Abstract
Background
The prevalence of atherosclerotic cardiovascular disease is rising, and its onset from childhood is widely studied. Prematurity and low birth weight were associated with higher atherogenic risk when assessed using some lipid ratios. However, the atherogenic index of plasma (AIP), a sensitive marker for atherosclerosis is understudied in newborns. Utilizing AIP, this study aimed to determine atherogenic risk prevalence among newborns and its association with gestational age and birth weight.
Methods
Newborns were consecutively recruited, and their lipid profiles were determined. The AIP was calculated as the logarithm to base 10 (log10) of the ratio of molar concentrations of triglyceride to high-density lipoprotein cholesterol. The atherogenic risk was operationalized using AIP: high, >0.24; medium, 0.1–0.24; and low/no risk, <0.1. The relationship between AIP values, gestational age, and birth weight was analyzed using Pearson correlation.
Results
The mean AIP of the 167 newborns studied was –0.35±0.34, which is within the global reference range. Three (1.8%), 10 (6.0%), and 154 (92.2%) newborns were in the high, medium, and low/no atherogenic risk categories, respectively. Hence, 13 newborns (7.8%) had medium to high atherogenic risk. AIP had a moderate significantly positive relationship only with gestational age (r=0.35, P<0.001).
Conclusions
The study found an atherogenic risk prevalence of 7.8% using AIP in newborns which, contrary to previous studies that used other ratios, has no significant association with birth weight, correlating positively with gestational age, though is lowest in late preterms. Follow-up studies will elucidate these findings.
Coronary heart disease (CHD) is a cardiovascular disease (CVD) that has been a leading cause of death worldwide with increasing prevalence in developing countries in which death increasingly occurs at a younger age [1]. In several sub-Saharan African countries, Onen [2] noted that a rising prevalence of dyslipidemia among other risk factors is a major precipitating factor for CHD. Ischemic heart disease was the leading cause of death in 2017 and is projected to still be the leading cause in 2040 [3].
Atherosclerosis resulting from dyslipidemia has been demonstrated to begin in utero, persisting into adulthood, and efforts are being made to assess this abnormality at birth for possible risk of CVD [4]. A programmed permanent alteration of fetal physiology and metabolism to adapt to intrauterine stress or malnutrition has been postulated as the cause of such fetal onset of adult disease [5]. An early manifestation of CVD in childhood is evidenced by myocardial infarction following homozygous familial hyperlipidemia which has occurred as early as 3 years of age, with death being common in adolescence [6]. Intrauterine myocardial infarction in a fetus with coronary artery stenosis has also been reported [7].
Atherosclerotic CHD has been associated with being born preterm and having low birth weight [5,8–10]. With the high magnitude of preterm and low birth weight deliveries in sub-Saharan Africa [11–13], it has become necessary to enact primary prevention of CVD by monitoring the trajectory of fetal dyslipidemia in the region.
Although fetal dyslipidemia was positively correlated with intima-media thickness (IMT), a subclinical marker of atherosclerosis [14], a considerable number of those with normal lipid values could have atherosclerosis leading to CVD. This is due to the wide variability of cholesterol within high-density lipoprotein (HDL) and low-density lipoprotein (LDL) molecules. Hence, the raw lipoprotein level does not match the cholesterol level always [15]. Hence, different atherogenic indices derived from lipid ratios have been used to better assess atherogenicity [16–18]. However, the atherogenic index of plasma (AIP) stands out as a sensitive marker for atherosclerosis that was studied across all age groups, including newborns (whose cord blood samples were studied), and it consists of easily assayed lipid fractions [19–21]. AIP is the logarithm ratio of the molar concentration of triglyceride (TG) to HDL cholesterol (HDL-C; log10 TG/HDL-C) and is positively correlated to CVD risk [19]. It is a statistically reliable index of atherogenicity, and AIP<0.1 is normal in newborns. Values of –0.3 to 0.1, 0.1 to 0.24, and above 0.24 depict low, medium, and high CVD risks respectively [21]. Since AIP comprises TG and HDL-C which are widely measured lipid fractions, and applicable in all age groups including newborns [21], its use will give room for wide reproducibility of data from other parts of Nigeria and the sub-Saharan region for systematic analysis. So far, there is a dearth of studies on AIP as a tool for risk assessment in newborns. AIP was evaluated by Umar et al. [22] in Northern Nigeria in only full-term neonates with a smaller sample size. This index study improved on the methodology of the previous Nigerian study in the following ways: firstly, a larger sample size was used to aid the epidemiological clarity of associations. Secondly, preterm newborns were also included to ensure a wide representation of different gestational age brackets. Thirdly, the evaluation of the relationship between atherogenic risk, gestational age, and birth weight was also considered.
From the foregoing, the index study aimed to determine the prevalence of atherogenic risk among apparently healthy newborns and highlight its relationship with gestational age and birth weight.
This study was approved by the Health Research Ethics Committee of the Federal Medical Centre (Umuahia, Nigeria) (No. FMC/QEH/G.596/Vol.10/461). Written informed consent was obtained at different phases from every parent preferably during the antenatal period (prenatal phase), or at a convenient time for the mother before the active phase of labor (prelabor phase), or post-sample collection for those who were either not earlier booked for antenatal care, or could not be reached in the antenatal period (post-collection phase) [23]. Parents’ telephone numbers were also obtained for further communication of study findings to them. Parents were assured of their right to post-survey information to make room for appropriate correspondence, especially for subjects whose findings would require further medical evaluation/follow-up. Efforts were made to contact parents whose newborns had medium to high atherogenic index: they were given a copy of the Cardiovascular Health Integrated Lifestyle Diet (CHILD) schedule [24] which the US National Heart, Lung, and Blood Institute (NHLBI) adopted in 2010 as a measure to reduce pediatric cardiovascular disease in all pediatric age groups, starting from birth in high-risk subjects. Since routine lipid screening for children less than 2 years is not generally recommended partly due to the feared adverse effect of interventions with lipid-altering medications, and the effect of dietary fat restriction on the developing brain, the parents were advised to adhere to the CHILD schedule and to bring their babies back for reevaluation after 2 years [25]. All the parents were given access to the researcher’s phone number to enhance free and prompt communication so that feedback in early infancy could be sustained beyond the time frame of the study.
The index article is a component of a wider investigation of the lipid profile of cord blood. Another article that has been published describes the study methods in detail [26]. However, a summary of the methodology relevant to this paper is hereby considered.
This was a hospital-based cross-sectional study. The participants were newborns delivered either in the labor ward or maternity theatre of a tertiary hospital in Nigeria. Obstetric history from mothers, physical examination of neonates, sample collection, and acquisition of other data were done at the labor ward and maternity theatre. The spinning and temporary storage of the separated serum samples were done at the labor ward side-laboratory before transfer to the Special Research Laboratory of the hospital.
A total of 2,102 live babies were born during the study period which lasted from March 4, 2019, to July 14, 2020, out of which 167 newborns were recruited consecutively as the mothers presented in labor and based on those that met the inclusion criteria after obtaining well-informed, written consent. Enrollment in the study was continued until the expected sample size was achieved.
The socioeconomic classes were assigned according to the classification by Oyedeji [27] including classes I, II, III, IV, and V with the highest class being I and the least V. These were further re-stratified into upper (classes I and II), middle (class III), and lower (classes IV and V) classes [28].
Participants (newborns) born in the labor ward or maternity theatre of the study hospital, irrespective of their gestational ages and birth weights, whose parents gave well-informed and written consent were consecutively recruited until the minimum calculated sample size of 167 was achieved. Overall, 184 newborns were recruited, but 10 had lysed blood samples, three samples had analytical errors in the laboratory, while four were rendered invalid by suboptimal storage temperature due to paused power supply. Newborns were excluded in the following cases: prolonged labor, or prolonged rupture of membrane >24 hours, chorioamnionitis or fetal distress, 5th minute APGAR (appearance [color], pulse [heart rate], grimace [reflexes], activity [muscle tone], and respiration) score <7 [29,30], gross congenital malformations, major illness obvious at birth requiring admission at the neonatal unit, family history of dyslipidemias, or CHD. Others excluded were babies whose mothers had been diagnosed with thyroid disease, hypertension, severe preeclampsia, diabetes mellitus, chronic ailments like chronic kidney disease and retroviral disease either before or during pregnancy [30], or whose mothers took lipid-altering drugs in pregnancy (e.g., statins, protease inhibitors, retinoic acid derivative, bile acid binding resins, diuretics, β-blockers like propranolol) [31,32]. Generally, the protocol of care in the study facility does not permit mothers to eat when they are in the active phase of labor. Hence the mothers are considered to be in fasting state at delivery, which is preferred for a reliable serum TG result.
Relevant obstetric and medical history were obtained from mothers, and each baby was thoroughly examined and findings documented. Data obtained include gestational age, birth weight, sex of the newborn, mother’s and father’s age, mother’s parity, head circumference, mode of delivery, mother’s booking status, and socioeconomic class. The cord blood samples from the placental end were collected from each newborn after cord clamping and emptied gently into a plain tube, allowed to clot, then spun to separate the sera which were stored at –20 °C. Lipid parameters including HDL-C and TG were analyzed within 2 weeks using an autoanalyzer (BiOLis 24i, Tokyo Boeki Medisys Inc) under the supervision of a chemical pathologist. The AIP was calculated as a logarithm ratio of molar concentrations of TG to HDL-C (log10 TG/HDL-C) [19]. This entailed the conversion of TG to millimoles per liter by multiplying the value in milligrams per decilitre by 0.01129, while HDL-C (mg/dL) was converted to millimoles per liter by multiplying by 0.02586 [33]. Participants were grouped based on their AIP values as low/no risk (AIP less than 0.1), medium risk (AIP 0.1 to less than 0.24), and high risk (AIP above 0.24) [21].
Data were analyzed using the IBM SPSS ver. 20 (IBM Corp). After data cleaning, the normality of the distribution of data was done using Shapiro-Wilk tests. The AIP was normally distributed. The relationship between AIP scores, gestational age, and birth weight was analyzed using analysis of variance (AIP categorized into low, medium, and high) correlation analysis (AIP as a continuous variable). The relationship between AIP and other lipid ratios was done using Pearson correlation. Bonferroni post hoc test was used to examine the differences between AIP scores across gestational age (i.e., preterm, late preterm and term). Multivariate stepwise linear regression analysis was used to determine the independent predictors of AIP scores with coefficient of determination being the percentage of variance of AIP scores explained by the independent variables. All tests of significance were two-tailed at the 5% level of significance and confidence interval (CI) estimation of 95% (with a P-value set at <0.05).
A total of 167 subjects were studied with a male to female ratio of 1:1.1. The gestational ages of the newborn ranged from 31 weeks to 43 completed weeks, with a modal gestational age of 36 weeks. This included 106 term newborns (gestational age up to 37 completed weeks) and 46 late-preterm newborns (whose gestational ages were up to 34 weeks but less than 37 completed weeks). The remaining 15 preterm babies were less than 34 completed weeks of gestational age.
Those that belonged to the upper socioeconomic class (classes I and II) were 64.1% of the subjects, while 32.3% and 3.6% were of the middle (class III) and lower (class IV and V) socioeconomic classes, respectively. The babies were predominantly from Igbo ethnic group (97.6%) (Table 1).
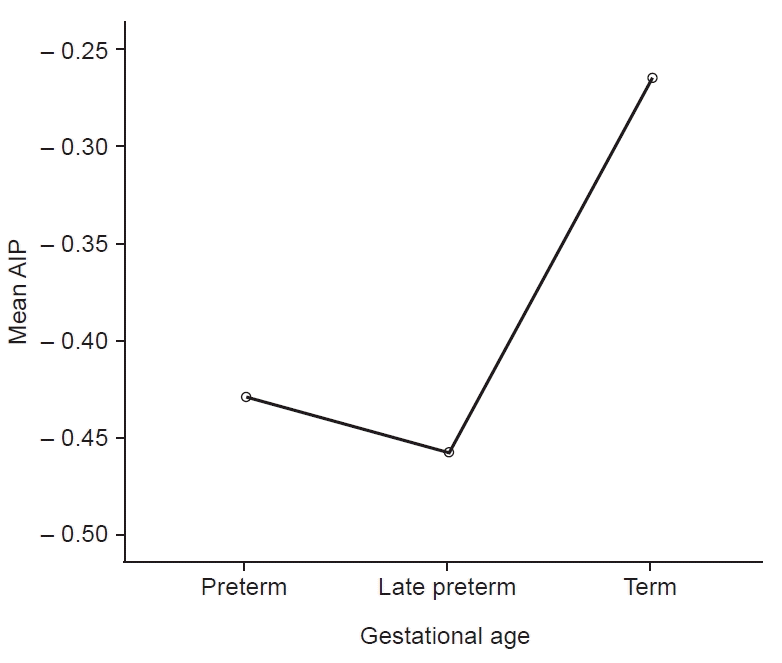
The prevalence of high, medium, and low atherogenic risks were 1.8% (95% CI, 0.4%–5.2%), 6.0% (95% CI, 2.9%–10.7%), and 92.2% (95% CI, 87.1%–95.8%), respectively (Table 2). AIP had a positive moderate significant correlation with gestational age which was statistically significant (r=0.35, P<0.001) (Table 3). The relationship between AIP and gestational age (categorized into preterm, late preterm, and term) was significant (F=7.98, P<0.001) as shown in Fig. 1. Bonferroni post hoc test shows that the difference was between preterm versus term (P=0.001) and preterm versus late preterm (P=0.001). The predictors of atherogenic risk are shown in Table 4. Out of the independent variables assessed (gestational age, birth weight, sex of the newborn, mother’s age, and socioeconomic class), gestational age was the only significant predictor of AIP with a P-value of <0.001. The relationship between AIP and other lipid ratios is shown in Table 5. The table shows a positive strong correlation between AIP and TG/HDL-C ratio (r=0.83, P<0.001), moderate correlation with total cholesterol (TC) to HDL-C ratio (r=0.40, P<0.001), and a weak correlation with LDL-C/HDL-C ratio (r=0.12, P=0.110).
Using AIP for assessment, the prevalence of high atherogenic risk among newborns was 1.8% while that of medium atherogenic risk was 6%, with an overall atherogenic risk prevalence of 7.8% in the studied population. Direct comparison with other studies is hampered by the paucity of atherogenic risk prevalence studies in newborns using AIP. However, the importance of assessing this risk prevalence cannot be overemphasized in the face of the increasing burden of cardiovascular diseases in younger age groups [1]. In the index study, the exclusion of subjects with possible risk factors for dyslipidemia like birth asphyxia, maternal chronic illnesses, prolonged rupture of membrane, severe preeclampsia, and others, may suggest a higher atherogenic risk prevalence in the overall population. The mean AIP of –0.35±0.34 in this study falls under the low/no atherogenic risk category and within the normal range for newborns which should be less than 0.1 [21]. The mean AIP in this study was lower than the mean value of –0.09 reported by Umar et al. [22], who evaluated only term babies in northern Nigeria. This may be due to the larger sample size and inclusion of preterm babies in this index study which gave a wider representation of the neonatal mean AIP. Comparing AIP with other lipid ratios like TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C, this study shows that only TG/HDL-C correlated strongly with AIP (Table 5), hence, suggesting that the two others should be used with caution as an alternative marker.
The positive correlation of AIP with gestational age in this study is in contrast to the finding of Pardo et al. [17] who reported higher atherogenic risk in the near-term compared to term newborns. The different methodology and study population in this present study may have contributed to the disparity. Whereas AIP was utilized in the index study which was calculated as the logarithm ratio of molar concentrations of TG to HDL-C (log10 TG/HDL-C), Pardo et al. [17] assessed atherogenicity using these ratios: TC to HDL-C, LDL-C to HDL-C, and apolipoprotein B to apolipoprotein A-1. Given that in other earlier reports [5,8–10], atherogenic risk was associated with being born preterm and having low birth weight, the increase of AIP with gestational age in this present study would appear to suggest that term or postterm deliveries pose a more atherogenic risk in newborns. However, AIP (calculated as log10 TG/HDL-C) is directly related to fetal TG which has been reported to increase with gestational age [26,34]. Hence, AIP will increase expectedly with gestational age, and would only reach the atherogenic risk threshold if the newborn has subclinical atherosclerosis. On the other hand, the fetal LDL-C and TC are known to be physiologically higher in early fetal life and decrease progressively towards term [35–37]. Hence, atherogenic risk assessment using LDL/HDL or TC/HDL (as used in some earlier studies) could have yielded exaggerated high atherogenic indices in preterm and low birth weight newborns because of the direct relationship of these ratios with LDL-C and TC respectively. However, larger multicenter studies involving the much lower gestational age group will be needed to elucidate the findings in this study. Further analysis showed that the mean AIP was lowest among the late-preterm category, and highest in the term category of newborns (Fig. 1). This finding of lower AIP among the late-preterm newborns would require further elucidation with longitudinal studies in order to have a reasonable conclusion. The uniqueness of this study remains the use of AIP to assess cardiovascular risk unlike in the aforementioned studies. The importance of AIP lies in its ability to sensitively predict risk for CHD as a marker for lipoprotein particle size, even in subclinical atherosclerosis [19].
The absence of a significant relationship between AIP and birth weight in the index study agrees with the finding of Aletayeb et al. [38] who assessed atherogenicity in cord blood using TC/HDL and LDL/HDL ratios and found no significant relationship with both birth weight and sex. Likewise, AIP had no significant relationship with sex, maternal age, and socioeconomic status in the index study.
Medical treatment for suspected atherogenic dyslipidemia in children below 2 years of age is not advised because of their peculiar lipid metabolism and widely variable lipid profile at this stage when lipids are employed in brain growth, hormonal metabolism, and other metabolic processes. However, breastfeeding reduces the risk of cardiovascular disease until about 13 to 16 years of age [25]. Breastfed adolescents have lower levels of C-reactive protein and a 14% lower LDL/HDL ratio (another atherogenic biomarker) than their non-breastfed counterparts [25]. CHILD has also been advocated from birth for at-risk newborns [24]. Control of maternal factors like hypertension, diabetes mellitus, intake of lipid-altering medications are possible measures that will prevent risk in fetuses, and elucidation of these will require further follow-up studies.
Despite being new in our context, the study was constrained by the use of a hospital-based sample. A community sample would have been more accurate, particularly for setting reference values. A longitudinal approach would have been more rigorous since it would have allowed the evaluation of the risk’s progression toward the diagnosis of clinical conditions, hence elucidating the predictive strength of AIP for CVD risk. However, efforts to follow-up on the newborns who had high atherogenic index (based on AIP) were hampered by apathy for further assessment in most of the parents who failed to bring back their infants for reassessment and were not reachable via telephone. Furthermore, enrollment of extremely preterm (less than 28 weeks gestational age) newborns was challenging because of the rarity of such births in an urban tertiary center with quality antenatal care and their inability to meet the inclusion criteria. Their inclusion (likely obtainable in a multicenter study) could affect the relationship of AIP with gestational age. Finally, the study did not measure maternal lipid values before and at delivery. Some authors reported that it may influence the cord lipid values. This is also an area for further research.
Despite the normal mean AIP value, an overall medium to high atherogenic risk of 7.8% was observed in this study. AIP had no significant association with birth weight but correlated positively with gestational age. However, AIP was lowest in late-preterm newborns. Whereas a larger multicenter study to elucidate this finding is needful, a cardiovascular risk assessment should be done for newborns irrespective of maturity and weight at birth in sub-Saharan Africa.
Notes
Author contributions
Conceptualization: all authors; Data curation: all authors; Formal analysis: OVO, JUO; Investigation: OVO, UCA, NKC, DKA; Methodology: all authors; Project administration: OVO, UCA, NKC, DKA; Resources: OVO; Supervision: all authors; Validation: OVO, JUO; Writing–original draft: all authors; Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010; 35:72–115.

2. Onen CL. Epidemiology of ischaemic heart disease in sub-Saharan Africa. Cardiovasc J Afr. 2013; 24:34–42.
3. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. IHME; 2018.
4. Kenchappa Y, Behera N. Assay of neonatal cord blood lipid levels and its correlation with neonatal gestational age, gender and birth weight: a single center experience. Int J Contemp Pediatr. 2016; 3:718–24.

6. Rohrs JH, Schatz D, Winter WE, Davis V. Pediatric lipid disorders in clinical practice treatment and management [Internet]. Medscape; [updated 2023 Apr 20; cited 2024 Feb 5]. Available from: https://emedicine.medscape.com/article/1825087-treatment.
7. Concheiro-Guisan A, Sousa-Rouco C, Fernandez-Santamarina I, Gonzalez-Carrero J. Intrauterine myocardial infarction: unsuspected diagnosis in the delivery room. Fetal Pediatr Pathol. 2006; 25:179–84.

8. Bezold LI, Pflieger K. Myocardial infarction in childhood [Internet]. Medscape; [updated 2020 Dec 7; cited 2022 Feb 22]. Available from: https://emedicine.medscape.com/article/897453-overview#a3.
9. Miyamoto K, Tsuboi T, Suzumura H, Arisaka O. Relationship between aortic intima-media thickening, serum IGF-I and low-density lipoprotein particle diameter in newborns with intrauterine growth restriction. Clin Pediatr Endocrinol. 2009; 18:55–64.

10. Stroescu R, Micle I, Marginean O, Bizerea T, Marazan M, Puiu M, et al. Is small for gestational age status associated with an increased risk of atherogenesis? Maedica (Bucur). 2013; 8:315–20.
11. Dahlui M, Azahar N, Oche OM, Aziz NA. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Glob Health Action. 2016; 9:28822.

12. Awoleke JO. Maternal risk factors for low birth weight babies in Lagos, Nigeria. Arch Gynecol Obstet. 2012; 285:1–6.

13. Umeigbo BC, Modebe IA, Iloghalu IC, Eleje GU, Okoro CC, Umeononihu OS, et al. Outcomes of preterm labor and preterm births: a retrospective cross-sectional analytical study in a Nigerian single center population. Obstet Gynecol Res. 2020; 3:17–28.

14. Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci. 2010; 7:278–83.

15. Kaneva AM, Potolitsyna NN, Bojko ER, Odland JO. The apolipoprotein B/apolipoprotein A-I ratio as a potential marker of plasma atherogenicity. Dis Markers. 2015; 2015:591454.

16. Nimmanapalli HD, Kasi AD, Devapatla PK, Nuttakki V. Lipid ratios, atherogenic coefficient and atherogenic index of plasma as parameters in assessing cardiovascular risk in type 2 diabetes mellitus. Int J Res Med Sci. 2016; 4:2863–9.

17. Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Atherogenic lipid profile of Brazilian near-term newborns. Braz J Med Biol Res. 2005; 38:755–60.

18. Kharb S, Kaur A, Nanda S. Comparison of cord blood atherogenic index in males and females. Int Cardiovasc Res J. 2010; 4:35–8.
19. Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic index of plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015; 29:240.
20. Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem. 2004; 50:1184–8.

21. Dobiasova M. AIP: atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006; 52:64–71.
22. Umar LW, Aliyu IS, Akuyam SA. Evaluation of lipid profile in cord blood of full-term Nigerian newborn infants. Sub Saharan Afr J Med. 2017; 4:9–14.

23. Armson BA, Allan DS, Casper RF. Umbilical cord blood: counselling, collection, and banking. J Obstet Gynaecol Can. 2015; 37:832–44.

24. Williams LA, Wilson DP. Nutritional management of pediatric dyslipidemia. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. MDText.com Inc; [updated 2023 Apr 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395582/.
25. Neal WA, John CC. Disorders of lipoprotein metabolism and transport. In : Kliegman RM, Stanton BF, St. Geme JW, Schor NF, editors. Nelson textbook of pediatrics. 20th ed. Elsevier;2016. p. 691–705.
26. Obaji OV, Chukwudi NK, Adiele DK, Adizua UC, Onu JU. Cord blood lipid profile of term and preterm newborns in a tertiary hospital in South East Nigeria: relationship with gestational age and birth weight. Niger Health J. 2023; 23:478–88.
27. Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesha. Niger J Paediatr. 1985; 12:111–7.
28. Ezenwosu OU, Emodi IJ, Ikefuna AN, Chukwu BF, Osuorah CD. Determinants of academic performance in children with sickle cell anaemia. BMC Pediatr. 2013; 13:189.

29. Yashodha HT, Anjum SK. Cord blood lipid profile in late preterm and term neonates. Int J Contemp Pediatr. 2018; 5:542–6.

30. Ghiasi A, Ziaei S, Faghihzadeh S. The relationship between levels of lipids and lipoprotein B-100 in maternal serum and umbilical cord serum and assessing their effects on newborn infants anthropometric indices. J Midwifery Reprod Health. 2014; 2:227–32.
31. Farinde A. Lipid-lowering agents [Internet]. Medscape; [updated 2023 Apr 4; cited 2024 Feb 5]. Available from: https://emedicine.medscape.com/article/2172172-overview.
32. Moll J. Medications that cause high cholesterol levels [Internet]. Verywell Health; [updated 2023 Jun 19; cited 2024 Feb 5]. Available from: https://www.verywell.com/which-drugs-can-raise-cholesterol-levels-698229.
33. Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Yu S, et al. Changes in high-density lipoprotein cholesterol and risks of cardiovascular events: a post hoc analysis from the PICASSO Trial. J Stroke. 2020; 22:108–18.

34. Yonezawa R, Okada T, Kitamura T, Fujita H, Inami I, Makimoto M, et al. Very low-density lipoprotein in the cord blood of preterm neonates. Metabolism. 2009; 58:704–7.

35. Shoji H, Murano Y, Mori M, Matsunaga N, Ohkawa N, Suganuma H, et al. Lipid profile and atherogenic indices soon after birth in Japanese preterm infants. Acta Paediatr. 2014; 103:22–6.
36. Parker CR Jr, Carr BR, Simpson ER, MacDonald PC. Decline in the concentration of low-density lipoprotein-cholesterol in human fetal plasma near term. Metabolism. 1983; 32:919–23.

Table 1.
Fetal and maternal characteristics
Table 2.
Prevalence of atherogenic risk among the participants (n=167)
| Prevalence of atherogenic risk | No. of patients (%) | 95% CI (%) |
|---|---|---|
| High risk | 3 (1.8) | 0.4–5.2 |
| Medium risk | 10 (6.0) | 2.9–10.7 |
| Low risk | 154 (92.2) | 87.1–95.8 |
Table 3.
Relationship between AIP, gestational age, and birth weight
Table 4.
Summary of stepwise regression result of sociodemographic and clinical predictors of atherogenic risk (n=167)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download