Abstract
Mucosa-associated lymphoid tissue (MALT) lymphoma, also known as extranodal marginal zone lymphoma, is a low-grade B-cell lymphoma that can develop in the mucosal layer of various organs, including the gastrointestinal tract, salivary glands, lungs, and skin. The most common site is the gastrointestinal tract, particularly the stomach. On the other hand, primary esophageal lymphomas are extremely rare. MALT lymphomas can undergo histological transformation into more aggressive B-cell lymphomas, such as diffuse large B-cell lymphoma, resulting in a poor prognosis. This paper reports a rare case of primary esophageal MALT lymphoma mimicking a subepithelial tumor located in the lower esophagus that was treated successfully with radiotherapy. MALT lymphoma should be included in a differential diagnosis when subepithelial tumors are found in the esophagus, particularly if endoscopic ultrasonography reveals the tumor to be located in the deep mucosal and submucosal layers. Following the precise diagnosis, accurate staging and appropriate treatment are crucial. Regular follow-up is necessary to assess the possibility of recurrence or transformation to high-grade lymphoma.
Mucosa-associated lymphoid tissue (MALT) lymphoma, also known as extranodal marginal zone lymphoma, is a low-grade B-cell lymphoma that can originate from the mucosal layer of various organs, such as the gastrointestinal tract, salivary gland, lung, and skin.1 The most common site is the gastrointestinal tract, particularly the stomach. On the other hand, primary esophageal lymphomas are extremely rare.2
Esophageal subepithelial tumors (SETs) are rare mesenchymal lesions frequently discovered incidentally during health checkup esophagogastroduodenoscopy (EGD).3,4 Various histologic types of esophageal SETs have been reported,3,5 including leiomyoma, granular cell tumor, gastrointestinal stromal tumor, duplication cyst, and lymphangioma. Of these, some SETs have malignant potential.6 Esophageal MALT lymphoma can present with a SET-like appearance,7 and might transform into more aggressive B-cell lymphomas, such as diffuse large B-cell lymphoma.8 Therefore, making a precise differential diagnosis of esophageal SETs is crucial, and appropriate treatment is necessary.
This paper reports a rare case of primary esophageal MALT lymphoma mimicking an SET in the lower esophagus in a 70-year-old woman treated successfully with radiotherapy.
A 70-year-old woman with no relevant medical history was referred to our hospital for the evaluation of an SET measuring approximately 2 cm in size in the lower esophagus, which was detected incidentally on a health checkup EGD (Fig. 1A). She had been taking medication for hypertension and dyslipidemia for several years and had no history of alcohol consumption or smoking. The patient did not present any specific symptoms or signs. Hematological and biochemical test results were within the normal ranges. The serological examinations for hepatitis B, hepatitis C, and human immunodeficiency viruses were negative, and the real-time polymerase chain reaction test for the Epstein–Barr virus was also negative. A rapid urease test for Helicobacter pylori (H. pylori) and the serum anti-H. pylori IgG antibody were negative. Endoscopic ultrasonography (EUS) was performed for a differential diagnosis of esophageal SET. EUS revealed a relatively homogenously hypoechoic lesion with latticework structures in the deep mucosal and submucosal layers (Fig. 1B). On EUS elastography, the consistency was not hard (Fig. 1C). Contrast-enhanced EUS revealed increased enhancement only in the septal portion of the latticework structures (Fig. 1D). Endoscopic forceps biopsies were performed using the bite-on-bite technique. A histological examination revealed atypical lymphocytes infiltrating the deep mucosa and submucosa (Fig. 2A), and small lymphocytes characterized by central nuclei, irregular nuclear membranes, and abundant clear cytoplasm invaded the epithelium of the esophagus (Fig. 2B). The proliferative lymphoid cells were positive for CD20 (Fig. 2C) and CD21, but negative for CD5 (Fig. 2D) and CD10 (Fig. 2E). The Ki-67 proliferation index was approximately 20% (Fig. 2F). Based on these findings, the patient was diagnosed with esophageal MALT lymphoma and referred to the hemato-oncology department for staging and treatment.
Chest and abdominal computed tomography (CT), positron emission tomography (PET)-CT, and bone marrow examination were performed for staging. The final stage was determined to be IE according to the LUGANO staging because no metastatic lesions were detected in areas other than the esophagus (Fig. 3A). She underwent radiotherapy (RT) for esophageal MALT lymphoma, with a total dose of 30 Gy in 15 fractions. Complete remission was achieved on the follow-up EGD (Fig. 1E) and PET-CT (Fig. 3B) performed one month after RT. During subsequent follow-up examinations every three months until the present (13 months from the initial diagnosis), the patient has remained asymptomatic, with no clinical or radiological signs of recurrence.
MALT lymphoma is a low-grade B-cell non-Hodgkin lymphoma that occurs primarily at mucosal sites containing marginal zone B cells. It is characterized by the infiltration of atypical lymphoid cells with follicular proliferation, lymphoepithelial lesions, and B-cell surface markers.1,2 According to previous reports, the stomach (35%) is the most common site of MALT lymphoma, followed by the ocular adnexa (13%), lungs (8.8%), salivary glands (8.3%), colorectum (5.2%), and small intestine (3.4%).8 Esophageal lymphomas arise mainly due to invasion from the cervical and mediastinal lymph nodes or contiguous infiltration from gastric lymphoma. Therefore, primary esophageal lymphoma is very rare, accounting for less than 1% of primary gastrointestinal lymphomas.9
The etiology is unclear owing to its rarity.1 Esophageal MALT lymphoma in H. pylori-infected individuals is rare. Therefore, H. pylori infection is not a prominent indicator of esophageal MALT lymphoma.10 In the present case, the patient also had no current H. pylori infection. Other potential contributing factors to developing primary esophageal MALT lymphoma may include mechanical stimuli from food, hot beverages, meal-related chemicals, additional infections, and reflux esophagitis. None of these possible causative factors were identified in the present case. The underlying mechanisms of esophageal MALT lymphoma remain a subject for further investigation.10
According to previous studies, some cases are discovered incidentally during an examination without prior symptoms, as in the present case. In contrast, others present with symptoms such as dysphagia, foreign body sensation, heartburn, melena, hematochezia, and hematemesis.1,7 Endoscopic manifestations of esophageal lymphomas lack specificity and exhibit considerable diversity, ranging from polypoid masses, ulcers, strictures, and thickening of the mucosal folds to SETs.11 Within these cases, esophageal MALT lymphoma is predominantly characterized by the appearance of an SET,7 as in the present case. EUS findings also suggest that MALT lymphomas lack pathognomonic features and can manifest in various findings, such as hypoechoic or hyperechoic masses. In previous studies on SET-like gastric MALT lymphomas, these tumors were observed as hypoechoic lesions with latticework structures in the deep mucosa and submucosa, suggesting the presence of germinal centers.2,12 Therefore, MALT lymphoma should be included in the differential diagnosis when SETs are detected in the esophagus, particularly when the lesion is located in the deep mucosal and submucosal layers, according to EUS. Other differential diagnoses for esophageal SETs originating from the deep mucosal or submucosal layers on EUS include leiomyoma, granular cell tumor, gastrointestinal stromal tumor, duplication cyst, and lymphangioma.5 A histopathological examination of endoscopic biopsies and resected specimens continues to be crucial for diagnosing esophageal SETs.1
MALT lymphomas generally exhibit slow and non-aggressive clinical progression. Nevertheless, 2% of MALT lymphomas may transform histologically into more aggressive B-cell lymphomas, such as diffuse large B-cell lymphoma, leading to a poor prognosis.8 Therefore, accurate staging and appropriate treatment are essential after a diagnosis. Currently, there are no established treatment modalities for primary esophageal MALT lymphomas. According to the NCCN guidelines, RT is preferred for stage IE extranodal marginal zone lymphoma at non-gastric sites. Several studies have suggested other approaches to treating primary esophageal MALT lymphoma, including endoscopic mucosal resection, endoscopic submucosal dissection, surgery, and chemotherapy (Table 1).1,11,13-22 In the present case, RT was administered to the patient, resulting in complete remission. After treatment, regular follow-up is essential to evaluate the recurrence or transformation into high-grade lymphoma. The NCCN guidelines recommend that clinical follow-up, including diagnostic and imaging tests previously used, be done every three to six months for five years and then yearly thereafter or as clinically indicated. In the present case, a follow-up examination was conducted one month after completing RT and then every three months; no recurrence was observed.
In conclusion, MALT lymphoma should be included in the differential diagnosis when SETs are detected in the esophagus, particularly if the lesion is in the deep mucosal and submucosal layers, according to EUS. Accurate staging and appropriate treatment are essential after diagnosis. Regular follow-up is mandatory to evaluate the recurrence or transformation into high-grade lymphoma.
Notes
REFERENCES
1. Ma Q, Zhang C, Fang S, et al. 2017; Primary esophageal mucosa-associated lymphoid tissue lymphoma: A case report and review of literature. Medicine (Baltimore). 96:e6478. DOI: 10.1097/MD.0000000000006478. PMID: 28353588. PMCID: PMC5380272.
2. Baek DH, Kim GH, Song GA, et al. 2012; Primary esophageal mucosa-associated lymphoid tissue lymphoma treated with endoscopic resection. Gastrointest Endosc. 75:1282–1283. DOI: 10.1016/j.gie.2011.06.003. PMID: 21820112.

3. Codipilly DC, Fang H, Alexander JA, Katzka DA, Ravi K. 2018; Subepithelial esophageal tumors: a single-center review of resected and surveilled lesions. Gastrointest Endosc. 87:370–377. DOI: 10.1016/j.gie.2017.07.043. PMID: 28782509.

4. Choe Y, Cho YK, Kim GH, et al. 2023; Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study. Clin Endosc. 56:744–753. DOI: 10.5946/ce.2023.005. PMID: 37621066. PMCID: PMC10665619.

5. Deprez PH, Moons LMG, OʼToole D, et al. 2022; Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 54:412–429. DOI: 10.1055/a-1751-5742. PMID: 35180797.

6. Lee KH, Yoo CK, Lee HL, et al. 2021; The pathologic confirmation in subepithelial tumors. Korean J Helicobacter Up Gastrointest Res. 21:215–219. DOI: 10.7704/kjhugr.2020.0067.

7. Kobayashi S, Iwamuro M, Nishida K, et al. 2018; Primary localized esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Intern Med. 57:2347–2352. DOI: 10.2169/internalmedicine.0487-17. PMID: 29607943. PMCID: PMC6148167.

8. Ishikawa E, Nakamura M, Satou A, Shimada K, Nakamura S. 2022; Mucosa-associated lymphoid tissue (MALT) lymphoma in the gastrointestinal tract in the modern era. Cancers (Basel). 14:446. DOI: 10.3390/cancers14020446. PMID: 35053607. PMCID: PMC8773811.

9. Moriya K, Tamura H, Nakamura K, Hosone M, Inokuchi K. 2016; A primary esophageal MALT lymphoma patient with Helicobacter pylori infection achieved complete remission after H. pylori eradication without anti-lymphoma treatment. Leuk Res Rep. 7:2–5. DOI: 10.1016/j.lrr.2016.12.001. PMID: 28053856. PMCID: PMC5196236.

10. Xia Y, Wang Y, Han J, Liu M. 2021; En Bloc resection of primary large esophageal mucosa-associated lymphoid tissue lymphoma by endoscopic submucosal dissection: A case report. Front Med (Lausanne). 8:757485. DOI: 10.3389/fmed.2021.757485. PMID: 34722592. PMCID: PMC8548363.

11. Oría IC, Pizzala JE, Villaverde AM, et al. 2021; Primary lymphoma of the entire esophagus diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Radiol Case Rep. 16:1242–1244. DOI: 10.1016/j.radcr.2021.02.051. PMID: 33868528. PMCID: PMC8041659.

12. Kim GH, Choi BG, Lee JN, et al. 2010; [2 cases of gastric mucosa-associated lymphoid tissue lymphoma presenting as a submucosal tumor-like lesion]. Korean J Gastroenterol. 56:103–108. Korean. DOI: 10.4166/kjg.2010.56.2.103. PMID: 20729622.

13. Bang JH, Kang JH, Kim SH, et al. 2023; Primary esophageal mucosa-associated lymphoid tissue lymphoma: A rare case report and review of other published data. Korean J Helicobacter Up Gastrointest Res. 23:207–213. DOI: 10.7704/kjhugr.2023.0026.

14. Choi J. 2022; Successful endoscopic resection of gastric mucosa-associated lymphoid tissue lymphoma unresponsive to Helicobacter pylori eradication therapy. Clin Endosc. 55:136–140. DOI: 10.5946/ce.2020.232. PMID: 33190433. PMCID: PMC8831412.

15. Kishi K, Maeda H, Nakamura Y, Shirai S, Sato M. 2012; Radiotherapy for mucosa-associated lymphoid tissue (MALT) lymphoma of the esophagus: a case report with a diagnostic and therapeutic discussion. Int J Clin Oncol. 17:174–180. DOI: 10.1007/s10147-011-0265-8. PMID: 21660505.

16. Kitamoto Y, Hasegawa M, Ishikawa H, et al. 2003; Mucosa-associated lymphoid tissue lymphoma of the esophagus: a case report. J Clin Gastroenterol. 36:414–416. DOI: 10.1097/00004836-200305000-00011. PMID: 12702984.

17. Yano S, Usui N, Dobashi N, et al. 2009; A case of primary esophageal mucosa-associated lymphoid tissue lymphoma with a numerical abnormality of 18q21 detected by fluorescence in situ hybridization. Ann Hematol. 88:703–704. DOI: 10.1007/s00277-008-0653-y. PMID: 19052745.

18. Hosaka S, Nakamura N, Akamatsu T, et al. 2002; A case of primary low grade mucosa associated lymphoid tissue (MALT) lymphoma of the oesophagus. Gut. 51:281–284. DOI: 10.1136/gut.51.2.281. PMID: 12117895. PMCID: PMC1773320.

19. Kudo K, Ota M, Narumiya K, Shirai Y, Ohki T, Yamamoto M. 2014; Primary esophageal mucosa-associated lymphoid tissue lymphoma treated by endoscopic submucosal dissection. Dig Endosc. 26:478–481. DOI: 10.1111/den.12138. PMID: 23772967.

20. Bardisi ES, Alghanmi N, Merdad AA. 2014; Primary mucosa-associated lymphoid tissue lymphoma of the esophagus masquerading as a benign tumor. Ann Med Surg (Lond). 3:39–42. DOI: 10.1016/j.amsu.2014.05.001. PMID: 25568784. PMCID: PMC4268481.

21. Miyazaki T, Kato H, Masuda N, et al. 2004; Mucosa-associated lymphoid tissue lymphoma of the esophagus: case report and review of the literature. Hepatogastroenterology. 51:750–753. PMID: 15143908.
22. Lee DS, Ahn YC, Eom DW, Lee SJ. 2016; Primary esophageal mucosa-associated lymphoid tissue lymphoma diagnosed by using stacked forceps biopsy. Dis Esophagus. 29:887–890. DOI: 10.1111/dote.12309. PMID: 25626120.

Fig. 1
Endoscopic and endosonographic findings of an esophageal subepithelial tumor. (A) A 2 cm-sized subepithelial tumor covered with normal mucosa was observed in the lower esophagus. (B) Endoscopic ultrasonography (EUS) shows a relatively homogenously hypoechoic lesion with latticework structures in the deep mucosal and submucosal layers. (C) On EUS elastography, its consistency is not hard. (D) Contrast-enhanced EUS shows increased enhancement only in the septal portion of the latticework structures. (E) Follow-up endoscopy one month after radiotherapy shows complete remission of the previous lesion.
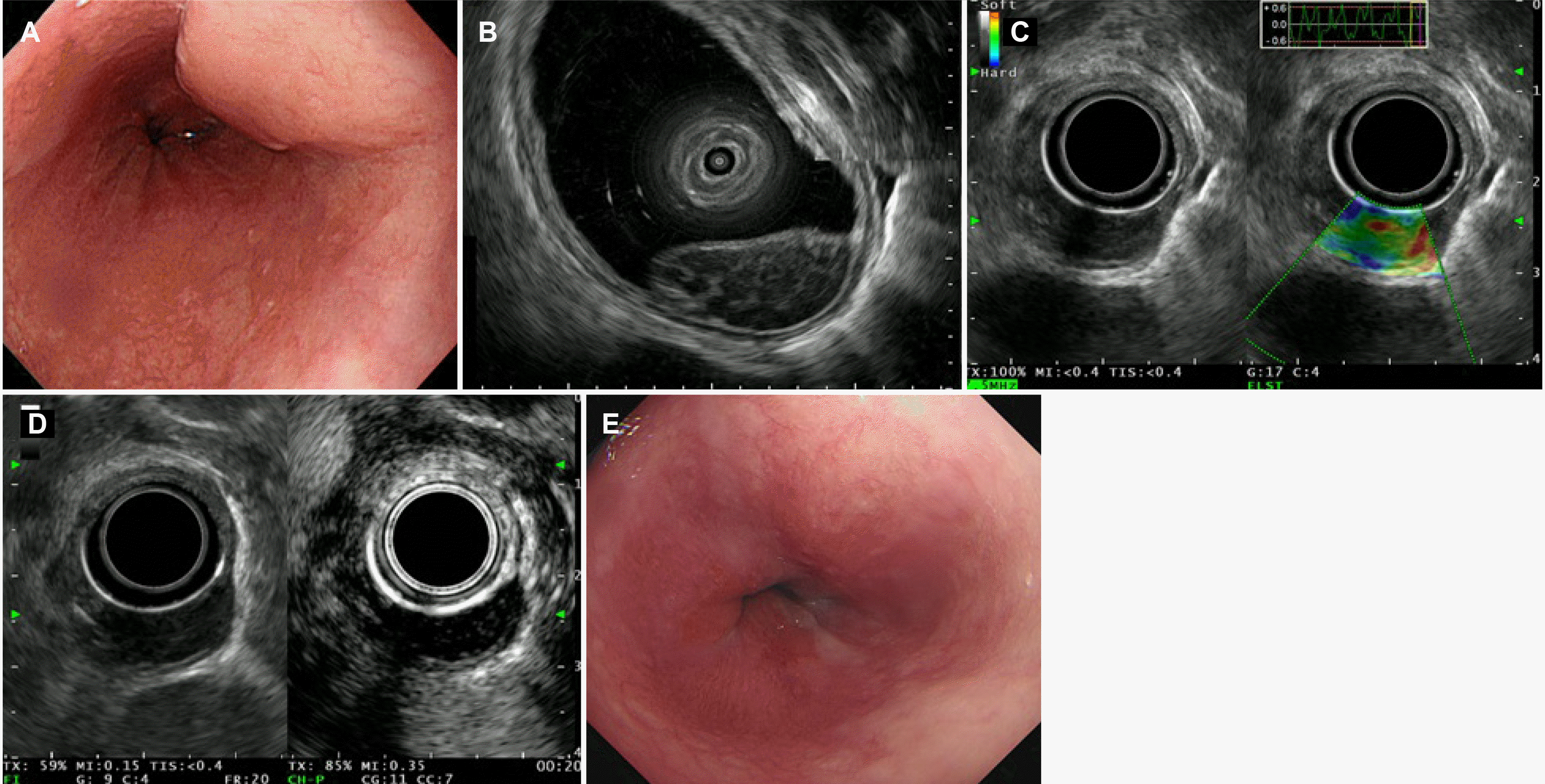
Fig. 2
Histopathological findings. (A) Atypical lymphocytes infiltrate the deep mucosa and submucosa (H&E stain, x100). (B) Small lymphocytes characterized by central nuclei, irregular nuclear membranes, and abundant clear cytoplasm invade the epithelium of the esophagus (H&E stain, x400). (C–E) Tumor cells are positive for CD20 (C) but negative for CD5 (D) and CD10 (E) (immunohistochemical stain, x100). (F) Ki-67 index is approximately 20% (Ki-67 stain, x100).

Fig. 3
PET-CT findings. (A) Initial PET-CT shows a hypermetabolic lesion (maximum standardized uptake value=5.4, arrow) in the lower thoracic esophagus. (B) Follow-up PET-CT one month after radiotherapy shows no abnormal glucose metabolism, confirming complete remission. PET-CT, positron emission tomography-computed tomography.
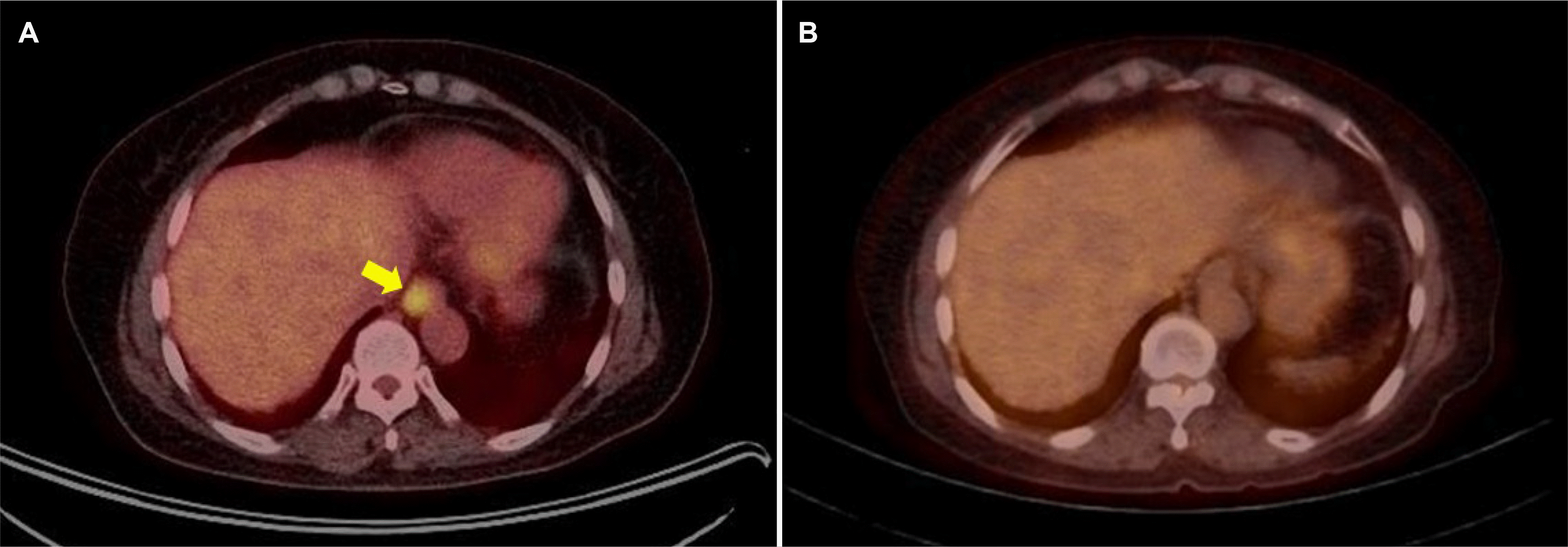
Table 1
Summary of Esophageal MALT Lymphoma Cases Reported to Date
| Authors | Sex | Age (yr) | Location | Size (cm) | Treatment | Follow-up period (mo) | Outcome |
|---|---|---|---|---|---|---|---|
| Kishi et al.15 | M | 59 | Upper to lower | 15 | Radiotherapy | 36 | CR |
| Kitamoto et al.16 | M | 74 | Middle | NA | EMR, Radiotherapy | NA | CR |
| Yano et al.17 | F | 70 | Middle | 2 | EMR, Radiotherapy | 36 | CR |
| Baek et al.2 | F | 59 | Upper | 1.4 | EMR | 24 | CR |
| Hosaka et al.18 | F | 83 | Upper | NA | EMR | 22 | CR |
| Kudo et al.19 | M | 66 | Lower | 1.5 | ESD | 12 | CR |
| Kobayashi et al.7 | F | 69 | Middle | 2 | ESD | 57 | CR |
| Xia et al.10 | F | 77 | Lower | 4.3 | ESD | 5 | CR |
| Bardisi et al.20 | M | 50 | Middle to lower | 10 | Surgical resection | 12 | CR |
| Miyazaki et al.21 | M | 49 | Middle | NA | Surgical resection | 12 | CR |
| Ma et al.1 | M | 75 | Upper to lower | 14 | Surgical resection | 8 | CR |
| Lee et al.22 | M | 49 | Middle | 0.8 | Chemotherapy | 6 | CR |
| Present case | F | 70 | Lower | 2 | Radiotherapy | 13 | CR |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download