Abstract
Background/Aims
Helicobacter pylori (H. pylori) is the most prevalent infection in the world and is strongly associated with gastric adenocarcinoma, lymphoma and gastric or duodenal ulcers. Different regimens have been used for H. pylori eradication. We aimed to compare the efficacy of two different regimens as first-line H. pylori eradication regimens, in an area with high antibiotic resistance.
Methods
In this RCT, we assigned 223 patients with H. pylori infection, who were naïve to treatment. They were randomly divided into two groups to receive either 12-day concomitant quadruple therapy (consisting of pantoprazole 40 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500 mg every 12 hours) or 14-day high dose dual therapy (consisting of esomeprazole 40 mg and amoxicillin 1 g TDS). H. pylori eradication was assessed eight weeks after the end of treatment.
Results
H. pylori eradication rate by PP analysis for 12-day concomitant quadruple therapy and 14-day high dose dual therapy were 90.4% and 79.1%, respectively (p=0.02). According to ITT analysis, the eradication rates were 86.2% and 76.3%, respectively (p=0.06). Adverse drug reactions were 12.3% in high dose dual therapy and 36.8% in concomitant quadruple therapy (p<0.001).
Conclusions
Twelve-day concomitant therapy seems to be an acceptable regimen for first-line H. pylori eradication in Iran, a country with a high rate of antibiotic resistance. Although, high dose dual therapy did not result in an ideal eradication rate, but it had fewer drug side effects than the 12-day concomitant regimen.
Go to : 
Almost 50% of the world’s population is infected with Helicobacter pylori (H. pylori). This organism plays an important role in the development of upper gastrointestinal tract diseases, such as peptic ulcer disease, gastric adenocarcinoma, and MALT lymphoma.1 H. pylori eradication accelerates the healing of peptic ulcers and prevents their recurrence.2 Despite many efforts to achieve a proper H. pylori eradication regimen in recent decades, up to 15% to 20% treatment failure is seen in H. pylori eradication regimens.3-5 Thus, the need for new clinical trials to determine an appropriate treatment is obvious. The most important challenge in the treatment of H. pylori infection is antibiotic resistance. Over the past 20 years, H. pylori antibiotic resistance has been increasing in different parts of Iran.6 In a systematic review in 2015, Khademi et al.7 showed that in Iran, the average H. pylori antibiotic resistance rate to metronidazole, clarithromycin, amoxicillin, and furazolidone were 66.6%, 22.4%, 16.0%, and 21.6%, respectively.
In another study in Iran by Saniee et al.8 H. pylori antibiotic resistance to metronidazole, clarithromycin, and amoxicillin was 74.9%, 34.4%, and 27.1%, respectively.
Previous studies from different parts of the world have reported acceptable H. pylori eradication with 14-days concomitant quadruple therapy.9-15 In Spain in 2013, the per-protocol (PP) eradication rates of the concomitant quadruple regimen was 96.1%.10 In the study in Italy in 2014, the PP eradication rate for 14-day concomitant quadruple therapy was 95%, while a five-day concomitant quadruple regimen did not produce the desired result.12 PP eradication rates by 10- and 14-day concomitant regimens in Korea were reported to be 95.6% and 98.5%, respectively.11 Another study in Korea in 2018 reported a 95.5% PP eradication rate by a 10-day concomitant quadruple regimen.13 Studies from Taiwan, a country with low antibiotic resistance, have also reported an acceptable eradication rate for 7-, 10-, and 14-day concomitant quadruple therapies.16 Two studies from Iran have also evaluated the effectiveness of concomitant quadruple therapy. In 2015, Alhooei et al.14 reported an unacceptable eradication rate (83.1%) with 10-day concomitant quadruple therapy. In 2020, Bari et al.15 reported H. pylori eradication rates of 88.8% and 92.6% for 10- and 12-day concomitant quadruple therapies, respectively, based on intention to treat (ITT) analysis.
In a review by Fakheri et al.6 H. pylori eradication rates with non-bismuth-based quadruple therapy regimens were 79.9% up to 92.9%. These low H. pylori eradication rates in our country compared to other regions of the world are due to the high H. pylori antibiotic resistance to metronidazole and clarithromycin.7,8 Therefore the high dose dual therapy of amoxicillin plus proton pomp inhibitor (PPI) in the absence of clarithromycin and metronidazole may be an alternative regimen for overcoming this problem. Various studies evaluated the effectiveness of high dose dual therapy regimen in the eradication of H. pylori and have shown that high dose dual therapies have good efficacy with better patient drug compliance, lower costs, and fewer side effects.17-21 However, these studies have been conducted mainly in areas with low H. pylori antibiotic resistance. As we know, Iran is a country with a high rate of multi-drug-resistant H. pylori infection.7,8
The result of our previous high dose dual therapy regimen in H. pylori eradication was unacceptable borderline and based on PP analysis was 82.2%.22 The optimal activity of amoxicillin depends on the pH of the environment and requires a pH of 6 or higher.23 As the pH increases above 6, the bacterium starts to multiply and in turn becomes more sensitive to amoxicillin.24 In this study, we aimed to assess H. pylori eradication rate by increasing the dose of esomeprazole in high dose dual therapy regimen from 40 mg BID to 40 mg TDS.
Go to : 
In this study, patients who were presented with a long term history of dyspepsia and showing signs and symptoms of high risk for gastric malignancy were included in the study (positive family history of gastric cancer, age greater than 40 years, IDA, dyspepsia non-responsive to PPI, hematemesis or melena) and upper GI endoscopy was done for all of them. After upper GI endoscopy, we enrolled all patients who have gastric or duodenal ulcers or erosion, those who have intestinal metaplasia in pathology and or have positive familial history of gastric cancer, and have H. pylori infection in giemsa staining of pathology samples. Patient who have previous history of gastric surgery, those who were given a course of H. pylori eradication regimen or, who have advanced heart, liver or lung disease and or a history of adverse reaction to drugs used in this porotocol were excluded from the study. Patients underwent upper GI endoscopy by expert collaborating gastroenterologists using a Pentax endoscope (version: EG2985; Pentax, Tokyo, Japan). During EGD, gastric mapping was done for all patients (two biopsies from the antrum, one biopsy from angularis, and two biopsies from the body). Also, biopsies were taken from any abnormal visible mucosal lesion. H. pylori infection was reported by pathologist co-workers by giemsa staining of pathology samples.
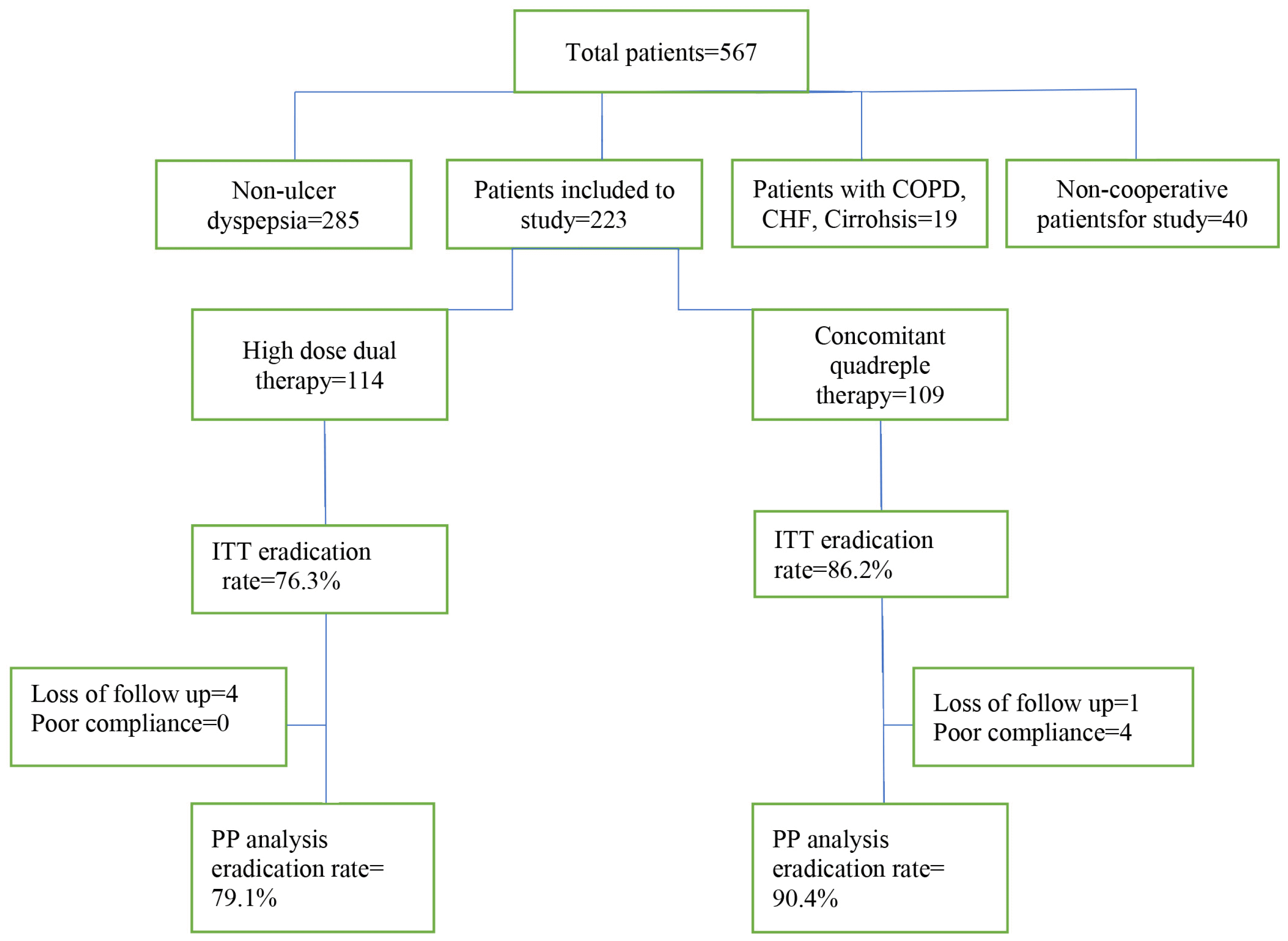
From 567 patients with signs and symptoms of dyspepsia, according to inclusion and exclusion criteria, finally, 223 patients were included in the study. Informed consents were obtained from all participants. patients were clustered into two groups and were taken two different regimens for H. pylori eradication. One group, including 109 patients were taken concomitant quadruple therapy regimen (first group) and another group of patients including 114 patients were taken high dose dual therapy regimen (second group). In first group, patients were given pantoprazole 40 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500, all twice daily for 12 days. And in the second group, patients were given high dose dual therapy composed of Ezonium (esomeprazole of Dr. Abidi Pharmaceutical Company) 40 mg/BID and amoxicillin 1 g TDS for 14-day.
For all patients a questionnaire including demographic information of age, sex, history of aspirin and NSAID usage and cigarette smoking as well as endoscopic findings, pathology results, and data of H. pylori infection were completed.
H. pylori eradication was assessed by an H. pylori stool antigen test at least eight weeks after the end of treatment. During treatment, compliance of patients for drug consumption and drug side effects were recorded for all patients. Severe drug side effects were defined when patients can not continued drug consumption. If the patients consumed more than 80% of the prescribed drugs: the compliance was considered good. Those who took 60% to 80% of the drugs and those who consumed less than 60% were categorized as intermediate and low compliance respectively.
The data was analyzed using SPSS-24 software (IBM Co., Armonk, NY, USA). Chi-square and t-tests were used for comparing qualitative and quantitative data, respectively. A p-value <0.05 was considered statistically significant. To calculate eradication rate based on intention to treat, all patients who were initially included in the study were analyzed. To calculate eradication rate based on per protocol, only patients who followed all the steps of the study protocol and took more than 80% of the drugs were included in the statistical analysis. The current study proposal was approved by the scientific members of the Gut and Liver Research Center and the Ethics Committee of Mazandaran University of Medical Sciences (ethics code: IR.MAZUMS.IMAMHOSPITAL.REC.1400.093). Moreover, this study was registered in the Iranian Registry of Clinical Trials with the IRCT number of IRCT20131124015510N5. Written informed consent was taken from the patients to include the clinical details.
Go to : 
A total of 223 patients were included in the study. One hundred and nine patients received 12-day concomitant quadruple therapy, and 114 patients received the 14-day high dose dual therapy regimen. In the concomitant regimen, 44% of the patients and in the high dose dual therapy regimen, 50.9% of patients were men (p=0.3) with the average age of 44.03 and 49.02, respectively (p=0.003). There was no significant difference between the two groups in smoking, but patients in high dose dual therapy group had more Aspirin and NSAID consumption than the concomitant group. The most common endoscopic finding in all patients was peptic ulcer disease (Table 1).
Demographic Characteristics and Endoscopic Findings of Patients of Two Groups
Based on ITT analysis, the H. pylori eradication rate in the concomitant quadruple therapy regimen and high dose dual therapy were 86.2% (95% confidence interval [CI], 78.5–91.6%) and 76.3% (95% CI, 70.4–84.1%), respectively (p=0.06). Two hundred fourteen patients of the 223 patients completed the study. One patient in the concomitant quadruple therapy regimen and 4 patients in the high dose dual therapy group did not complete the study protocol due to improvement in clinical symptoms and lack of cooperation in conducting the final test for evaluating H. pylori eradication. Four patients in concomitant quadruple therapy regimen had low compliance for drug consumption and were excluded from the study. Therefore, according to PP analysis, H. pylori eradication rate in the concomitant quadruple therapy and high dose dual therapy were 90.4% (95% CI, 82.3–95.8%) and 79.1% (95% CI, 72.8–87.5%), respectively (p=0.02) (Fig. 1). In none of the treatment groups, there was any correlation between endoscopic findings and treatment success rate. The rate of drug side effects was 35.8% in the concomitant quadruple and 12.3% in high dose dual therapy regimens (p<0.001). The most common side effect was bitter taste, (observed in 16% of patients in concomitant regimen but none of patients in high dose dual therapy regimen) and followed by headache and nausea.
Moreover, the rate of treatment acceptance was 87.2% in the concomitant quadruple therapy regimen and 93% in the high dose dual therapy group (p=0.04) (Table 2).
Frequency and Severity of Side Effects and Treatment Acceptance Rate in Two Groups
Go to : 
Considering that H. pylori is an infectious disease, an ideal treatment regimen is one that can eradicate H. pylori in more than 95% of cases, with less than 5% severe side effects.25
According to the evidence-based study by Graham et al.26 for evaluation of new H. pylori eradication regimen, based on PP analysis, the success rate of ≥95% is considered an excellent eradication rate, 90–95% is considered good, 85–89% is considered fairly good (borderline acceptable), 81–84% is considered poor eradication, and <80% is considered borderline unacceptable. Therefore, according to the results of our study, the 12-day concomitant quadruple regimen is considered as a treatment regimen with a good eradication rate (with PP eradication rate of 90.4%), but the eradication rate of 14-day high dose dual therapy regimen is unacceptable in our region (with PP eradiction rate of 79.1%).
H. pylori antibiotic resistance rate has increased in different regions of Iran.6 In 2005, the highest antibiotic resistance rate in Iran was related to metronidazole (57.4%), while resistance to amoxicillin was very low (1.6%).27 In the study in 2019, H. pylori antibiotic resistance was evaluated and 82.4% of the isolated organisms were resistant to metronidazole, followed by 33.8% resistance to clarithromycin and 30.9% resistance to amoxicillin. Overally, 75% of the isolated oragnisms were resistant to at least two antibiotics (aka. multidrug resistance).28
In recent years, researchers have conducted various studies to provide a successful regimen as the first line treatment of H. pylori infection. One of the promising regimens they investigated is high dose dual therapy regimen by using amoxicillin plus PPI. Considering the increasing prevalence of H. pylori resistance to clarithromycin and metronidazole, the administration of high dose amoxicillin may be a suitable alternative to the mentioned antibiotics.17
The optimal activity of amoxicillin is pH dependent and requires pH of 6 or higher. On the other hand, PPIs by reducing gastric acidity increase the concentration of amoxicillin in the stomach to more than the minimum inhibitory concentration for H. pylori. Therefore, they significantly increase the H pylori eradication effect of amoxicillin.24
A systematic review showed that high dose dual therapy had similar successful eradication rate compared to bismuth- containing concomitant regimen, while having fewer side effects.18 In the study by Zullo et al.17 in Italy, a 87.5% H. pylori eradication rate was reported with high dose dual therapy regimen of esomeprazole and amoxicillin. Also, the dual therapy regimen of rabeprazole plus high dose amoxicillin had an eradication rate of 95.3% as the first line regimen for H. pylori infection eradication in Taiwan in 2015.20
Contrary to these successful results of H. pylori eradication by high dose dual therapy, studies from Korea and China, similar to the results of our study, reported the ineffectiveness of high dose dual therapy regimen as the first line of H. pylori eradication. Their study showed excellent compliance and minimal side effects, similar to our study.29,30 However, Yu et al.21 reported an eradication rate of >90% in China in 2019 with a dual therapy regimen of esomeprazole plus high dose amoxicillin. Amoxicillin resistance was 0% in this study.
In another study in the same year in China, Yang et al.18 evaluated the efficacy of a modified dual therapy regimen (esomeprazole plus Amoxicillin) for H. pylori eradication, and achieved H. pylori eradication rate of 91.1% based on PP analysis. In this study, H. pylori antibiotic resistance to amoxicillin was similar to the study of Yu et al.21 and was 0% resistance.18
In a study conducted by Yadollahi and I and our colleagues in 2022, H. pylori eradication rate based on PP analysis for 14-day concomitant quadruple therapy and 14-day high dose dual therapy regimen (amoxicillin 1,000 mg three times a day, esomeprazole 40 mg twice a day) were 88.6% and 81.6% respectively.22
The conflicting results of the efficacy of high dose dual therapy in past studies may be largely attributed to differences in the dose and frequency of administration of PPI and amoxicillin. Higher PPI doses,29 and longer treatment durations30,31 significantly improve success rate.32
In this study, we increased the frequency of administration of esomeprazole compared to our previous study (increasing the dose of esomeprazole 40 mg BID to 40 mg TDS) but didn’t observe an increased H. pylori eradication rate. Resistance to amoxicillin is probably the cause of this failure. The second goal in choose of a regimen for eradication of H. pylori is reduction of drug side effects and better patient's compliance for drug consumption.25 Similar to the result of our study, in other studies conducted with high dose dual therapy regimen, the frequency of drug side effects was low and the patient's compliance for drug consumption was high.17,19,29 This can be the advantage of the high dose dual therapy regimen of amoxicillin and PPI compared to the concomitant quadruple therapy regimen in the treatment of H. pylori eradication.
The main limitation of the present study was the unavailability of H. pylori culture and antibiotic susceptibility/resistance test. However, the strength of this study is that this is one of the first studies evaluating the efficacy of high dose dual therapy for H. pylori eradication in Iran, a region with high resistance to metronidazole and clarithromycin.
Twelve days concomitant quadruple therapy can be considered an acceptable regimen for H. pylori eradication in areas of high clarithromycin and metronidazole resistance. But 14-day high dose dual therapy regimen with esomeprazole and amoxicillin seems to be unable to the achieve ideal eradication rate. Further studies with high dose PPI and amoxicillin plus another antibiotic such as bismuth are recommended to be evaluated as first line H. pylori eradication therapy in regions with high clarithromycin and metronidazole resistance.
Go to : 
Notes
Financial support
This study was supported by the Vice Chancellor of Research at the Mazandaran University of Medical Sciences (Grant no: 093). The members involved in this study are faculty members of Mazandaran University of Medical Sciences. To conduct the study, we have the financial support of the University's Research Vice-Chancellor, but this financial support does not affect the results of the study.
Go to : 
REFERENCES
1. Go MF. 2002; Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 16 Suppl 1:3–15. DOI: 10.1046/j.1365-2036.2002.0160s1003.x. PMID: 11849122.
2. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006; (2):CD003840. DOI: 10.1002/14651858.CD003840.pub4.

3. Gisbert JP, Calvet X. 2011; Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 34:604–617. DOI: 10.1111/j.1365-2036.2011.04770.x. PMID: 21745241.

4. Gisbert JP, Calvet X, O'Connor A, Mégraud F, O'Morain CA. 2010; Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 44:313–325. DOI: 10.1097/MCG.0b013e3181c8a1a3. PMID: 20054285.
5. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; Aug. 8. doi: 10.1136/gutjnl-2022-327745. DOI: 10.1136/gutjnl-2022-327745. PMID: 35944925.
6. Fakheri H, Saberi Firoozi M, Bari Z. 2018; Eradication of Helicobacter pylori in Iran: A review. Middle East J Dig Dis. 10:5–17. DOI: 10.15171/mejdd.2017.84. PMID: 29682242. PMCID: PMC5903928.

7. Khademi F, Poursina F, Hosseini E, Akbari M, Safaei HG. 2015; Helicobacter pylori in Iran: A systematic review on the antibiotic resistance. Iran J Basic Med Sci. 18:2–7.
8. Saniee P, Hosseini F, Kadkhodaei S, Siavoshi F, Khalili-Samani S. 2018; Helicobacter pylori multidrug resistance due to misuse of antibiotics in Iran. Arch Iran Med. 21:283–288.
9. Zullo A, Scaccianoce G, De Francesco V, et al. 2013; Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 37:647–650. DOI: 10.1016/j.clinre.2013.04.003. PMID: 23747131.

10. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. 2013; Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 145:121–128.e1. DOI: 10.1053/j.gastro.2013.03.050. PMID: 23562754.

11. Park SM, Kim JS, Kim BW, Ji JS, Choi H. 2017; Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 32:589–594. DOI: 10.1111/jgh.13510. PMID: 27505301.

12. De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. 2014; Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 63:748–752. DOI: 10.1099/jmm.0.072322-0. PMID: 24586031.

13. Choe JW, Jung SW, Kim SY, et al. 2018; Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter. 23:e12466. DOI: 10.1111/hel.12466. PMID: 29369454.

14. Alhooei S, Tirgar Fakheri H, Hosseini V, et al. 2016; A Comparison between hybrid and concomitant regimens for Helicobacter pylori eradication: A randomized clinical trial. Middle East J Dig Dis. 8:219–225. DOI: 10.15171/mejdd.2016.24. PMID: 27698972. PMCID: PMC5045675.

15. Bari Z, Fakheri H, Taghvaei T, Yaghoobi M. 2020; A Comparison between 10-day and 12-day concomitant regimens for Helicobacter pylori eradication: A randomized clinical trial. Middle East J Dig Dis. 12:106–110. DOI: 10.34172/mejdd.2020.169. PMID: 32626563. PMCID: PMC7320987.

16. Yao CC, Kuo CM, Hsu CN, et al. 2019; First-line Helicobacter pylori eradication rates are significantly lower in patients with than those without type 2 diabetes mellitus. Infect Drug Resist. 12:1425–1431. DOI: 10.2147/IDR.S194584. PMID: 31239721. PMCID: PMC6554512.
17. Zullo A, Ridola L, Francesco VD, et al. 2015; High-dose esomeprazole and amoxicillin dual therapy for first-line Helicobacter pylori eradication: a proof of concept study. Ann Gastroenterol. 28:448–451.
18. Yang X, Wang JX, Han SX, Gao CP. 2019; High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: A systematic review and meta-analysis. Medicine (Baltimore). 98:e14396. DOI: 10.1097/MD.0000000000014396. PMID: 30762742. PMCID: PMC6408008.
19. Yang J, Zhang Y, Fan L, et al. 2019; Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 114:437–445. DOI: 10.14309/ajg.0000000000000132. PMID: 30807294.

20. Yang JC, Lin CJ, Wang HL, et al. 2015; High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 13:895–905.e5. DOI: 10.1016/j.cgh.2014.10.036. PMID: 25460556. PMCID: PMC4404168.
21. Yu L, Luo L, Long X, et al. 2019; High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: A randomized trial. Helicobacter. 24:e12596. DOI: 10.1111/hel.12596. PMID: 31111580.

22. Yadollahi B, Valizadeh Toosi SM, Bari Z, et al. 2022; Efficacy of 14-day concomitant quadruple therapy and 14-day high-dose dual therapy on H. pylori eradication. Gastroenterol Hepatol Bed Bench. 15:172–178.
23. Cheng A, Sheng WH, Liou JM, et al. 2015; Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J Microbiol Immunol Infect. 48:72–79. DOI: 10.1016/j.jmii.2012.08.021. PMID: 23036269.

24. Salcedo JA, Al-Kawas F. 1998; Treatment of Helicobacter pylori infection. Arch Intern Med. 158:842–851. DOI: 10.1001/archinte.158.8.842. PMID: 9570169.

25. Graham DY, Lu H, Yamaoka Y. 2007; A report card to grade Helicobacter pylori therapy. Helicobacter. 12:275–278. DOI: 10.1111/j.1523-5378.2007.00518.x. PMID: 17669098.
26. Graham DY, Lee YC, Wu MS. 2014; Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 12:177–186.e3. DOI: 10.1016/j.cgh.2013.05.028. PMID: 23751282. PMCID: PMC3830667.

27. Mohammadi M, Doroud D, Mohajerani N, Massarrat S. 2005; Helicobacter pylori antibiotic resistance in Iran. World J Gastroenterol. 11:6009–6013. DOI: 10.3748/wjg.v11.i38.6009. PMID: 16273615. PMCID: PMC4436725.
28. Farzi N, Yadegar A, Sadeghi A, et al. 2019; High prevalence of antibiotic resistance in Iranian Helicobacter pylori Isolates: Importance of functional and mutational analysis of resistance genes and virulence genotyping. J Clin Med. 8:2004. DOI: 10.3390/jcm8112004. PMID: 31744181. PMCID: PMC6912791.

29. Hu JL, Yang J, Zhou YB, Li P, Han R, Fang DC. 2017; Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol. 23:275–280. DOI: 10.4103/sjg.SJG_91_17. PMID: 28937021. PMCID: PMC5625363.

30. Zhang Y, Zhu YJ, Zhao Z, et al. 2020; Efficacy of modified esomeprazole-amoxicillin dual therapies for Helicobacter pylori infection: an open-label, randomized trial. Eur J Gastroenterol Hepatol. 32:563–568. DOI: 10.1097/MEG.0000000000001646. PMID: 31851093.

31. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013; (12):CD008337. DOI: 10.1002/14651858.CD008337.pub2. PMCID: PMC10114080.

32. Ierardi E, Losurdo G, Fortezza RF, Principi M, Barone M, Leo AD. 2019; Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J Gastroenterol. 25:5097–5104. DOI: 10.3748/wjg.v25.i34.5097. PMID: 31558859. PMCID: PMC6747288.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download