Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is characterized by an increase in hepatic triglyceride content and increased inflammatory macrophage infiltration through the C-C motif chemokine receptor (CCR) 5 pathway in the liver. DA-6034 (7-carboxymethyloxy-3',4',5-trimethoxy flavone), is a synthetic derivative of eupatilin that exhibits anti-inflammatory activity in inflammatory bowel disease. However, the effect of DA-6034 on the inflammatory response in NAFLD is not well elucidated. Therefore, we aimed to determine the effect of DA-6034 on hepatic steatosis and inflammation.
Methods
Forty male C57BL/6J mice were divided into the following four groups: (1) regular diet (RD), (2) RD with DA-6034, (3) high fat diet (HFD), and (4) HFD with DA-6034. All mice were sacrificed 12 weeks after the start of the experiment. The effects of DA-6034 on macrophages were assessed using RAW 264.7 cells.
Results
DA-6034 not only reduced hepatic triglyceride levels and lipid accumulation but also macrophage infiltration and proinflammatory cytokines in HFD-fed mice. According to fluorescence-activated cell sorter analysis, DA-6034 reduced the CD8+ T cell fraction in the liver of HFD-fed mice. DA-6034 also reduced CCR5 expression and the migration of liver macrophages in HFD-fed mice and inhibited CCR2 ligand and CCR4 ligand, which stimulated the migration of macrophages.
The liver plays a crucial role in glucose homeostasis in the body; however, its function is reduced by lipid accumulation in the liver or liver diseases. Nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH) is commonly caused by fat accumulation in the liver due to obesity [1]. NAFLD and NASH are closely associated with type 2 diabetes and insulin resistance [2]. According to recent studies, the accumulated fat accelerates inflammation by increasing immune cell accumulation and proinflammatory cytokine secretion [3].
In obesity, there is an increase in various mediators, such as triglycerides (TGs) and cytokines, as well as the upregulation of the transcription factor, nuclear factor kappa B (NF-κB). These factors can contribute to the development of inflammation [4]. Activated NF-κB induces the activation of various inflammatory genes and regulates various immune cells [4]. In recent studies, the NF-κB signaling pathway in the liver has been highlighted to play an important role in the development of metabolic diseases [5]. The function of NF-κB in inflammation has mainly been examined in macrophages (a family of innate immune cells), which can be phenotypically differentiated into different states, such as classically activated (M1) and alternatively activated (M2) macrophages [5,6]. M1 macrophages secrete proinflammatory cytokines involved in various inflammatory processes and promote inflammation by enhancing the differentiation of inflammatory T cells [7,8].
Eupatilin, an active flavone, has been reported to exhibit antioxidant, anti-inflammatory, and anti-cancer effects [9-11]. Further, eupatilin has been demonstrated to ameliorate chronic pancreatitis in a mouse model, inhibit NF-κB activation, and reduce the production of proinflammatory cytokines [12]. DA-6034, a synthetic derivative of eupatilin, has the ability to alleviate inflammation in gastritis and reduce the expression of NF-κB in gastric epithelial cells, where inflammation occurs [13]. The purpose of this study was to determine whether DA-6034 ingestion affects NAFLD by assessing its role in regulating inflammation in high fat diet (HFD)-fed mice.
Ethical statements: This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University (Wonju Campus) (IACUC No: YWC-160804-1).
Six-week-old male C57BL/6 mice (n=40) were purchased from DBL Co. Ltd (Eumseong, Korea) and allowed to acclimate to the environment for 2 weeks. Animals were fed 60% HFD (60% of calories from fat, n=20; Research Diets Inc., New Brunswick, NJ, USA) for 10 weeks. The DA-6034 treatment dose was determined according to the study by Chung et al. [14]. Each group of mice was administered either HFD or HFD mixed with DA-6034 (50 mg/kg per day) for 12 weeks. The amount of DA-6034 added to HFD was adjusted according to the changes in body weight of each mouse. Food and water intake and body weight were measured weekly. Experimental mice were anesthetized with 250 mg/kg 2,2,2-tribromoethanol (Avertin, T48402; Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection. Then, blood was collected by cardiac puncture, and serum was separated by centrifugation at 2,000 rpm (377 relative centrifugal force) for 20 minutes using a refrigerated centrifuge at 4°C. Extracted liver tissues and separated serum were immediately frozen in liquid nitrogen and stored at –80℃ until further analysis.
Hepatic TG content was assayed via saponification in ethanolic poysddium hydroxide. Glycerol content was measured using a free glycerol determination kit (FG0100, Sigma-Aldrich) after neutralization with MgCl2. The glycerol content was calculated from the tissue TG values, and the resulting values were adjusted to account for liver weight.
The liver was dissected and immediately frozen in liquid nitrogen. Each liver tissue was homogenized with 500 μL of lysis buffer (pH 7.4) supplemented with protease and phosphatase inhibitors. Lipids were removed via centrifugation at 10,000 g for 20 minutes. Total protein (50 mg) was subjected to western blot analysis using a polyclonal antibody to anti-fatty acid synthase (anti-FAS; sc-55580, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-sterol regulatory element-binding protein 1 (anti-SREBP-1; sc-365531, Santa Cruz Biotechnology), anti-inhibitor of kappa B (IκB; 4814, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-NF-κB (3033, Cell Signaling Technology), anti-NF-κB (4764, Cell Signaling Technology), anti-C-C motif chemokine receptor 2 (anti-CCR2; sc-13563, Santa Cruz Biotechnology), anti-CCR5 (sc-17833, Santa Cruz Biotechnology), and β-actin (sc-47778, Santa Cruz Biotechnology).
Liver tissues were fixed overnight at room temperature in 4% formaldehyde and embedded in paraffin. Thereafter, the tissue sections (8 μm thick) were stained with hematoxylin and eosin (H&E) and mounted on glass slides. The stained sections were viewed under an Axiostar Plus light microscope (Carl Zeiss, Gottingen, Germany).
To assess macrophage infiltration in the liver, 4% paraformaldehyde-fixed liver tissues were stained with anti-CD68 (sc-59103, Santa Cruz Biotechnology). The stained tissue slides were observed under an Axiostar Plus light microscope.
Mice were sacrificed after an 8-hour fast, and their blood was collected via cardiac puncture. Serum glucose, total cholesterol, TG, alanine aminotransferase (ALT), and aspartate transaminase (AST) concentrations were determined using commercially available enzymatic assay kits (Asan Pharmacology, Seoul, Korea). Serum insulin and adiponectin levels were measured with an ultrasensitive mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Shibayagi, Gunma, Japan) and mouse adiponectin ELISA kit (ALPCO Diagnostics, Salem, NH, USA), respectively.
To obtain liver-infiltrating cells, the liver tissue was homogenized with a gentleMACS dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) and digested at 37°C for 15 minutes with shaking in RPMI 1640 medium supplemented with 20 mg/mL collagenase type 2 (Sigma-Aldrich). The digested tissue was filtered through a 100-µm nylon mesh to remove undigested tissue. Cells were harvested via gentle pipetting and exposed to a 40% to 70% percoll gradient to enrich mononuclear cells.
Immune cells isolated from the liver tissues were incubated with Fc Block (BD Biosciences, San Jose, CA, USA) in the dark at 4°C on a bidirectional shaker for 30 minutes. The cells were then double-stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (11-0118-42), phycoerythrin (PE)-conjugated anti-F4/80 (12-4801-82), PE-cyanine7-conjugated anti-CD11c (25-0114-82), allophycocyanin-conjugated anti-CD206 (17-2069-42), PE-cyanine7-conjugated anti-CD3 (25-0037-42), PE-conjugated anti-CD4 (12-0047-42), and FITC-conjugated anti-CD8 (11-0080-81) antibodies (eBioscience, San Diego, CA, USA). T cells were washed with fluorescence-activated cell sorting buffer and quantitated using the BD FACSVerse flow cytometer (BD Biosciences) and analyzed with the FlowJo software (Tree Star, Ashland, MA, USA).
The levels of inflammatory cytokines, including interleukin (IL)-6, IL-10, monocyte chemoattractant protein 1 (MCP1), interferon gamma (IFN-γ), tumor necrosis factor (TNF), and IL-12p70, in serum were analyzed using cytometric bead array mouse inflammation kit (BD Biosciences).
AML12 hepatocytes (ATCC, Manassas, VA, USA) were cultured at 37℃ in 5% CO2 in Dulbecco’s modified Eagle medium (DMEM)/F12 (Gibco BRL, NY, USA) containing 10% fetal bovine serum (FBS), 10 mL/L penicillin streptomycin (Invitrogen, Carlsbad, CA, USA), and 100X insulin-transferrin-selenium (ITS-G, Gibco BRL, Grand Island, NY, USA). RAW 264.7 cells were cultured at 37.5°C in DMEM containing 25.5 mM glucose, 1% antibiotics, and 5% FBS.
The viability of AML12 cells was determined using the thiazolyl blue tetrazolium bromide (MTT) assay according to the concentration of DA-6034 and eupatilin. The MTT solution (5 mg/mL in phosphate-buffered saline) was added to the cells, which were incubated for 2 hours at 37℃. Finally, the viability was measured at a wavelength of 540 nm.
To determine whether DA63034 affects diet-induced obesity, we evaluated the body weight of mice fed either a regular diet (RD) or HFD, in the presence or absence of DA-6034, for 12 weeks. The body and liver weights were found to be increased by HFD. Notably, DA-6034 did not statistically inhibit bodyweight gain (Fig. 2A). And food intake was not significantly different among experimental groups (Fig. 2B). The liver weights were significantly increased by HFD but, DA-6034 treatment in HFD did not statistically increase liver weight compared to RD (Fig. 2C). We proceeded to perform a histological analysis of the liver and quantified the liver TG content. H&E staining revealed significant lipid accumulation in the liver of HFD-fed mice; however, treatment with DA-6034 was found to significantly reduce the lipid accumulation (Fig. 2D, 2E). DA-6034 also regulated the levels of lipogenesis-related factors such as FAS and SREBP-1, in the liver (Fig. 2F–2H). Overall, DA-6034 has no significant effect on HFD-induced weight change but improves hepatic steatosis in HFD-induced obese mice.
To elucidate the effects of DA-6034 on metabolic parameters, we measured the serum levels of these parameters in mice for 12 weeks. Based on the results, the metabolic parameters did not differ between the wild-type (WT) and DA-6034–treated RD groups. However, the fasting glucose and serum TG levels in HFD-fed mice were decreased by DA-6034 treatment. To determine whether DA-6034 could improve hepatic steatosis, we measured the serum levels of ALT and AST. In HFD mice, serum ALT levels were slightly decreased while AST levels were significantly decreased by DA-6034 treatment (Table 1).
To determine the effects of DA-6034 on inflammation, we measured the levels of proinflammatory cytokines (TNF-α, MCP1, IL-6, IFN-γ, and IL-12p70) and anti-inflammatory cytokines (IL-10) in the liver of mice. Results showed that the levels of proinflammatory cytokines such as TNF-α, MCP1, and IL-6 were significantly increased in HFD-fed obese mice and these cytokine levels were significantly attenuated by DA-6034 treatment. However, the concentrations of IFN-γ and IL-12p70 level were not thought to be affected by HFD feeding or DA-6034 treatment (Table 2). In addition, the level of the anti-inflammatory cytokine, IL-10, was significantly increased by DA-6034 treatment (Table 2). We proceeded to investigate the effects of DA-6034 on the NF-κB pathway. Our findings revealed a significantly lower level of phosphorylated NF-κB and recovered IκB expression in the liver of DA-6034–treated obese mice compared to the HFD group (Fig. 2F, 2I, 2J). These data suggest that DA-6034 regulates liver inflammation by inhibiting NF-κB activation.
We determined the effect of DA-6034 on RAW 264.7 cells migration using a transwell system. RAW 264.7 cells were treated with CCR2 ligand (CCL2) and CCR5 ligand (CCL4). Treatment with CCL2 and CCL4 significantly induced macrophage migration; however, DA-6034 inhibited this effect (Fig. 3A, 3B). We proceeded to examine the effect of DA-6034 on macrophage infiltration in the liver. HFD activated macrophage infiltration into the liver; however, DA-6034 administration significantly inhibited HFD-induced macrophage infiltration (Fig. 3C). The CCR plays an important role in macrophage migration, and DA-6034 was found to decrease the level of CCR5 (Fig. 3D–3F). Furthermore, lipopolysaccharides treatment also significantly induced NF-κB phosphorylation, while DA-6034 treatment markedly inhibited its phosphorylation in RAW 264.7 cells (Fig. 3G, 3H). Moreover, we examined the phenotype of liver immune cells after DA-6034 treatment and used antibodies against CD11c and CD206 to distinguish between M1 and M2 macrophages (Fig. 4A). The infiltration of M1 macrophages, which is associated with hepatic inflammation, was significantly decreased by DA-6034 treatment (Fig. 4B). In addition, the percentage of CD8+ T cells in the liver was markedly reduced in DA-6034–treated obese mice compared to that in WT obese mice (Fig. 4C, 4D). These findings indicate that DA-6034 can inhibit macrophage migration.
In this study, we revealed that DA-6034 regulates inflammatory macrophage activation, which plays an important role in the development of fatty liver disease (FLD). The administration of DA-6034 did not affect body weight and plasma glucose levels, but attenuated HFD-induced hepatic steatosis based on the reduced accumulation of hepatic TG and decreased plasma AST and ALT levels. NAFLD is caused by various pathogenic factors, including obesity, insulin resistance, and inflammatory cytokines [16], and is characterized by excessive fat accumulation in the liver. Since hepatic steatosis can chronically cause liver diseases such as cirrhosis and hepatocellular carcinoma, it is important to control it at early stage. SREBP-1 is a major nuclear transcription factor that plays an important role in regulating lipid metabolism and conditions such as macrophage-induced inflammation or insulin resistance can promote the activation of SREBP-1 [16,17]. Here, treatment with DA-6034 reduced the protein levels of SREBP-1c in the liver of mice, suggesting that DA-6034 regulates hepatic lipid homeostasis. Kuan et al. [18] reported that inhibition of heat shock protein 90 (HSP90) improved lipid disorders by regulating SREBP-1, and another study by Ko et al. [19] reported that DA-6034 inhibited HSP90 in Helicobacter pylori-infected MKN-45. Therefore, it is expected that DA-6034 can regulate SREBP-1 by regulating HSP90, and for further studies, we plan to conduct research on the regulation of HSP90 by DA-6034 in lipid homeostasis.
Several studies have evaluated the crosstalk between metabolism and immune cells [20-22]. Macrophages play a key role in innate immunity and liver inflammation, and M1 phenotype polarization of macrophages can lead to increased hepatic steatosis. M1 macrophages have reduced mitochondrial oxidative capacity, increased rates of glycolysis and de novo FAS, and contain TG-rich lipid droplets (LDs), which are involved in the development of hepatic steatosis and are a major cause of increased inflammation in the liver [23,24]. In various disease states, macrophages with LDs serve as stores of TGs. TG lipolysis releases fatty acids for mitochondrial oxidation; however, in macrophages, proinflammatory signals decrease fatty acid oxidation and TG synthesis with LD development [25].
Obesity commonly results in adipose tissue dysfunction, which increases free fatty acids (FFA) levels, leading to the development of FLD [26]. FFA acts as a ligand for toll-like receptor 4 and activates NF-κB to induce cytokine and chemokine production related to inflammation [6,16]. NF-κB is an important transcription factor that regulates inflammation in both innate and adaptive immune cells [4]. Blocking the NF-κB pathway has been demonstrated to reduce oxidative stress and decrease cell death due to liver damage [27,28]. In the present study, DA-6034 significantly reduced the level of phosphorylated NF-κB in vitro and in vivo. When NF-κB is activated in the liver, various cytokines, including IFN-γ, IL-6, and TNF-α, which stimulate monocytes to differentiate into M1 macrophages, are released [5,29]. Therefore, NF-κB plays a vital role in the differentiation of monocytes into M1 or M2 macrophages and acts as an important factor that regulates inflammation associated with metabolic diseases. To alleviate exacerbated hepatic inflammation and accumulated TG, strategies that inhibit M1 polarization of macrophages and/or induce alternative M2 activation are needed. Herein, the proportion of macrophages with the M1 phenotype was found to be significantly reduced in the liver of DA-6034–treated obese mice.
CCR is an important factor in macrophage migration to the site of inflammation and is differentially expressed in each cell [30]. Among the CCRs, CCR2 and CCR5 are known to be involved in macrophage migration [31]. Based on previous studies, obesity and metabolic diseases are alleviated in CCR2-deficient mice [32,33]. Additionally, CCR2 was demonstrated to promote NAFLD via the recruitment of myeloid cells [34]. In CCR2-deficient mice, obesity-induced adipose tissue inflammation was found to be attenuated [35]. In our previous studies, metabolic diseases were confirmed to be alleviated after CCR2 inhibition or dual CCR2/CCR5 inhibition [36,37]. A recent study showed that CCR2/CCR5 dual inhibition prevented alcohol-related liver diseases, including steatosis and inflammation [38]. In this study, CCR5 levels were significantly reduced in the liver of DA-6034–treated obese mice, suggesting that DA-6034 decreases macrophage migration in the liver via CCR5 inhibition.
When activated, M1 macrophages produce various cytokines, such as TNF-α, IL-6, IL-12, and IFN-γ [39,40]. Kupffer cell depletion has been shown to significantly decrease TNF-α and IL-6 levels [40]. In the current study, we determined whether DA-6034 affects inflammatory cytokine production in an HFD-induced obese mouse model. TNF-α and IL-6 levels were found to be significantly decreased in DA-6034–treated obese mice. These cytokines accumulate in other innate immune cells, including T lymphocytes. Interestingly, the proportion of F4/80+/CD11c+/CD206– M1 macrophages and CD3+/CD8+ T lymphocytes was significantly decreased in DA-6034–treated obese mice. Therefore, DA-6034 is very critical for preventing inflammation in the liver.
In conclusion, this study suggests that DA-6034 can inhibit inflammation and fatty liver production in the progression to hepatic steatosis or early stage of NASH.
Notes
Funding
This research was supported by the Basic Science Research Program through the National Research Program of the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (NRF-2021R1A2B5B01002354) and Ministry of Education (NRF-2019R1I1A1A01042030).
Author contributions
Conceptualization, Data curation: all authors; Formal analysis, Methodology, Software: HMK, MHK; Funding acquisition: HMK; Project administration: HMK, MHK, CHC; Investigation: MHK, ESL, KBH; Visualization: HMK, ESL; Writing-original draft: HMK; Writing-review & editing: MHK, ESL, KBH, CHC.
References
1. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010; 5:145–71.

2. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011; 332:1519–23.

4. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023.

5. Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011; 13:11–22.

6. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.
7. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014; 1842:446–62.

8. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010; 2010:289645.

9. Fei X, Chen C, Kai S, Fu X, Man W, Ding B, et al. Eupatilin attenuates the inflammatory response induced by intracerebral hemorrhage through the TLR4/MyD88 pathway. Int Immunopharmacol. 2019; 76:105837.

10. Du L, Chen J, Xing YQ. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed Pharmacother. 2017; 85:136–40.

11. Zhong WF, Wang XH, Pan B, Li F, Kuang L, Su ZX. Eupatilin induces human renal cancer cell apoptosis via ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol Lett. 2016; 12:2894–9.

12. Lee S, Lee M, Kim SH. Eupatilin inhibits H(2)O(2)-induced apoptotic cell death through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Food Chem Toxicol. 2008; 46:2865–70.
13. Kim YW, Lee WH, Choi SM, Seo YY, Ahn BO, Kim SH, et al. DA6034 promotes gastric epithelial cell migration and wound-healing through the mTOR pathway. J Gastroenterol Hepatol. 2012; 27:397–405.

14. Chung HJ, Choi YH, Choi HD, Jang JM, Shim HJ, Yoo M, et al. Pharmacokinetics of DA-6034, an agent for inflammatory bowel disease, in rats and dogs: contribution of intestinal first-pass effect to low bioavailability in rats. Eur J Pharm Sci. 2006; 27:363–74.

15. Kim YS, Son M, Ko JI, Cho H, Yoo M, Kim WB, et al. Effect of DA-6034, a derivative of flavonoid, on experimental animal models of inflammatory bowel disease. Arch Pharm Res. 1999; 22:354–60.

16. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005; 135:2503–6.

17. Pettinelli P, Obregón AM, Videla LA. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr Hosp. 2011; 26:441–50.
18. Kuan YC, Hashidume T, Shibata T, Uchida K, Shimizu M, Inoue J, et al. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein. J Biol Chem. 2017; 292:3016–28.

19. Ko SH, Yoo DY, Kim YJ, Choi SM, Kang KK, Kim H, et al. A mechanism for the action of the compound DA-6034 on NF-κB pathway activation in Helicobacter pylori-infected gastric epithelial cells. Scand J Immunol. 2011; 74:253–63.

20. Stienstra R, Netea-Maier RT, Riksen NP, Joosten LA, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 2017; 26:142–56.

21. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017; 13:633–43.

22. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–808.

23. Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010; 51:511–22.

24. Morgan PK, Huynh K, Pernes G, Miotto PM, Mellett NA, Giles C, et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J Biol Chem. 2021; 297:101341.

25. Castoldi A, Monteiro LB, van Teijlingen Bakker N, Sanin DE, Rana N, Corrado M, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020; 11:4107.

26. Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018; 22:23–37.

27. Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003; 19:725–37.

28. Luedde T, Assmus U, Wüstefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005; 115:849–59.

29. Luedde T, Schwabe RF. NF-κB in the liver: linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:108–18.

30. Stone MJ, Hayward JA, Huang C, E Huma Z, Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci. 2017; 18:342.

31. Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020; 19:41.

32. Parker R, Weston CJ, Miao Z, Corbett C, Armstrong MJ, Ertl L, et al. CC chemokine receptor 2 promotes recruitment of myeloid cells associated with insulin resistance in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2018; 314:G483–93.

33. Lee SJ, Kang JS, Kim HM, Lee ES, Lee JH, Chung CH, et al. CCR2 knockout ameliorates obesity-induced kidney injury through inhibiting oxidative stress and ER stress. PLoS One. 2019; 14:e0222352.

34. Gutierrez DA, Kennedy A, Orr JS, Anderson EK, Webb CD, Gerrald WK, et al. Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes. 2011; 60:2820–9.

35. Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012; 61:1680–90.

36. Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ, et al. Dual CCR2/5 antagonist attenuates obesity-induced insulin resistance by regulating macrophage recruitment and M1/M2 status. Obesity (Silver Spring). 2018; 26:378–86.

37. Kim HM, Kim YM, Huh JH, Lee ES, Kwon MH, Lee BR, et al. α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS One. 2017; 12:e0179204.

38. Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology. 2019; 69:1105–21.

Fig. 1.
Cytotoxicity of eupatilin and DA-6034 in AML12 cells. (A) Chemical structures of eupatilin and DA-6034. (B) MTT cytotoxicity assay results of cell viability based on DA-6034 and eupatilin administered at different doses. Measurement of dissolved MTT in AML12 cells after DA-6034 and eupatilin treatment. *p<0.05, compared with 0 μM. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
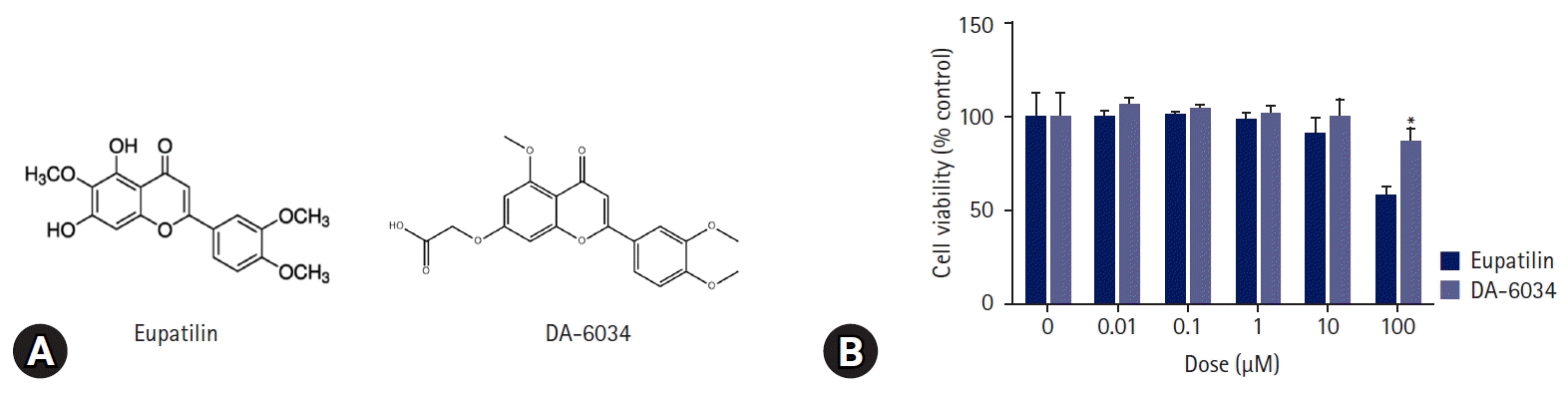
Fig. 2.
Weight gain and liver change owing to DA-6034 in HFD-fed mice. WT and DA-6034–treated mice were fed HFD or RD for 12 weeks. The change in (A) body weight, (B) food intake, and (C) liver weight. (D) Paraffin-embedded liver tissue was stained with hematoxylin and eosin. (E) Reduction of hepatic TG by DA-6034 in the HFD group. (F–J) Protein levels of (F, G) FAS, (F, H) SREBP-1, (F, I) phosphorylated NK-κB, and (F, J) IκB in the liver based on western blotting. Cropped membranes were used for western blotting. Data are presented as mean±standard error of the mean (n=10 animals per group). *p<0.05, compared with RD-fed mice. †p<0.05, compared with HFD-fed mice. RD, regular diet; HFD, high fat diet; WT, wild type; DA, DA-6034; TG, triglycerides; FAS, fatty acid synthesis; SREBP-1, sterol regulatory element-binding protein 1; IκB, inhibitor of kappa B; NF-κB, nuclear factor kappa B; p-NF-κB, phospho-NF-κB.
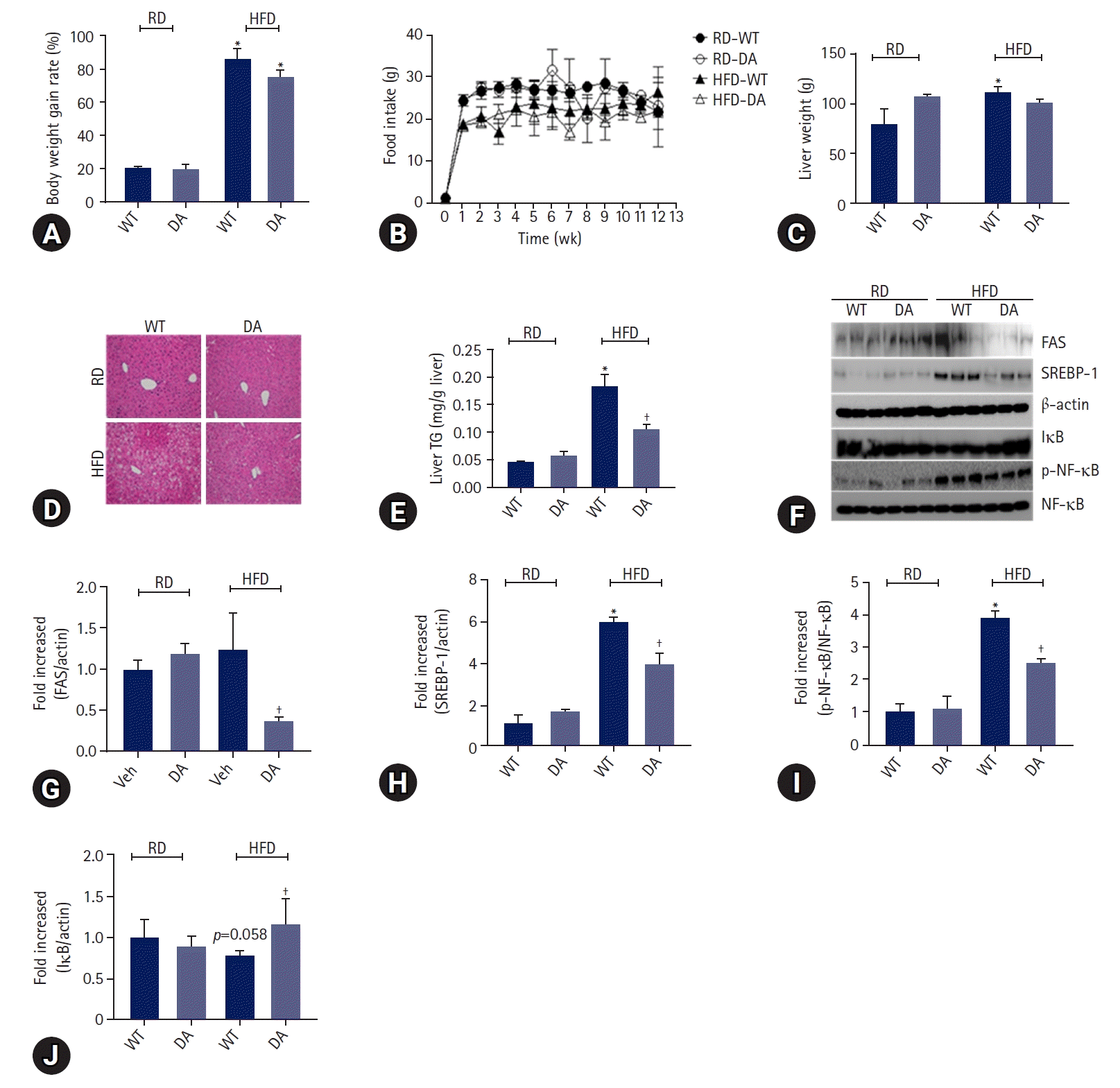
Fig. 3.
DA-6034 effects on inflammation and macrophage migration. RAW 264.7 cells were treated with CCL2 (10 ng/mL) or CCL4 (10 ng/mL) in the presence and absence of DA-6034 (20 µM/mL) for 24 hours (n=4). (A, B) The number of transmigrated RAW 264.7 cells in the presence or absence of DA-6034 was measured using the migration assay. Original magnification, 200× (scale bar, 100 µm). (C) Macrophages infiltrating liver cells were detected via CD68 staining (C). (D–F) Protein levels of CCR2 and CCR5 in the liver based on western blotting. The protein levels of phosphorylated NK-κB in the RAW 264.7 cells (G, H) based on western blotting. Cropped membranes were used for western blotting. Data are presented as mean±standard error of the mean (n=10 animals per group). *p<0.05, compared with RD-fed mice or Veh. †p<0.05, compared with HFD-fed mice or LPS. CON, control; DA, DA-6034; CCL2, CCR2 ligand; CCL4, CCR4 ligand; Veh, vehicle; RD, regular diet; HFD, high fat diet; WT, wild type; CCR, C-C motif chemokine receptor; NF-κB, nuclear factor kappa B; p-NF-κB, phospho-NF-κB; LPS, lipopolysaccharides.
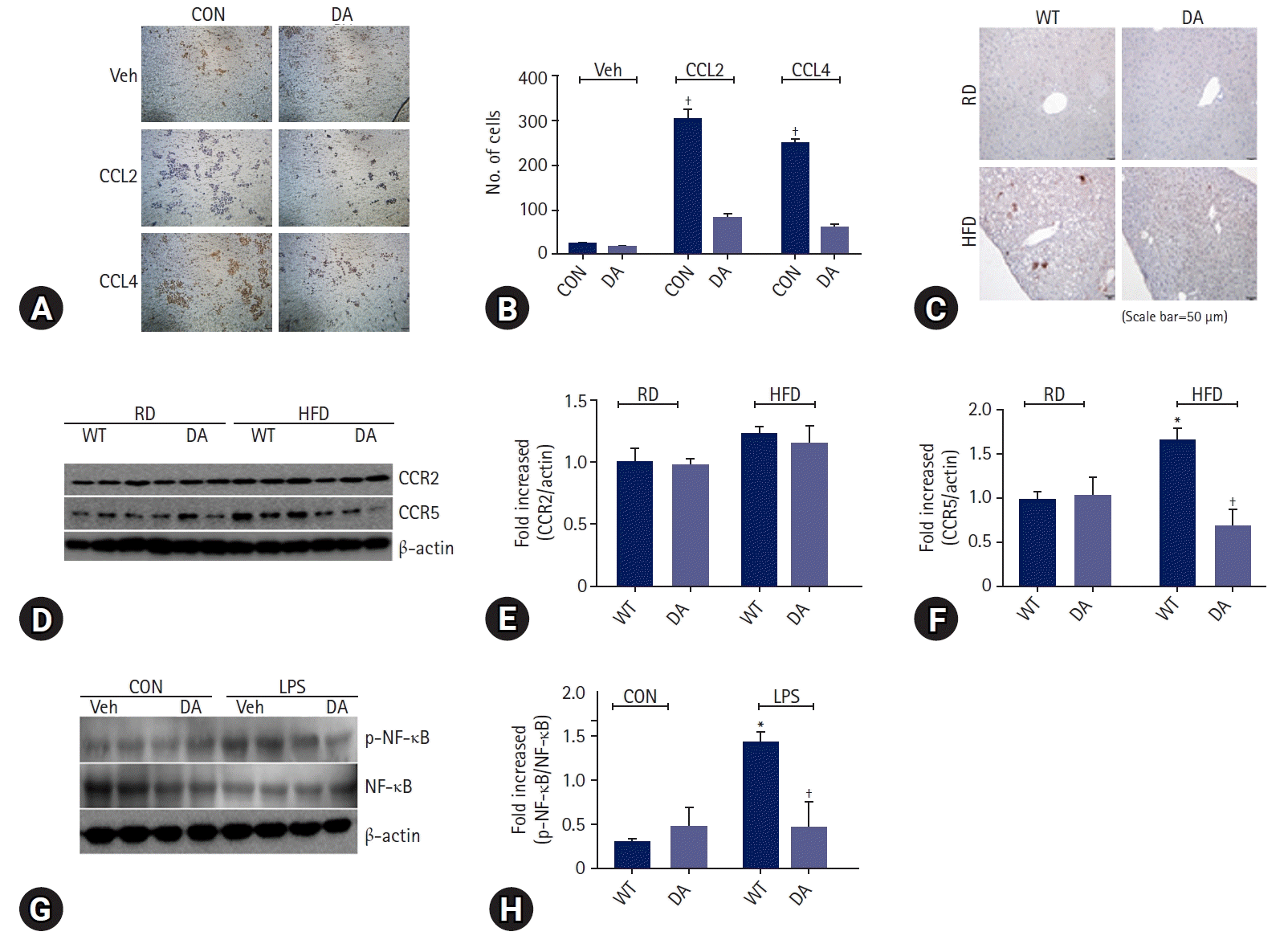
Fig. 4.
DA-6034 reduces the level of proinflammatory immune cells in the liver. Flow cytometry analysis of immune cells from the liver of mice. (A) CD11c+/CD206– or CD11c–/CD206+ cell population was analyzed in the liver of RD-fed mice. (B) Percentages of M1 and M2 adipose tissue macrophages. (C) Fluorescence-activated cell sorting plots and gating of immune cells to detect the percentage of T cells (CD3+ cells) in the liver. Representative flow cytometric plots of the percentage of CD8+ T cells (CD3+/CD4–/CD8+) and CD4+ T cells (CD3+/CD4+/CD8–). (D) Percentages of CD8+ T cells and CD4+ T cells. Data are presented as mean±stanard error of the mean. *p<0.05 compared with RD-fed mice. †p<0.05 compared with HFD-fed mice. WT, wild type; DA, DA-6034; RD, regular diet; HFD, high fat diet.
Table 1.
Effect of DA-6034 on metabolic parameters at 12 weeks
| Parameter | RD group | RD+DA group | HFD group | HFD+DA group |
|---|---|---|---|---|
| Glucose (mg/dL) | 139.20±7.51 | 143.76±7.41 | 187.67±5.90a) | 150.69±10.14b) |
| Insulin (ng/dL) | 0.19±0.01 | 0.19±0.06 | 0.39±0.05a) | 0.34±0.03 |
| TC (mg/dL) | 155.80±3.92 | 156.40±4.11 | 266.13±3.36a) | 236.59±8.78 |
| TG (mg/dL) | 34.20±2.70 | 29.44±2.37 | 70.97±3.66a) | 48.08±1.82b) |
| Adiponectin (ng/mL) | 32.30±0.89 | 31.03±1.05 | 19.87±0.37 | 20.86±0.50 |
| ALT (IU/L) | 3.20±0.43 | 2.43±0.44 | 4.40±0.38 | 3.01±0.71 |
| AST (IU/L) | 16.70±0.88 | 17.79±1.08 | 29.64±1.37a) | 22.79±0.91b) |
Table 2.
Effect of DA-6034 on inflammatory cytokine secretion
| Variable | RD group | RD+DA group | HFD group | HFD+DA group |
|---|---|---|---|---|
| TNF-α (pg/mL) | 390.88±11.21 | 432.44±8.49 | 479.55±10.68b) | 434.48±13.79c) |
| MCP1 (pg/mL) | 571.93±12.36 | 545.76±6.65 | 850.19±92.89a) | 532.41±12.99c) |
| IL-6 (pg/mL) | 816.11±29.18 | 832.29±23.35 | 1199.72±201.68a) | 848.91±59.59c) |
| IL-10 (pg/mL) | 2,812.15±38.17 | 2,768.64±38.02 | 2,542.93±21.91b) | 2,806.69±37.26d) |
| IFN-γ (pg/mL) | 134.00±0.75 | 143.69±5.44 | 143.71±7.21 | 137.22±3.03 |
| IL-12p70 (pg/mL) | 355.23±8.60 | 355.85±6.00 | 343.77±3.73 | 337.45±7.91 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download