Abstract
Aortic dissection in pregnant patients results in an inpatient mortality rate of 8.6%. Owing to the pronounced mortality rate and speed at which aortic dissections progress, efficient early detection methods are crucial. Here, we highlight the importance of early chest computed tomography (CT) for differentiating aortic dissection from pulmonary embolism in pregnant patients with dyspnea. We present the unique case of a 38-year-old pregnant woman with elevated D-dimer and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, initially suspected of having a pulmonary embolism. Initial transthoracic echocardiography did not indicate aortic dissection. Surprisingly, after an emergency cesarean section, a chest CT scan revealed a DeBakey type I aortic dissection, indicating a diagnostic error. Our findings emphasize the need for early chest CT in pregnant patients with dyspnea and elevated D-dimer and NT-proBNP levels. This case report highlights the critical importance of considering both aortic dissection and pulmonary embolism in the differential diagnosis of such cases, which will inform future clinical practice.
Aortic dissection occurs in 5.5 out of every 100,000 hospitalized pregnant patients or those in the postpartum period, with an inpatient mortality rate of 8.6% [1]. This rate is significantly higher than the 0.0026% in-hospital all-cause mortality rate in patients without cardiovascular disease [2]. Notably, aortic dissection tends to occur most frequently during the final stages of pregnancy and early postpartum period, owing to peak enhancements in maternal cardiac output and hormonal activation [3]. Because the mortality rate associated with aortic dissection is pronounced and increases by 1% with each passing hour [4], early detection is of paramount importance. Although chest and back pain are typical symptoms, dyspnea is a prominent symptom in cases of pulmonary embolism, and the incidence is notably higher in pregnant patients than in non-pregnant individuals [5]. However, the mortality rate for pulmonary embolism is 0.002%, which is lower than that for aortic dissection [5]. Although there is existing case report on late-pregnancy aortic dissection, previous reports have featured typical chest pain, making the diagnosis less challenging [6].
Here, we report a case of DeBakey type I aortic dissection that primarily manifested as dyspnea without chest pain. The patient underwent an emergency cesarean section with suspected pulmonary embolism but was later diagnosed with aortic dissection. Overall, both aortic dissection and pulmonary embolism should be considered in the differential diagnosis of pregnant patients with dyspnea, as the management and outcomes of these conditions differ significantly.
Ethical statements: This case report was approved by the Institutional Review Board (IRB) of Keimyung University Dongsan Hospital (IRB No: 2023-07-063) on July 28, 2023, and the need for patient informed consent was waived.
A 38-year-old pregnant woman in her third trimester (gestational age, 36 weeks; gravida two, para one; height, 171 cm; weight, 81.7 kg; body mass index, 27.9 kg/m2) visited the emergency department because she had been experiencing exertional dyspnea for the past 5 days. The patient had a history of gestational diabetes mellitus and hypertension. Because there was no record of genetic testing, the presence of genetic disorders could not be determined. Her initial vital signs included elevated blood pressure (130/70 mmHg), normal peripheral oxygen saturation (97%), and normal serum blood glucose levels (120 mg/dL). Although she was tachycardic (113 beats/minute), her heart rhythm by electrocardiography was normal. The patient’s initial D-dimer and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were elevated, measuring 4.43 μg/mL and 5,387 pg/mL, respectively. No abnormalities were noted on chest radiograph (Fig. 1). Bedside transthoracic echocardiography (TTE) was performed in a limited manner, acquiring only apical four-chamber and parasternal short-axis view at mid-ventricle level, which revealed a dilated right ventricle and reduced right ventricular systolic function without pericardial effusion (Fig. 2). The cardiologist suspected pulmonary thromboembolism considering the patient’s symptoms, elevated D-dimer level, and right ventricular dysfunction on TTE. However, considering the possible harm to the unborn child from ionizing radiation, a decision was made to prioritize emergency cesarean section.
Upon admission to the operating room, the patient’s initial blood pressure was 99/52 mmHg, heart rate was 95 beats/minute, and peripheral oxygen saturation was 97% on 3 L/minute of oxygen via a nasal cannula. Spinal anesthesia was performed in the sitting position using 0.5% bupivacaine 10 mg and fentanyl 15 μg. During the surgery, phenylephrine 200 μg was administered when the patient’s systolic blood pressure fell below 80 mmHg. The surgery lasted 80 minutes, and chest computed tomography (CT) was performed immediately afterward. Unexpectedly, the anticipated pulmonary embolism could not be found; however, a DeBakey type I aortic dissection was detected (Fig. 3). The patient was immediately transferred to the operating room, where she underwent the Bentall procedure, hemiarch replacement, and coronary artery bypass graft surgery that lasted 7 hours. These procedures were necessary because extension of the dissection flap to the aortic valve caused severe aortic regurgitation (Fig. 4), and transection of the right coronary artery. The patient was discharged on postoperative day 11 without any complications. Two months after surgery, genetic testing confirmed the diagnosis of Marfan syndrome.
Aortic dissection is classified as hyperacute, acute, subacute, or chronic, based on the timing of symptom onset [7]. As illustrated in this case, aortic dissection is defined as acute when the diagnosis is made within 2 weeks of symptom onset. In contrast, pulmonary embolism, as initially suspected in this case, is typically characterized by sudden onset dyspnea within 48 hours [8], which was not consistent with the observations of this case. Dyspnea can be a primary symptom of aortic dissection due to airway compression, cardiac tamponade, or aortic regurgitation, as observed in our case. The risk factors for aortic dissection and pulmonary embolism differ [1,9]. The risk factors associated with our patient were hypertension and gestational diabetes for aortic dissection, and age >35 years for pulmonary embolism (Table 1) [1,9]. These factors also favor the diagnosis of aortic dissection.
According to Li et al. [10], D-dimer levels are significantly elevated in patients with acute aortic dissection and those with pulmonary embolism. Therefore, D-dimer levels cannot serve as a differentiating marker as there is no distinct variance in the values between the two conditions, with reported median values of 0.38 and 2.72 μg/mL, respectively [10]. High NT-proBNP levels are observed in both diseases. In acute aortic dissection, elevated NT-proBNP (>600 pg/mL) is observed owing to increased ventricular wall afterload caused by uncontrolled hypertension and is associated with a poor outcome, with a prognostic sensitivity of 96% and specificity of 55% [11,12]. Similarly, in pulmonary embolism cases, an initial NT-proBNP level >600 pg/mL has a sensitivity of 100% and specificity of 33% for predicting mortality, indicative of right ventricular dysfunction [13].
A definitive diagnosis is made based on radiographic examination. The 2018 European Society of Cardiology guidelines recommend TTE for patients to manage cardiovascular diseases during pregnancy, and chest CT examination to rule out the possibility of pulmonary embolism [14]. However, it is important to note that TTE has a sensitivity of 78% to 90% for diagnosing ascending aortic dissection, indicating a risk of misdiagnosis [15]. A characteristic finding of TTE in patients with pulmonary embolism is right ventricular dysfunction [16]. Therefore, the cardiologist in this case examined only the apical four-chamber and parasternal short-axis view at the mid-ventricle level. Moreover, the use of TTE for the diagnosis of pulmonary embolism is limited owing to its low sensitivity (70%) and specificity (33%) [17]. In this case, the aortic dissection involving the right coronary artery manifested as right ventricular dysfunction, further complicating the differentiation between these two conditions with TTE. In addition, the recently published pregnancy-adapted YEARS algorithm recommends performing chest CT to diagnose pulmonary embolism, even in the absence of symptoms, when the D-dimer level is >1,000 ng/mL [18]. In this case, only TTE was performed before the cesarean section because of concerns regarding fetal radiation exposure from the CT scan. However, given the low sensitivity of TTE in differentiating between aortic dissection and pulmonary embolism, coupled with the patient’s elevated D-dimer and NT-proBNP levels, both critical indicators for assessing patient outcomes, a prompt chest CT scan may have been more beneficial. Furthermore, radiation doses <0.5 Gy are safe for women in their third trimester of pregnancy. Since the radiation dose for a CT scan to diagnose aortic dissection is <0.5 Gy, it can be considered safe [19]. A direct CT scan in this case might have allowed for a more definitive diagnosis and the immediate management of DeBakey type I aortic dissection.
Unlike previous report describing typical chest pain in pregnant patients with aortic dissection [6], this case report offers valuable insights into the differential diagnosis and management of aortic dissection and pulmonary embolism in pregnant patients with atypical symptoms. This report uniquely combines the examination of various laboratory parameters, such as D-dimer and NT-proBNP levels, with the evaluation of patient symptoms and history, highlighting the importance of early comprehensive assessment with an early CT scan in reaching an accurate diagnosis. In addition, this differential diagnosis adheres to established guidelines and recently published algorithms, thereby demonstrating an up-to-date approach for disease diagnosis.
This case report has several limitations. It reports observations from a single case, which limits its applicability to a broader patient population. In this case, the initial evaluation with TTE was conducted within a limited scope, without observing the aortic valve and ascending aorta. Notably, detecting an intimal flap, tear, or hematoma in the proximal aorta has a significant diagnostic value for ascending aortic dissection [20]. Therefore, the lack of a comprehensive examination in these areas may have contributed to the delay in diagnosis. However, this limitation underscores the importance of thorough initial assessments to distinguish between aortic dissection and pulmonary embolism during early evaluation. This case report also does not provide a comparative analysis with similar cases. Further research should be conducted to verify whether the initial application of chest CT in pregnant patients exhibiting dyspnea and elevated D-dimer and NT-proBNP levels truly improves patient outcomes by facilitating the early differential diagnosis of aortic dissection and pulmonary embolism.
In conclusion, this case report underscores the importance of considering both aortic dissection and pulmonary embolism in the differential diagnosis of dyspnea in pregnant patients. Specifically, owing to the potential implications of elevated D-dimer and NT-proBNP levels for adverse outcomes in both conditions, immediate implementation of a chest CT scan is particularly crucial in pregnant patients exhibiting dyspnea and elevated biomarkers. In addition, when conducting TTE in these patients, a thorough examination that includes the ascending aorta should be performed to enhance diagnostic accuracy.
References
1. Beyer SE, Dicks AB, Shainker SA, Feinberg L, Schermerhorn ML, Secemsky EA, et al. Pregnancy-associated arterial dissections: a nationwide cohort study. Eur Heart J. 2020; 41:4234–42.

2. Majmundar M, Doshi R, Patel KN, Zala H, Kumar A, Kalra A. Prevalence, trends, and outcomes of cardiovascular diseases in pregnant patients in the USA: 2010-19. Eur Heart J. 2023; 44:726–37.

3. Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol. 1967; 83:336–41.
4. Harris KM, Strauss CE, Eagle KA, Hirsch AT, Isselbacher EM, Tsai TT, et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011; 124:1911–8.

5. Farmakis IT, Barco S, Hobohm L, Braekkan SK, Connors JM, Giannakoulas G, et al. Maternal mortality related to pulmonary embolism in the United States, 2003-2020. Am J Obstet Gynecol MFM. 2023; 5:100754.

6. Huang KL, Leung-Chit Tsang L, Yen HT, Chou YL, Tsai CC, Cheng HH, et al. Lethal acute aortic dissection complicated with ischemic bowels in the third trimester of pregnancy: a case report. Taiwan J Obstet Gynecol. 2020; 59:740–3.

7. Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020; 71:723–47.

8. Miniati M, Cenci C, Monti S, Poli D. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One. 2012; 7:e30891.

9. Gris JC, Guillotin F, Chéa M, Bourguignon C, Bouvier S. The risk of thrombosis around pregnancy: where do we stand? Front Cardiovasc Med. 2022; 9:901869.

10. Li W, Huang B, Tian L, Yang Y, Zhang W, Wang X, et al. Admission D-dimer testing for differentiating acute aortic dissection from other causes of acute chest pain. Arch Med Sci. 2017; 13:591–6.

11. Vrsalovic M, Vrsalovic Presecki A, Aboyans V. N-terminal pro-brain natriuretic peptide and short-term mortality in acute aortic dissection: a meta-analysis. Clin Cardiol. 2020; 43:1255–9.

12. Wen D, Jia P, Du X, Dong JZ, Ma CS. Value of N-terminal pro-brain natriuretic peptide and aortic diameter in predicting in-hospital mortality in acute aortic dissection. Cytokine. 2019; 119:90–4.

13. Kostrubiec M, Pruszczyk P, Kaczynska A, Kucher N. Persistent NT-proBNP elevation in acute pulmonary embolism predicts early death. Clin Chim Acta. 2007; 382:124–8.

14. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018; 39:3165–241.

15. Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010; 11:645–58.

16. Shahabi J, Zavar R, Amirpour A, Bidmeshki M, Barati-Chermahini M. Right ventricular (RV) echocardiographic parameters in patients with pulmonary thromboembolism (PTE). ARYA Atheroscler. 2018; 14:78–84.
17. Casazza F, Bongarzoni A, Capozi A, Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005; 6:11–4.

18. van der Pol LM, Tromeur C, Bistervels IM, Ni Ainle F, van Bemmel T, Bertoletti L, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019; 380:1139–49.

19. Yoon I, Slesinger TL. Radiation exposure in pregnancy [updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2023. [cited 2023 Dec 4]. https://www.ncbi.nlm.nih.gov/books/NBK551690/.
Fig. 2.
Transthoracic echocardiography shows dilated right ventricle (arrowheads) and reduced right ventricular systolic function. (A) Apical four-chamber view. (B) Parasternal short-axis view at mid-ventricle level.
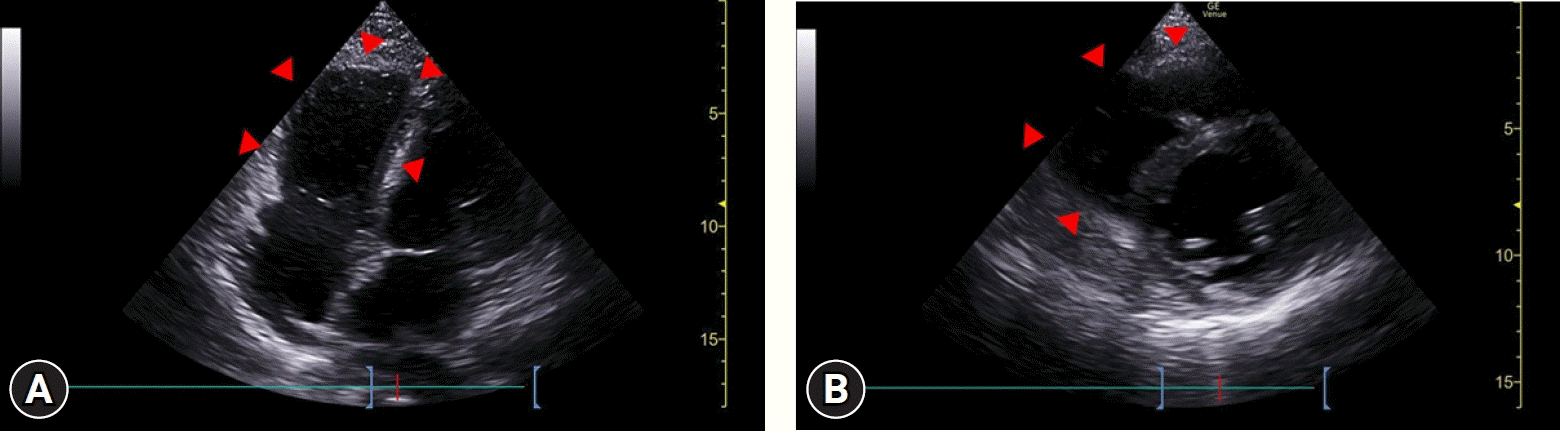
Fig. 3.
Chest computed tomography shows (A) ascending (coronal view) and (B) abdominal aortic dissection (transverse view). Arrowheads indicate the dissection flap.
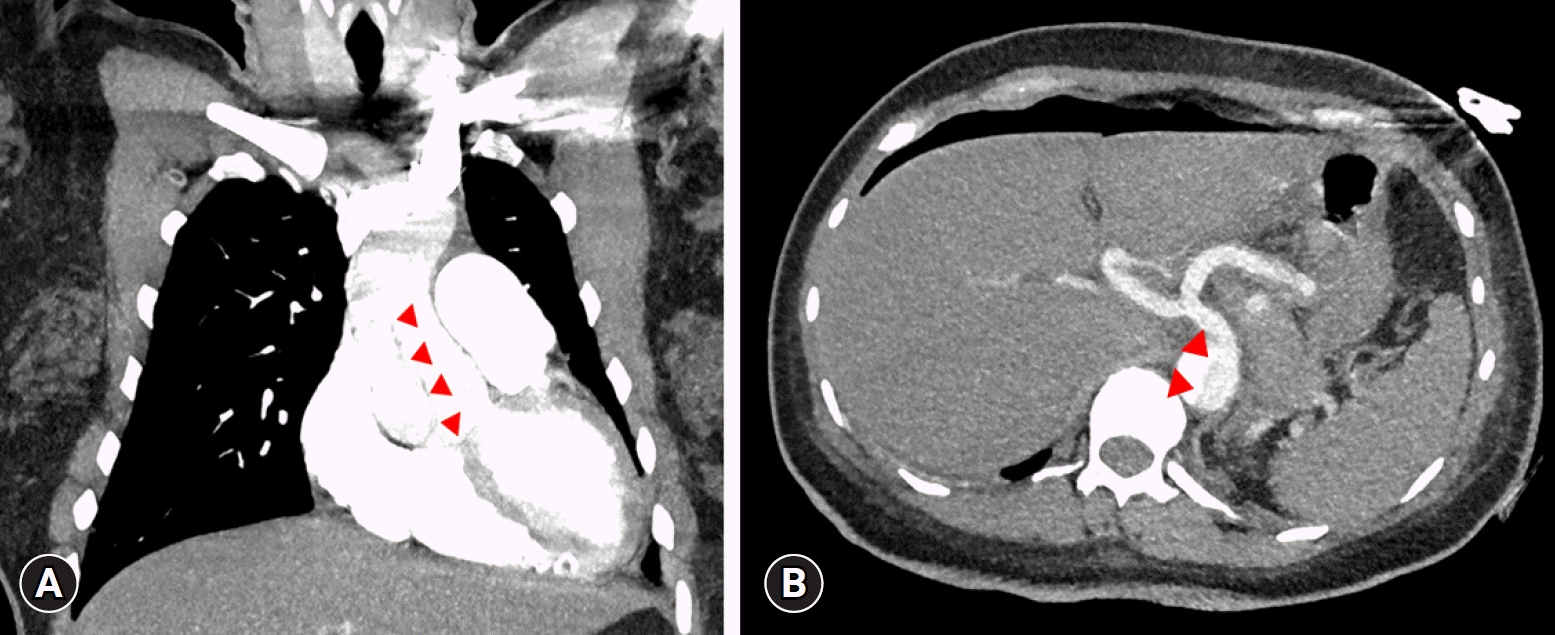
Fig. 4.
(A) Intraoperative transesophageal echocardiography (mid-esophageal long-axis view). The dissection flap (arrowheads) is seen in the proximal ascending aorta. (B) Systolic aortic regurgitation is also seen.
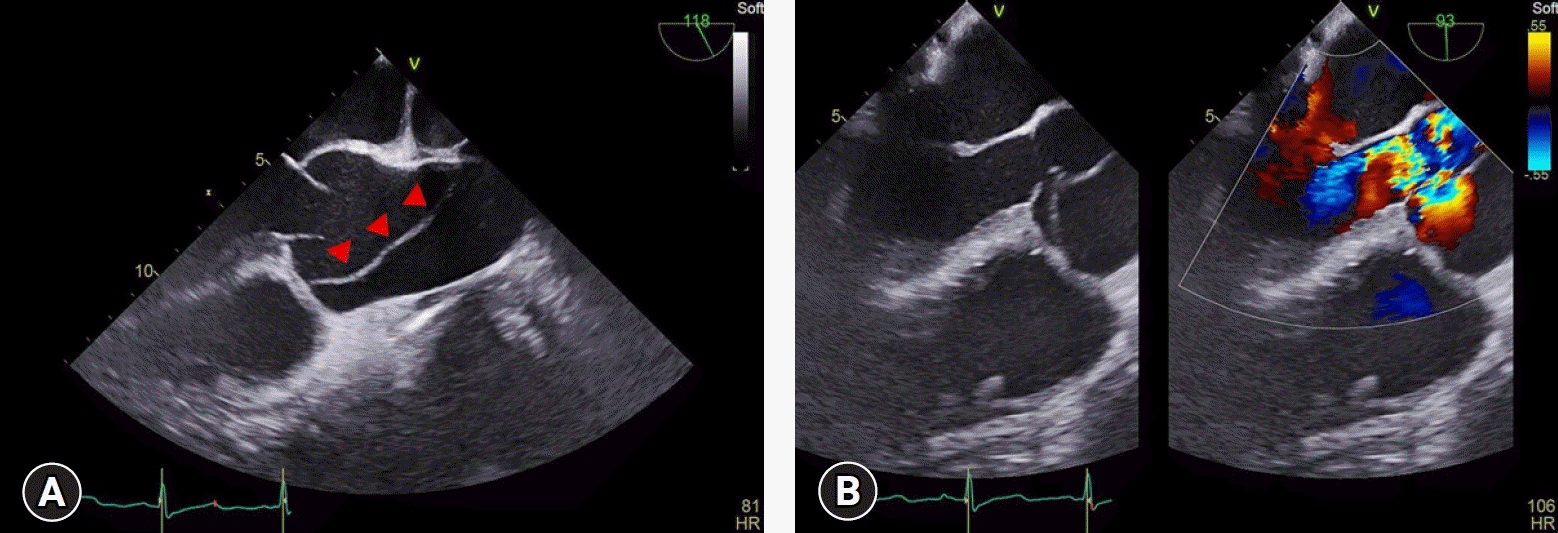
Table 1.
Risk factors associated with aortic dissection and pulmonary embolism
| Risk factors for aortic dissection [1] | Risk factors for pulmonary emboli [9] |
|---|---|
| Hypertension | Prior venous thromboembolism |
| Body mass index, >30 kg/m2 | Body mass index, >30 kg/m2 |
| Marfan syndrome | Familial venous thromboembolism |
| Ehlers-Danlos syndrome | Age, >35 years |
| Gestational diabetes | Parity, >3 |
| Preeclampsia/eclampsia | Preeclampsia |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download