Abstract
Background
Missing isoniazid (INH) resistance during tuberculosis (TB) diagnosis can worsen the outcomes of INH-resistant TB. The BD MAX MDR-TB assay (BD MAX) facilitates the rapid detection of TB and INH and rifampin (RIF) resistance; however, data related to its performance in clinical setting remain limited. Moreover, its effect on treatment outcomes has not yet been studied.
Methods
We compared the performance of BD MAX for the detection of INH/RIF resistances to that of the line probe assay (LPA) in patients with pulmonary TB (PTB), using the results of a phenotypic drug sensitivity test as a reference standard. The treatment outcomes of patients who used BD MAX were compared with those of patients who did not.
Results
Of the 83 patients included in the study, the BD MAX was used for an initial PTB diagnosis in 39 patients. The sensitivity of BD MAX for detecting PTB was 79.5%. The sensitivity and specificity of BD MAX for INH resistance were both 100%, whereas these were 50.0% and 95.8%, respectively, for RIF resistance. The sensitivity and specificity of BD MAX were comparable to those of LPA. The BD MAX group had a shorter time interval from specimen request to the initiation of anti-TB drugs (2.0 days vs. 5.5 days, p=0.001).
Tuberculosis (TB) is a major global health concern. In 2022, 7.5 million people were newly diagnosed with TB, and approximately 1.13 million people died due to this infection worldwide [1]. Among these, multidrug- or rifampin (RIF)-resistant TB (MDR/RR-TB) was observed in 410,000 patients. Moreover, the prevalence of isoniazid (INH)-resistant TB (Hr-TB) was higher than that of MDR/RR-TB. Approximately 1.3 million people may have Hr-TB and RIF-susceptible TB, which is generally missed in clinical settings as the detection of RIF resistance is often prioritized [1]. In 2022, 16,264 patients with TB were newly diagnosed in South Korea. Among these, 1.9% (308 patients) had MDR-TB [2], and approximately 10% of newly diagnosed patients and >30% of previously treated patients had Hr-TB [3].
As the identification of Mycobacterium tuberculosis (MTB) and drug resistance using the acid-fast bacilli (AFB) culture and phenotypic culture-based drug sensitivity test (DST) is time-consuming [4], several molecular methods are now being widely used. The Xpert MTB/RIF assay (Xpert; Cepheid, Santa Clara, CA, USA) exhibits high sensitivity and specificity for the detection of RIF resistance and a shorter turnaround time than the phenotypic culture-based DST [5]. Accordingly, the World Health Organization (WHO) has recommended Xpert as an initial diagnostic test for detecting TB and RIF resistance in patients with signs and symptoms of pulmonary TB (PTB) [6]. However, it cannot detect INH resistance. The line probe assay (LPA) shows comparable sensitivity and specificity for the rapid detection of INH/RIF resistance [7]. However, considering the low sensitivity of LPA in AFB smear-negative specimens [8], the current WHO guidelines recommend the use of LPA for INH/RIF resistance only in AFB smear-positive specimens or cultured isolates of TB [6]. Moreover, the sensitivity of LPA for detecting INH resistance is lower than that for RIF resistance [9,10]. Therefore, according to WHO, phenotypic DST for INH may be used to evaluate patients when INH resistance is not detected using LPA [6]. As current guidelines recommend the inclusion of quinolone at the initiation of Hr-TB treatment [11], detecting Hr-TB quickly to treat it effectively from the beginning is crucial to improving poor treatment outcomes [12,13] and preventing MDR-TB emergence [14].
Recently, several novel molecular platforms using nucleic acid amplification tests have been developed for the rapid detection of INH/RIF resistance and the diagnosis of TB. Among these, the BD MAX MDR-TB assay (BD MAX; BD, Franklin Lakes, NJ, USA) showed high sensitivity and specificity for the detection of TB and INH/RIF resistance in a multicenter large-scale study [15]. Additionally, the diagnostic performance of BD MAX has been evaluated using the preserved samples [16-18]. However, to date, compared to TB-polymerase chain reaction (PCR) and LPA, data on BD MAX performance in clinical practice for detecting TB and INH/RIF resistance remains insufficient. Moreover, its effects on treatment outcomes in patients with TB have not yet been studied. Therefore, this study aimed to investigate the applicability of BD MAX in the treatment of patients with TB in clinical settings.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Wonkwang University Sanbon Hospital (IRB No: WMCSB202208-74-200861). The requirement for informed consent was waived by the IRB because of the retrospective design of the study and the use of medical records only.
We conducted a retrospective cohort study of patients diagnosed with PTB at a secondary referral hospital. All the patients diagnosed with TB between January 2019 and July 2022 were screened for eligibility. Patients who did not provide specimens at the time of TB diagnosis and those who did not undergo TB treatment were excluded from the study. Further, patients diagnosed with extrapulmonary TB and patients whose diagnosis changed from TB to other diseases during the course of treatment were also excluded. In addition, those who had negative AFB culture results but were clinically diagnosed and treated for TB were excluded from the analysis.
Anthropometric characteristics of the patients including age, sex, body weight, and height, were collected from the medical records. Smoking status, TB treatment history, and the presence of comorbidities were also reviewed.
Respiratory specimens were collected from the patients suspected of having PTB. Thereafter, an AFB smear and culture test, and an AdvanSure TB-PCR assay (LG Chemistry, Seoul, Korea) were performed. Our hospital introduced and started using the BD MAX in March 2020. Subsequently, patients suspected of having PTB were tested using the BD MAX. As the Xpert test is not performed at our hospital, it was not included in this study. If a patient suspected of having PTB was not diagnosed using a sputum sample, a bronchoscopy was performed to obtain a respiratory specimen.
Active TB was diagnosed based on the positive results of the AFB culture of the obtained specimens. Treatment success was defined as “cured” or “treatment completed” according to the 2021 WHO updated definition [19].
Specimens treated with a 4% solution of sodium hydroxide/standard N-acetyl-L-cysteine were stained using the Ziehl-Neelsen method. The specimens were inoculated in 3% Ogawa agar (Shinyang Chemical, Busan, Korea) and BBL mycobacteria growth indicator tubes (MGIT; BD), and then cultured at 37°C in an incubator and the MGIT 960 system (BD) for 6 and 8 weeks, respectively. All positive cultures were identified to the species level using the AdvanSure Mycobacterium GenoBlot Assay (LG Chemistry). Phenotypic DST was performed using the conventional absolute concentration method.
TB-PCR was performed using the AdvanSure TB/NTM real-time PCR assay (LG Chemistry) following the manufacturer’s instructions. A pre-treatment solution (3 mL) was added to the specimen. Thereafter, DNA was extracted from the precipitate, following centrifugation. The IS6110 region of MTB and the internal transcribed spacer region of mycobacteria were detected using a SLAN real-time quantitative PCR detection system (LG Chemistry).
LPA was performed when an AFB smear yielded a positive result. MolecuTech REBA MTB-MDR (YD Diagnostics, Yongin, Korea) was used to detect mutations in rpoB, katG, and inhA using a reverse blot hybridization assay. LPA was also performed on specimens that were AFB smear-negative but culture-positive.
BD MAX was performed following the manufacturer’s instructions. The concentrated specimens were added to the sample treatment reagent and shaken manually. After incubation, the samples were transferred to a BD MAX system and processed for further analysis, including automated PCR amplification and detection.
We calculated the sensitivities of AFB smear, AdvanSure TB-PCR, and BD MAX for PTB detection. We described them separately when analyzing the whole specimens and when analyzing the specimens obtained using bronchoscopy.
To determine the diagnostic accuracy of BD MAX for detecting INH/RIF resistance, we compared the sensitivity and specificity of this assay with those of LPA, using the results of phenotypic DST as the reference standard.
We also compared the treatment outcomes of patients for whom BD MAX was used with those for whom BD MAX was not used. The time intervals from specimen request to the initiation of anti-TB drugs and from specimen request to negative culture conversion were compared, along with the treatment success rates.
All statistical analyses were performed using the the R software version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria; https://r-project.org). All statistical tests were two-sided, and differences with p<0.05 were considered statistically significant.
During the study period, 146 patients were diagnosed with or treated for TB. Of these, eight were diagnosed with TB but were transferred to other hospitals before initiating treatment. Four patients were diagnosed with TB at other hospitals and were transferred to our hospital; therefore, their initial diagnostic results were unavailable. Further, 17 patients diagnosed with extrapulmonary TB and seven patients whose diagnoses were changed to other diseases, including nontuberculous mycobacterial lung disease and pneumonia, were excluded. Additionally, 27 patients with negative AFB culture results who were clinically diagnosed and treated for TB were also excluded. Finally, 83 patients were included in this study (Fig. 1).
Among the included patients, BD MAX was used during PTB diagnosis for 39 patients (BD MAX group), whereas it was not used for 44 patients (non-BD MAX group). No significant differences were observed in the anthropometric characteristics and comorbidities between the two groups. The presence of cavitary lesions did not differ between the groups; however, higher numbers of bronchoscopies were performed in the BD MAX group (Table 1).
For all patients, the sensitivities of AFB smear, AdvanSure TB-PCR, and BD MAX for PTB detection were 45.8% (38 of 83), 71.1% (59 of 83), and 79.5% (31 of 39), respectively. On limiting the analysis to patients who underwent bronchoscopy at the time of PTB diagnosis, the sensitivity of each test was 27.8% (10 of 36), 61.1% (22 of 36), and 73.1% (19 of 26), respectively. In both cases, the BD MAX exhibited the highest sensitivity for detecting PTB (Table 2).
In the BD MAX group, positive results were reported for 31 patients. Further, in five patients, only TB-PCR performed using BD MAX was positive. Notably, INH/RIF resistance profiles could not be obtained due to insufficient sample volume. Consequently, 26 patients were included in the BD MAX performance analysis for detecting INH/RIF resistance.
The sensitivity and specificity of BD MAX for detecting INH resistance were both 100%. The sensitivity and specificity of LPA were 100% and 95.7%, respectively. Of the three patients in whom INH resistance was detected using BD MAX, two had inhA mutations, and one had a katG mutation.
The sensitivity of BD MAX for detecting RIF resistance was 50.0%, discriminating one RR-TB case as RIF-susceptible TB. The specificity of BD MAX was 95.8%. The results of LPA were similar to those of BD MAX (Table 3).
The time interval from specimen request to the initiation of anti-TB drugs was significantly shorter in the BD MAX group than in the non-BD MAX group (2 days vs. 5.5 days, p=0.001).
The time intervals from specimen request to negative culture conversion were 18 days and 46 days in the BD MAX and non-BD MAX groups, respectively. However, this difference was not significant (p=0.106).
Regarding treatment outcomes, although 35 (89.7%) and 38 patients (86.4%) were successfully treated in the two groups, respectively, the difference was not significant (Table 4).
BD MAX has been extensively studied among novel molecular platforms for the rapid detection of INH/RIF resistance. A large-scale study involving over 1,000 participants showed high sensitivity and specificity of BD MAX in detecting INH/RIF resistance [15], and several other studies using preserved samples have also reported encouraging results [16-18]. To the best of our knowledge, this study is the first to document the use of BD MAX in actual clinical scenarios and report its performance and impact on treatment outcomes.
Our results indicate that the sensitivity of BD MAX in the PTB diagnosis is superior to that of the AFB smear and AdvanSure TB-PCR. Previously, the diagnostic accuracy of Xpert using unprocessed specimens has been reported to be more sensitive than that of the AdvanSure TB-PCR method using decontaminated and concentrated specimens, especially using lower respiratory tract specimens (LRTS), which are more sterile and concentrated than sputum specimens [20,21]. Moreover, although our study excluded patients with negative AFB culture results but clinically diagnosed PTB, previous studies showed enhanced diagnostic usefulness of Xpert for AFB culture-negative LRTS [22,23]. As unprocessed specimens are used in BD MAX, similar to Xpert, it may be useful for the detection of patients with TB who are not diagnosed using AdvanSure TB-PCR, especially when LRTS is used. Considering that TB treatment in patients with negative AFB smear and TB-PCR results is delayed [24], BD MAX as well as Xpert may be valuable for detecting TB, especially when performed using LRTS in patients with a low bacterial load.
In addition to the diagnostic advantages, novel molecular platforms may reduce turnaround time owing to rapid diagnosis and culture conversion. In our study, the turnaround time to initiate TB treatment was shorter in the BD MAX group than in the non-BD MAX group. Notably, turnaround time is also reduced using Xpert [25], and a similar effect can be achieved using BD MAX. The finding that the use of BD MAX did not affect the time interval to culture conversion may be attributed to the small number of patients with drug-resistant TB in this study.
In our study, BD MAX showed excellent performance in detecting INH resistance (100%), in accordance with the phenotypic DST. This result is better than that of previous studies that showed a sensitivity of 58.3% to 82% in detecting INH resistance [15,18]. Considering that several months are required to obtain the results of phenotypic DST [4] and that monodrug-resistant TB as well as MDR-TB can lead to poor outcomes [26], using BD MAX for detecting INH resistance simultaneously with TB diagnosis may be beneficial. Although our study did not include a sufficiently large number of patients with Hr-TB, future large-scale studies may reveal a shorter time interval from specimen request to the initiation of quinolone treatment in patients with Hr-TB using BD MAX, thereby contributing to improved treatment outcomes.
The sensitivity of BD MAX for detecting RIF resistance in our study was 50.0%, with one false-negative result. One patient with negative BD MAX and positive phenotypic DST results for RIF resistance also showed negative LPA results for RIF resistance. The causes of false-negative results of molecular methods for RIF resistance have rarely been studied, but previous studies have suggested that some strains with S531W and S531F mutations within the rpoB gene are non-interpretable via LPA [27], and some strains with L533P mutation susceptible via Xpert [28]. Additionally, approximately 4% of RIF resistances are caused by mutations outside the rpoB gene [29]. Furthermore, TB infection with multiple strains can also negatively affect Xpert’s performance [30]. No single test is perfect, and molecular tests may be inaccurate sometimes; therefore, combined molecular and phenotypic DST results must be considered.
Our study has some limitations. First, owing to the retrospective design of this study, factors other than the implementation of BD MAX may have affected the reduction in turnaround time. For example, the high sensitivity of bronchoscopy specimens may also have resulted in a short turnaround time because the proportion of patients who underwent bronchoscopy was higher in the BD MAX group. However, the superior sensitivity of the BD MAX in detecting PTB was maintained only when patients who underwent bronchoscopy were analyzed. Nevertheless, future prospective studies are required under similar conditions. Second, although comparing the BD MAX performance with that of Xpert, for which extensive data are available, is desirable according to previously published guidelines for studies evaluating the accuracy of rapid TB drug-susceptibility tests [31], we could not examine the clinical advantages of BD MAX over Xpert, as our hospital does not use Xpert. As the advantage of BD MAX over Xpert lies in identifying INH resistance, future studies must be conducted to compare BD MAX and Xpert in a larger patient population, including a sufficient number of patients with Hr-TB. Third, Although BD MAX performance for detecting INH/RIF resistance was comparable to LPA, only 26 patients were included in our analysis. Despite the insufficient sample size to accept the sensitivity and specificity of BD MAX as reference values, our study is the first report in clinical settings, and further research in this area is warranted.
In conclusion, the BD MAX showed comparable performance to conventional tests for detecting PTB and INH/RIF resistance. Further, the implementation of BD MAX as a diagnostic tool for PTB resulted in a shorter turnaround time for the initiation of TB treatment.
References
1. World Health Organization (WHO). Global tuberculosis report 2023 [Internet]. Geneva, Switzerland: WHO;2023. [cited 2024 Jan 4]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023.
2. Korea Disease Control and Prevention Agency (KDCA). Annual report on the notified tuberculosis in Korea, 2022 [Internet]. Cheongju, Korea: KDCA;2023. [cited 2024 Jan 4]. https://www.kdca.go.kr/board/board.es?mid=a31001000000&bid=0130&act=view&list_no=723921&tag=&nPage=1.
3. Jhun BW, Koh WJ. Treatment of isoniazid-resistant pulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2020; 83:20–30.

4. Jhun BW, Huh HJ, Koh WJ. Diagnosis of pulmonary tuberculosis. J Korean Med Assoc. 2019; 62:18–24.

5. Kim YW, Seong MW, Kim TS, Yoo CG, Kim YW, Han SK, et al. Evaluation of Xpert(®) MTB/RIF assay: diagnosis and treatment outcomes in rifampicin-resistant tuberculosis. Int J Tuberc Lung Dis. 2015; 19:1216–21.

6. World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 3: diagnosis–rapid diagnostics for tuberculosis detection [Internet]. Geneva, Switzerland: WHO;2020. [cited 2024 Jan 4]. https://www.who.int/publications/i/item/9789240029415.
7. Miotto P, Tessema B, Tagliani E, Chindelevitch L, Starks AM, Emerson C, et al. A standardised method for interpreting the association between mutations and phenotypic drug resistance in Mycobacterium tuberculosis. Eur Respir J. 2017; 50:1701354.
8. Yadav RN, Kumar Singh B, Sharma R, Chaubey J, Sinha S, Jorwal P. Comparative performance of line probe assay (version 2) and Xpert MTB/RIF assay for early diagnosis of rifampicin-resistant pulmonary tuberculosis. Tuberc Respir Dis (Seoul). 2021; 84:237–44.
9. Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017; 49:1601075.

10. World Health Organization (WHO). The use of molecular line probe assay for the detection of resistance to isoniazid and rifampicin: policy update [Internet]. Geneva, Switzerland: WHO;2016. [cited 2024 Jan 4]. https://apps.who.int/iris/handle/10665/250586.
11. World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment [Internet]. Geneva, Switzerland: WHO;2020. [cited 2024 Jan 4]. https://www.who.int/publications/i/item/9789240007048.
12. Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009; 6:e1000150.

13. Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017; 17:223–34.

14. Romanowski K, Campbell JR, Oxlade O, Fregonese F, Menzies D, Johnston JC. The impact of improved detection and treatment of isoniazid resistant tuberculosis on prevalence of multi-drug resistant tuberculosis: a modelling study. PLoS One. 2019; 14:e0211355.

15. Shah M, Paradis S, Betz J, Beylis N, Bharadwaj R, Caceres T, et al. Multicenter study of the accuracy of the BD MAX multidrug-resistant tuberculosis assay for detection of mycobacterium tuberculosis complex and mutations associated with resistance to rifampin and isoniazid. Clin Infect Dis. 2020; 71:1161–7.

16. Beutler M, Plesnik S, Mihalic M, Olbrich L, Heinrich N, Schumacher S, et al. A pre-clinical validation plan to evaluate analytical sensitivities of molecular diagnostics such as BD MAX MDR-TB, Xpert MTB/Rif Ultra and FluoroType MTB. PLoS One. 2020; 15:e0227215.

17. Ciesielczuk H, Kouvas N, North N, Buchanan R, Tiberi S. Evaluation of the BD MAX™ MDR-TB assay in a real-world setting for the diagnosis of pulmonary and extra-pulmonary TB. Eur J Clin Microbiol Infect Dis. 2020; 39:1321–7.

18. Hofmann-Thiel S, Plesnik S, Mihalic M, Heiß-Neumann M, Avsar K, Beutler M, et al. Clinical evaluation of BD MAX MDR-TB assay for direct detection of mycobacterium tuberculosis complex and resistance markers. J Mol Diagn. 2020; 22:1280–6.

19. Linh NN, Viney K, Gegia M, Falzon D, Glaziou P, Floyd K, et al. World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J. 2021; 58:2100804.

20. Ko Y, Lee HK, Lee YS, Kim MY, Shin JH, Shim EJ, et al. Accuracy of Xpert(®) MTB/RIF assay compared with AdvanSure™ TB/NTM real-time PCR using bronchoscopy specimens. Int J Tuberc Lung Dis. 2016; 20:115–20.

21. Son E, Jang J, Kim T, Jang JH, Chung JH, Seol HY, et al. Head-to-head comparison between Xpert MTB/RIF assay and real-time polymerase chain reaction assay using bronchial washing specimens for tuberculosis diagnosis. Tuberc Respir Dis (Seoul). 2022; 85:89–95.

22. Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, et al. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013; 17:917–21.

23. Jo YS, Park JH, Lee JK, Heo EY, Chung HS, Kim DK. Discordance between MTB/RIF and real-time tuberculosis-specific polymerase chain reaction assay in bronchial washing specimen and its clinical implications. PLoS One. 2016; 11:e0164923.

24. Kwak SH, Choi JS, Lee EH, Lee SH, Leem AY, Lee SH, et al. Characteristics and risk factors associated with missed diagnosis in patients with smear-negative pulmonary tuberculosis. Korean J Intern Med. 2021; 36(Suppl 1):S151–9.

25. Kwak N, Choi SM, Lee J, Park YS, Lee CH, Lee SM, et al. Diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay in routine clinical practice. PLoS One. 2013; 8:e77456.

26. Kim J, Kwak N, Lee HY, Kim TS, Kim CK, Han SK, et al. Effect of drug resistance on negative conversion of sputum culture in patients with pulmonary tuberculosis. Int J Infect Dis. 2016; 42:64–8.

27. Singh BK, Sharma R, Kodan P, Soneja M, Jorwal P, Nischal N, et al. Diagnostic evaluation of non-interpretable results associated with rpoB gene in genotype MTBDRplus ver 2.0. Tuberc Respir Dis (Seoul). 2020; 83:289–94.

28. Rufai SB, Kumar P, Singh A, Prajapati S, Balooni V, Singh S. Comparison of Xpert MTB/RIF with line probe assay for detection of rifampin-monoresistant Mycobacterium tuberculosis. J Clin Microbiol. 2014; 52:1846–52.

29. Hughes D, Brandis G. Rifampicin resistance: fitness costs and the significance of compensatory evolution. Antibiotics (Basel). 2013; 2:206–16.

Fig. 1.
Inclusion flow chart. BD MAX, BD MAX MDR-TB assay (BD, Franklin Lakes, NJ, USA). TB, tuberculosis; AFB, acid-fast bacilli.
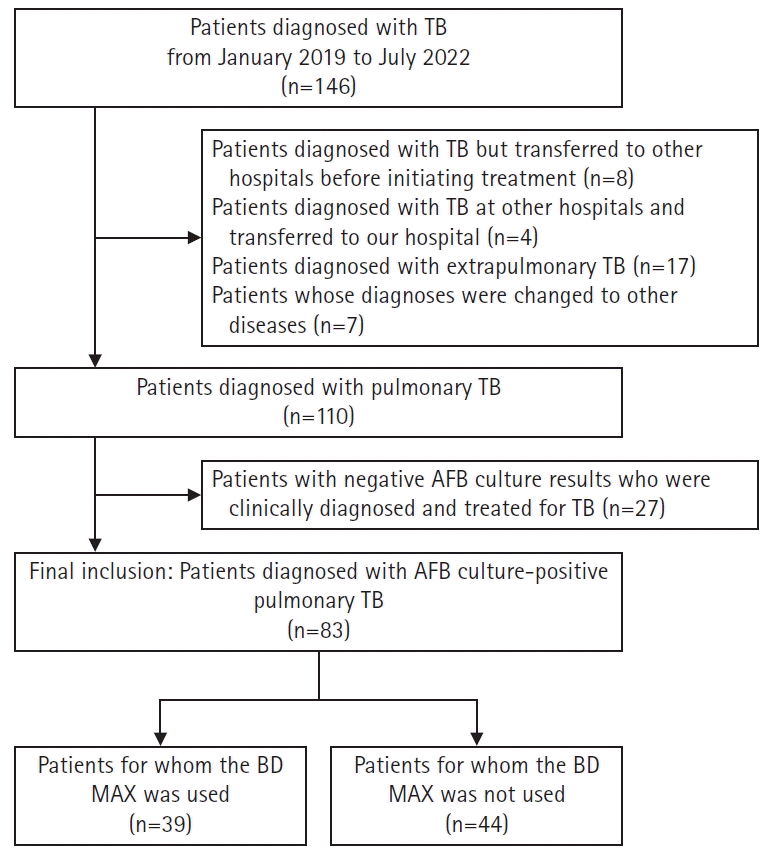
Table 1.
Baseline characteristics of the patients
Table 2.
Diagnostic accuracy of AFB smear, AdvanSure TB-PCR, and BD MAX MDR-TB assays for the diagnosis of pulmonary tuberculosis using whole specimens and specimens obtained using bronchoscopy
Table 3.
Diagnostic accuracy of BD MAX MDR-TB and line probe assays for detecting isoniazid and rifampin resistance
Table 4.
Comparison of treatment outcomes between patients for whom the BD MAX MDR-TB assay was used and those for whom this assay was not used
| Variable | BD MAX group | Non-BD MAX group | p-value |
|---|---|---|---|
| Interval from the specimen request to initiate antituberculosis drugs (day) | 2.0 (1.0–4.5) | 5.5 (2.0–21.0) | 0.001 |
| Interval from the specimen request to negative culture conversion (day) | 18.0 (15.0–62.0) | 46.0 (36.0–60.0) | 0.106 |
| Treatment outcomes | 0.893 | ||
| Treatment success | 35 (89.7) | 38 (86.4) | |
| Unfavorable outcomesa) | 4 (10.3) | 6 (13.6) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download