Abstract
Background/Aims
Colorectal adenomas are precancerous lesions that may lead to colorectal cancer. Recent studies have shown that colorectal adenomas are associated with atherosclerosis. The cardio-ankle vascular index (CAVI) and ankle-brachial index (ABI) are noninvasive methods for evaluating atherosclerosis. This study examined the association between atherosclerosis and high-risk colorectal adenomas based on the CAVI and ABI.
Methods
The data of patients aged ≥50 years who had a colonoscopy and CAVI and ABI measurements from August 2015 to December 2021 at the Kangwon National University Hospital were analyzed retrospectively. After the colonoscopy, subjects were divided into no, overall, and high-risk (size ≥1 cm, high-grade dysplasia or villous adenoma, three or more adenomas) adenoma groups based on the pathology findings. The data were subjected to univariate and multivariate logistic regression analyses.
Results
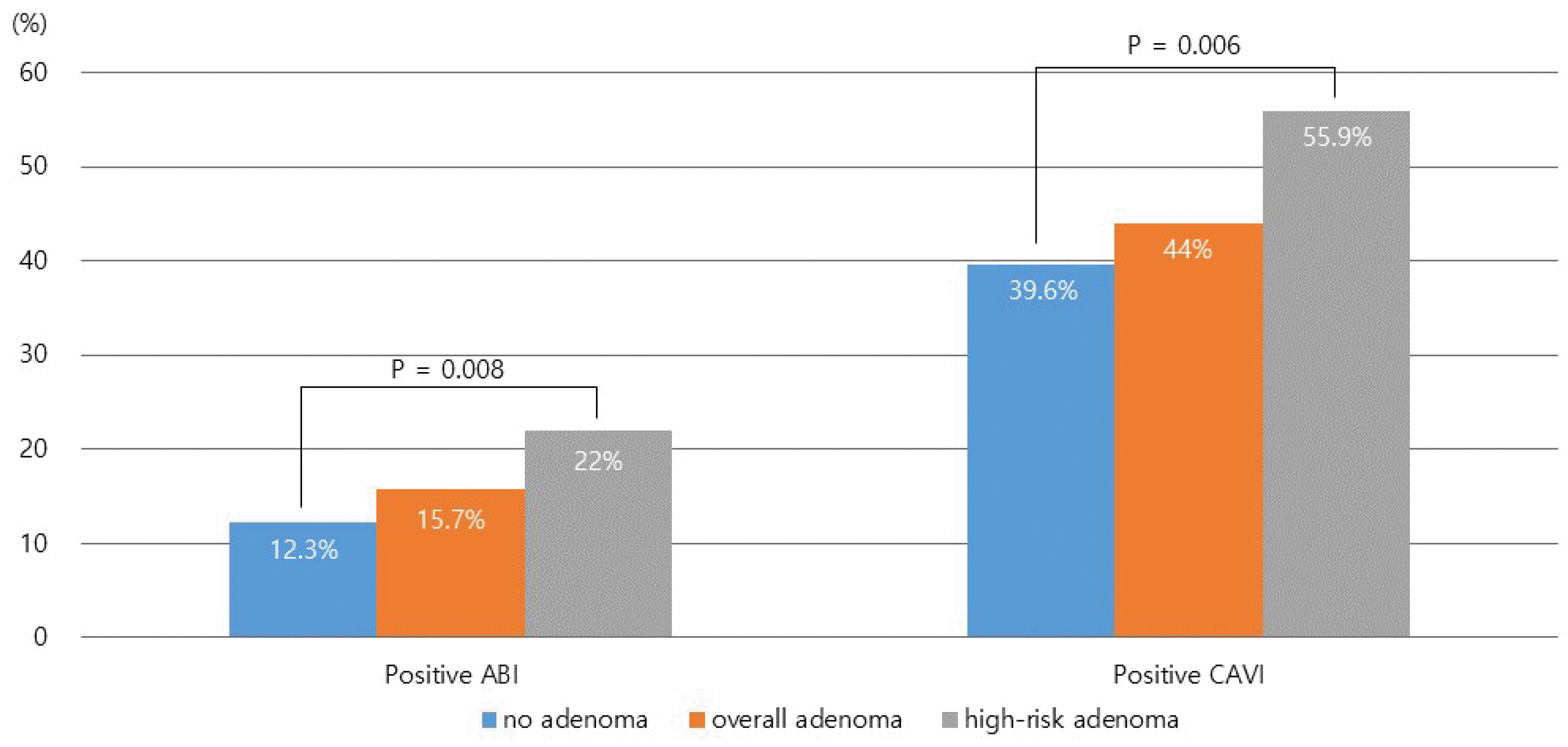
Among the 1,164 subjects, adenomas and high-risk adenomas were found in 613 (52.6%) and 118 (10.1%) patients, respectively. The rate of positive ABI (<0.9) and positive CAVI (≥9.0) were significantly higher in the high-risk adenoma group (22.0% and 55.9%) than in the no adenoma (12.3% and 39.6%) and the overall adenoma group (15.7% and 44.0%) (p=0.008 and p=0.006, respectively). Multivariate analysis revealed a positive CAVI and smoking status to be significantly associated with high-risk adenoma with an odds ratio of 1.595 (95% confidence interval 1.055–2.410, p=0.027) and 1.579 (1.072–2.324, p=0.021), respectively.
Go to : 
Colorectal cancer (CRC) is a common malignancy and a major cause of cancer-related death. Colorectal adenomas are precursor lesions of CRC. Colonoscopy facilitates the identification and removal of colorectal adenomas, as well as the early detection of CRC, reducing the incidence and mortality from CRC.1
Previous studies have reported an association between the occurrence of CRC and conditions such as diabetes mellitus, obesity, and metabolic syndrome.2,3 The accumulation of visceral fat and related metabolic disturbances in metabolic syndrome is believed to trigger atherosclerosis, leading to cardiovascular or cerebrovascular diseases. Therefore, patients with cardiovascular disease caused by atherosclerosis have a high risk of colorectal neoplasms.4
The ankle-brachial index (ABI), pulse wave velocity (PWV), and cardio-ankle vascular index (CAVI) are noninvasive methods used to assess atherosclerosis, a condition characterized by the stiffening and narrowing of arteries caused by plaque buildup.5,6 The ABI measures the ratio of blood pressure in the ankles and arms to detect peripheral artery disease. The PWV quantifies the speed at which pressure waves travel through the arteries, reflecting arterial stiffness and cardiovascular risk. The CAVI is a comprehensive index that evaluates vascular health from the heart to the ankles by considering arterial stiffness and blood pressure. These techniques collectively provide insights into the presence and severity of atherosclerosis, aiding in risk assessments and preventive strategies for cardiovascular and peripheral artery diseases. On the other hand, PWV depends on the patient's blood pressure when measured, while the CAVI is a more accurate and reproducible index of arteriosclerosis.7,8
Previous studies reported the relationship between peripheral vascular diseases and colorectal adenomas using ABI and PWV.9-12 Additionally, a recent study reported an association of arterial stiffness measured by PWV with the occurrence of high-risk adenomas and serrated lesions.13 However, no studies have assessed the association between CAVI and adenoma risk. Therefore, this study investigated the association between atherosclerosis and high-risk colorectal adenomas using CAVI and ABI.
Go to : 
The medical data from Korean subjects aged 50 years and above who underwent both CAVI and ABI measurements, as well as colonoscopy for health check-ups at Kangwon National University Hospital between August 2015 and December 2021, were analyzed retrospectively. Subjects with poor bowel preparation (n=65), failure of cecum intubation (n=5), inflammatory bowel disease (n=6), or a prior diagnosis of colorectal or other cancers (n=78) were excluded. This study was approved by the Institutional Committee on Human Research (Institutional Review Board No. KNUH 2023-02-013).
Clinical data, including demographic information, medical history, and relevant laboratory results, were extracted from the electronic medical records. The age, sex, body mass index (BMI), lipid profile, smoking, alcohol consumption, atherosclerosis indicators (CAVI, ABI), and various medical conditions were analyzed to determine their potential associations with colorectal adenoma. Based on the colonoscopy and pathology findings, the subjects were divided into no and overall adenoma groups, and a subgroup with high-risk adenomas was analyzed separately. High-risk adenomas were defined as those with a size of ≥1 cm with high-grade dysplasia, villous adenoma, or the presence of three or more adenomas.14
The CAVI was measured using a VaSera VS-1500N (Fukuda Denshi, Tokyo, Japan). The procedure involves wearing blood pressure cuffs on the upper arms and ankles while simultaneously recording the electrocardiogram signals. This index was used to reflect vascular stiffness, where lower values indicated healthier vessels and higher values suggested increased arterial stiffness. A CAVI of 9.0 or higher was considered an abnormal finding and indicated an increased risk of cardiovascular disease.
The ABI was measured using a handheld Doppler ultrasound device (Colin VP-1000 plus; Omron Healthcare, Kyoto, Japan, or VaSera VS-1500N; Fukuda Denshi, Tokyo, Japan). The systolic blood pressure at the ankle was compared with that at the upper arm, and the ABI was calculated as the ankle pressure divided by the brachial pressure. An ABI value below 0.9 was considered abnormal and indicated a higher risk of cardiovascular events.
Data management and statistical analyses were performed using SPSS for Windows (version 21.0; SPSS Inc., Armonk, NY, USA). The categorical variables are reported as numbers and percentages (%), and continuous variables are presented as the mean ± standard deviation. Logistic regression analysis was performed to determine the odds ratios (ORs) with the 95% confidence intervals (CI) for each outcome, including age, sex, smoking history, and alcohol consumption. Univariate and multivariate analyses were conducted to compare the positive diagnostic rates of ABI and CAVI between the groups diagnosed with adenoma and high-risk adenoma and the group without adenoma. A p-value <0.05 was considered significant.
Go to : 
This study enrolled 1,164 subjects. The mean age was 65.8±10.2 years (range 42–96 years); 726 (62.4%) were male and 438 (37.6%) were female. Of all subjects, adenomas were detected in 613 (52.6%). Among these adenomas, 118 were classified as high-risk (10.1%) (Table 1). Table 1 lists the clinical characteristics of the individuals across the different groups. Compared to the no adenoma group, the overall adenoma group and the subgroup with high-risk adenoma were significantly older and more likely to be male. In addition, they had a higher BMI and higher rates of smoking and alcohol consumption.
Clinical Characteristics
The prevalence of a positive ABI (ABI <0.9) was higher in the high-risk adenoma subgroup (22.0%) than in the no adenoma group (12.3%) and overall adenoma group (15.7%) (p=0.008; Fig. 1). Similarly, the prevalence of positive CAVI (CAVI ≥9.0) was significantly higher in the high-risk adenoma subgroup (55.9%) than in the no adenoma group (39.6%) and overall adenoma group (44.0%; p=0.006).
Tables 2 and 3 list the results of univariate and multivariate logistic regression analyses for overall and high-risk adenoma, respectively. Age, sex, BMI, ABI, CAVI, lipid profile, smoking status, alcohol consumption, and various medical conditions were evaluated for their potential influence on the presence of adenomas and high-risk adenomas.
Univariate and Multivariate Logistic Regression Analysis of Variables for the Overall Adenoma
Univariate and Multivariate Logistic Regression Analysis of Variables for the High-risk Adenoma
Multivariate analysis revealed male sex, smoking, and alcohol consumption to be significantly associated with overall adenoma with an OR of 1.636 (95% CI 1.276–2.096, p<0.001), 1.488 (95% CI 1.140–1.942, p=0.003), and 1.263 (95% CI 0.960–1.660, p=0.095), respectively. Two variables were found to be significantly associated with high-risk adenoma: positive CAVI (≥9.0) and smoking with an OR of 1.595 (95% CI 1.055–2.410, p=0.027) and 1,579 (95% CI 1.072–2.324, p=0.021), respectively.
Go to : 
This study showed that individuals with high-risk colorectal adenomas had higher rates of positive CAVI and ABI than those with no adenomas or overall adenomas. In multivariate analysis, male, sex, smoking, and alcohol consumption were significant factors associated with overall adenoma, and positive CAVI and smoking were significantly associated with high-risk adenoma. These results highlight the potential link between cardiovascular health and high-risk colorectal adenomas. Therefore, the CAVI could serve as a valuable predictor of high-risk colorectal adenoma.
Previous studies reported that colorectal adenomas exhibit an intriguing link with arterial stiffness, a well-known indicator of cardiovascular disease.9-13 PWV is a direct indicator of arterial wall elasticity and the most traditional measure used to gauge the degree of vascular rigidity. On the other hand, it is dependent on the patient's blood pressure at the time of measurement.15,16 CAVI has been proposed as an alternative method to evaluate arterial stiffness, which specifically evaluates the stiffness of the arteries from the heart to the ankle.17,18 The CAVI increases with arterial stiffness, with higher CAVI values suggesting increased arterial rigidity, often linked to hypertension and atherosclerosis. The CAVI is calculated using the Bramwell–Hill equation, which corrects for blood pressure, enhancing its efficacy as a marker of arterial stiffness and providing a more accurate and reproducible indicator than PWV.6,7 Moreover, a recent study showed that the CAVI is more closely associated with arterial damage and cardiovascular disease risk in patients with diabetes than brachial- ankle PWV (baPWV).8 ABI is a simple and commonly used test to assess peripheral arterial disease by measuring the ratio of the blood pressure in the ankle to that in the arm.19 A lower ABI value suggests decreased blood flow to the lower extremities, which can be a sign of blockages or narrowing in the arteries due to atherosclerosis. ABI is primarily used to assess lower-limb functional impairment or disease severity. In addition, low ABI is associated with atherosclerosis and cardiovascular events.5
A previous study investigating the association between arterial stiffness and colorectal adenomas reported a positive relationship between elevated baPWV levels and adenomas, but did not distinguish high-risk adenomas separately.10 In another study, heart-femoral PWV, reflecting the aortic stiffness, was found to be associated with colorectal adenoma, but the baPWV showed no association.9 On the other hand, another study revealed an association between baPWV and colorectal adenoma, as well as between ABI and advanced colorectal neoplasia, including CRC.11 A recent study suggested an association of arterial stiffness, measured using baPWV, with high-risk colorectal adenoma and high-risk serrated lesions.13 These results align with those obtained using CAVI in this study, showing that arterial stiffness and atherosclerosis are associated with colorectal adenoma, and the CAVI may be useful for assessing the risk of adenoma. Multivariate analysis revealed the CAVI and smoking to have significant associations with high-risk adenomas, whereas ABI did not demonstrate a significant association. This may be because the CAVI, which measures arterial stiffness, is a more sensitive indicator of atherosclerosis than the ABI, which evaluated the peripheral vascular occlusion. The ABI was reported to have high specificity in predicting the cardiovascular outcome but did not have high sensitivity.20 Hence, normal ABI levels do not exclude high-risk patients. Nevertheless, further research with a larger number of subjects will be needed to confirm this.
Arterial stiffness, a risk factor for cardiovascular disease, is known to be mediated by insulin resistance and elevated inflammation. Therefore, arterial stiffness may affect the development of colorectal adenoma through the following mechanisms. Insulin resistance triggers hyperinsulinemia, which stimulates the production of insulin-like growth factor-1 (IGF-1).21,22 Insulin and IGF-1 promote the proliferation of cells within the colon, potentially leading to the formation of colorectal adenoma. In addition, chronic inflammation may affect the risk of colonic adenoma directly or indirectly through other risk factors.23,24 Inflammatory signals characterized by the hypermethylation of DNA methylation valleys that show reduced CpG density and active chromatin marks contribute to tumor formation.25 This, in turn, increases the likelihood of adenoma.
This study had several limitations. First, the retrospective nature of this study introduced potential biases and limited the ability to examine the causal relationship between arterial stiffness and colorectal adenomas. Second, this study was conducted among participants who visited a single hospital, which limits the generalizability of the findings. Third, the study focused on individuals aged 50 years and above who are likely to be significantly affected by atherosclerosis and arterial stiffness. Hence, generalizing the results to all age groups may be challenging. Fourth, factors influencing colorectal adenomas, such as diet, exercise, and a history of taking medications, such as aspirin and nonsteroidal anti-inflammatory drugs, were not analyzed. Finally, owing to the relatively small number of participants included, the comparatively low number of serrated lesions, except for hyperplastic polyps, could not be included in the analysis.
In conclusion, this study revealed a significant correlation between a positive CAVI and high-risk colorectal adenomas, suggesting an association with atherosclerosis. These results also suggest the potential utility of the CAVI as a significant predictor of high-risk colorectal adenoma. Further research with a larger number of subjects will be needed to explore the underlying mechanisms of the association between atherosclerosis and colorectal adenoma, as well as its implications in clinical practice.
Go to : 
REFERENCES
1. Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Italian Multicentre Study Group. 2001; Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 48:812–815. DOI: 10.1136/gut.48.6.812. PMID: 11358901. PMCID: PMC1728339.

2. Trabulo D, Ribeiro S, Martins C, et al. 2015; Metabolic syndrome and colorectal neoplasms: An ominous association. World J Gastroenterol. 21:5320–5327. DOI: 10.3748/wjg.v21.i17.5320. PMID: 25954106. PMCID: PMC4419073.

3. Lee J, Lee KS, Kim H, et al. 2020; The relationship between metabolic syndrome and the incidence of colorectal cancer. Environ Health Prev Med. 25:6. DOI: 10.1186/s12199-020-00845-w. PMID: 32075578. PMCID: PMC7031951.

4. Yamamoto K, Miyata T, Nagawa H. 2007; The high prevalence of colorectal neoplasms in preoperative patients with abdominal aortic aneurysm or peripheral artery disease. Eur J Vasc Endovasc Surg. 33:397–400. DOI: 10.1016/j.ejvs.2006.10.020. PMID: 17137804.

5. Papamichael CM, Lekakis JP, Stamatelopoulos KS, et al. 2000; Ankle-brachial index as a predictor of the extent of coronary atherosclerosis and cardiovascular events in patients with coronary artery disease. Am J Cardiol. 86:615–618. DOI: 10.1016/S0002-9149(00)01038-9. PMID: 10980210.

6. Namba T, Masaki N, Takase B, Adachi T. 2019; Arterial stiffness assessed by cardio-ankle vascular index. Int J Mol Sci. 20:3664. DOI: 10.3390/ijms20153664. PMID: 31357449. PMCID: PMC6695820.

7. Takaki A, Ogawa H, Wakeyama T, et al. 2008; Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res. 31:1347–1355. DOI: 10.1291/hypres.31.1347. PMID: 18957805.

8. Saigusa T, Watanabe K, Hada Y, et al. 2022; Cardio-ankle vascular index is more closely associated than brachial-ankle pulse wave velocity with arterial damage and risk of cardiovascular disease in patients with diabetes. BMC Cardiovasc Disord. 22:365. DOI: 10.1186/s12872-022-02800-9. PMID: 35945498. PMCID: PMC9364514.

9. Lim YJ, Kwack WG, Lee YS, Hahm KB, Kim YK. 2010; Increased pulse wave velocity reflecting arterial stiffness in patients with colorectal adenomas. J Clin Biochem Nutr. 47:261–265. DOI: 10.3164/jcbn.10-70. PMID: 21103036. PMCID: PMC2966937.

10. Kim HB, Lee HR, Shim JY, et al. 2011; The association between arterial stiffness and colorectal adenomatous polyp in women. J Womens Health (Larchmt). 20:765–769. DOI: 10.1089/jwh.2010.2538. PMID: 21453035.

11. Yamaji Y, Mitsushima T, Koike K. 2014; Pulse-wave velocity, the ankle-brachial index, and the visceral fat area are highly associated with colorectal adenoma. Dig Liver Dis. 46:943–949. DOI: 10.1016/j.dld.2014.05.012. PMID: 24953207.

12. Lee J, Seo JW, Sim HC, et al. 2018; Predictors of high-risk adenoma occurrence at surveillance colonoscopy in patients who undergo colorectal adenoma removal. Dig Dis. 36:354–361. DOI: 10.1159/000489925. PMID: 29969782.

13. Chen HY, Lee WH, Hsu HL, et al. 2022; Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population. J Cardiol. 80:139–144. DOI: 10.1016/j.jjcc.2022.03.013. PMID: 35469715.

14. Kim SY, Kwak MS, Yoon SM, et al. 2022; Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition. Clin Endosc. 55:703–725. DOI: 10.5946/ce.2022.136. PMID: 36225130. PMCID: PMC9726446.

15. Soska V, Frantisova M, Dobsak P, et al. 2013; Cardio-ankle vascular index in subjects with dyslipidaemia and other cardiovascular risk factors. J Atheroscler Thromb. 20:443–451. DOI: 10.5551/jat.15420. PMID: 23459505.

16. Palombo C, Kozakova M. 2016; Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 77:1–7. DOI: 10.1016/j.vph.2015.11.083. PMID: 26643779.

17. Okura T, Watanabe S, Kurata M, et al. 2007; Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res. 30:335–340. DOI: 10.1291/hypres.30.335. PMID: 17541212.

18. Kadota K, Takamura N, Aoyagi K, et al. 2008; Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circ J. 72:304–308. DOI: 10.1253/circj.72.304. PMID: 18219171.

19. Sebastianski M, Narasimhan S, Graham MM, et al. 2014; Usefulness of the ankle-brachial index to predict high coronary SYNTAX scores, myocardium at risk, and incomplete coronary revascularization. Am J Cardiol. 114:1745–1749. DOI: 10.1016/j.amjcard.2014.09.010. PMID: 25306553.

20. Doobay AV, Anand SS. 2005; Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: a systematic review. Arterioscler Thromb Vasc Biol. 25:1463–1469. DOI: 10.1161/01.ATV.0000168911.78624.b7. PMID: 15879302.

21. Yoon YS, Keum N, Zhang X, Cho E, Giovannucci EL. 2015; Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metabolism. 64:1324–1333. DOI: 10.1016/j.metabol.2015.06.013. PMID: 26169471.

22. Jung IS, Shin CM, Park SJ, et al. 2019; Association of visceral adiposity and insulin resistance with colorectal adenoma and colorectal cancer. Intest Res. 17:404–412. DOI: 10.5217/ir.2018.00072. PMID: 30419640. PMCID: PMC6667358.

23. Godos J, Biondi A, Galvano F, et al. 2017; Markers of systemic inflammation and colorectal adenoma risk: Meta-analysis of observational studies. World J Gastroenterol. 23:1909–1919. DOI: 10.3748/wjg.v23.i10.1909. PMID: 28348498. PMCID: PMC5352933.

24. Kigawa N, Budhathoki S, Yamaji T, Iwasaki M, Inoue M, Tsugane S. 2017; Association of plasma C-reactive protein level with the prevalence of colorectal adenoma: the Colorectal Adenoma Study in Tokyo. Sci Rep. 7:4456. DOI: 10.1038/s41598-017-04780-9. PMID: 28667300. PMCID: PMC5493621.

25. Abu-Remaileh M, Bender S, Raddatz G, et al. 2015; Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 75:2120–2130. DOI: 10.1158/0008-5472.CAN-14-3295. PMID: 25808873.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download