (C: 3,000×, D: 10,000×). E, F. Deposition of inorganic matter in the inner side of the cell (white arrows; E: 3,000×, F: 20,000×). G, H. Clusters of prismatic and hexagonal hydroxyapatite crystals (G: 3,000×, H: 20,000×).
Table 1
Elemental composition of third sialolith at the peripheral, middle and core regions




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download