Abstract
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
Fig. 1.
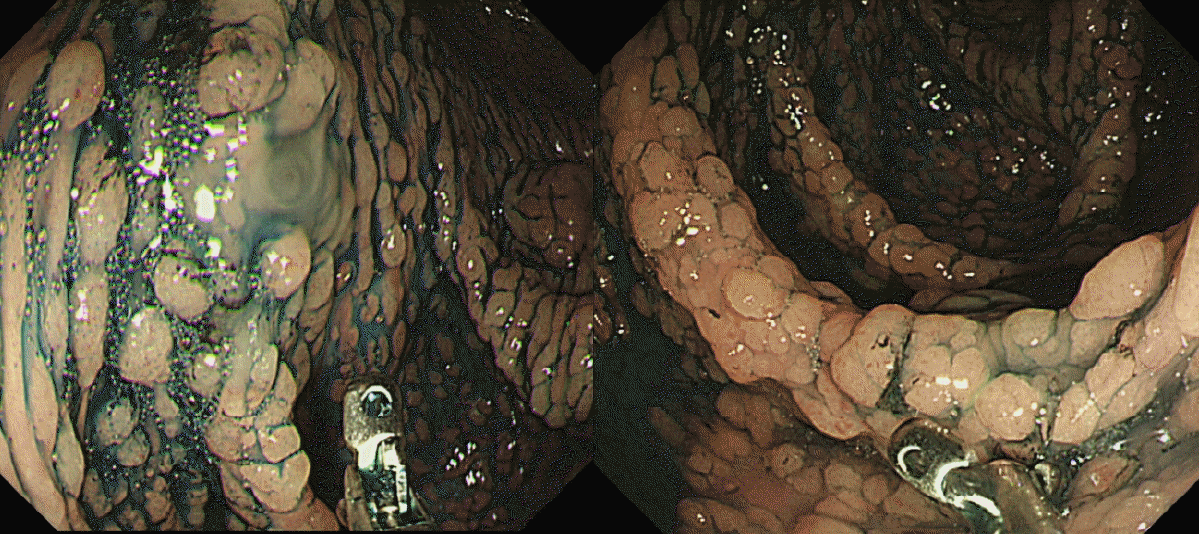
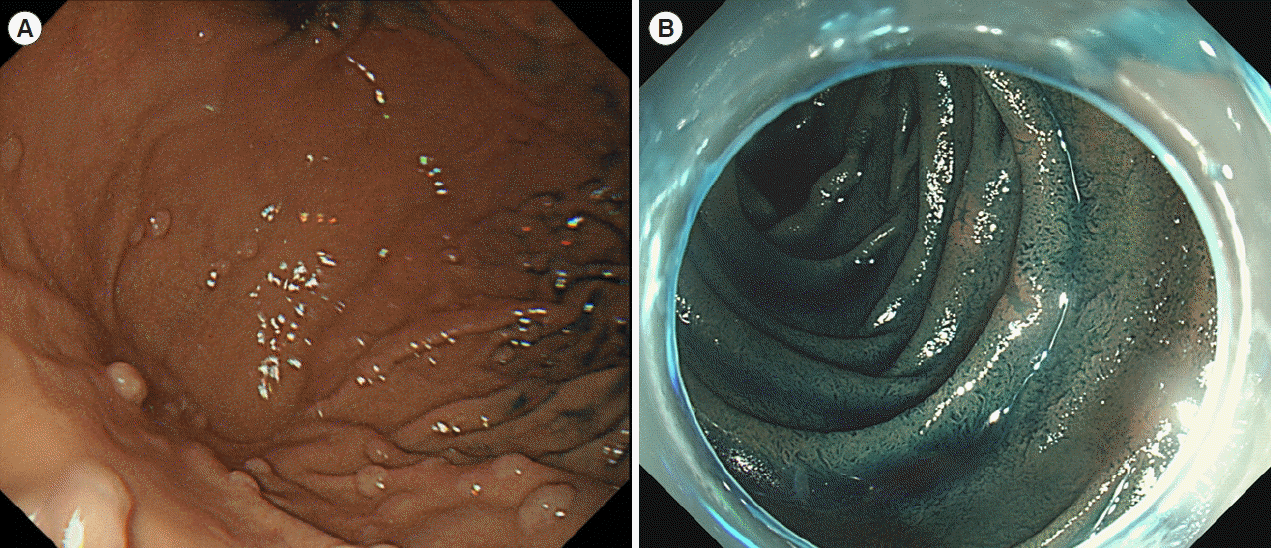
Fig. 4.
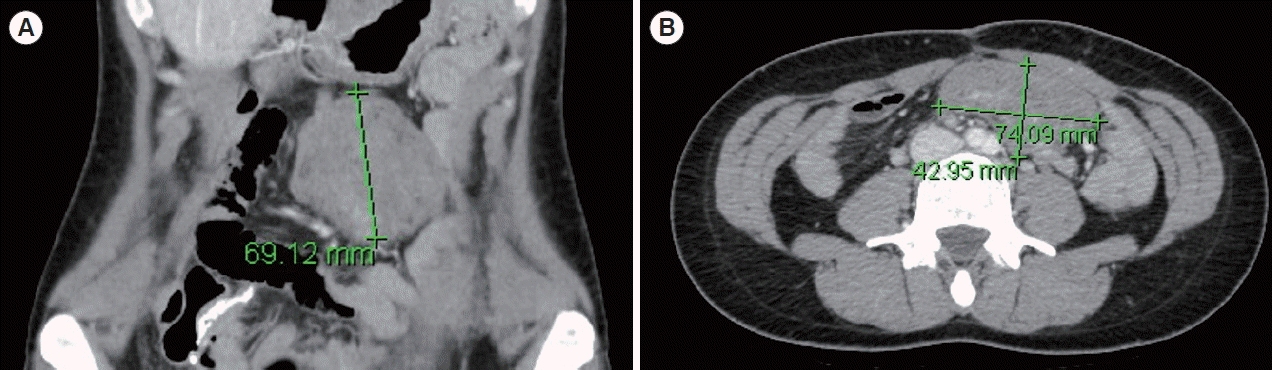
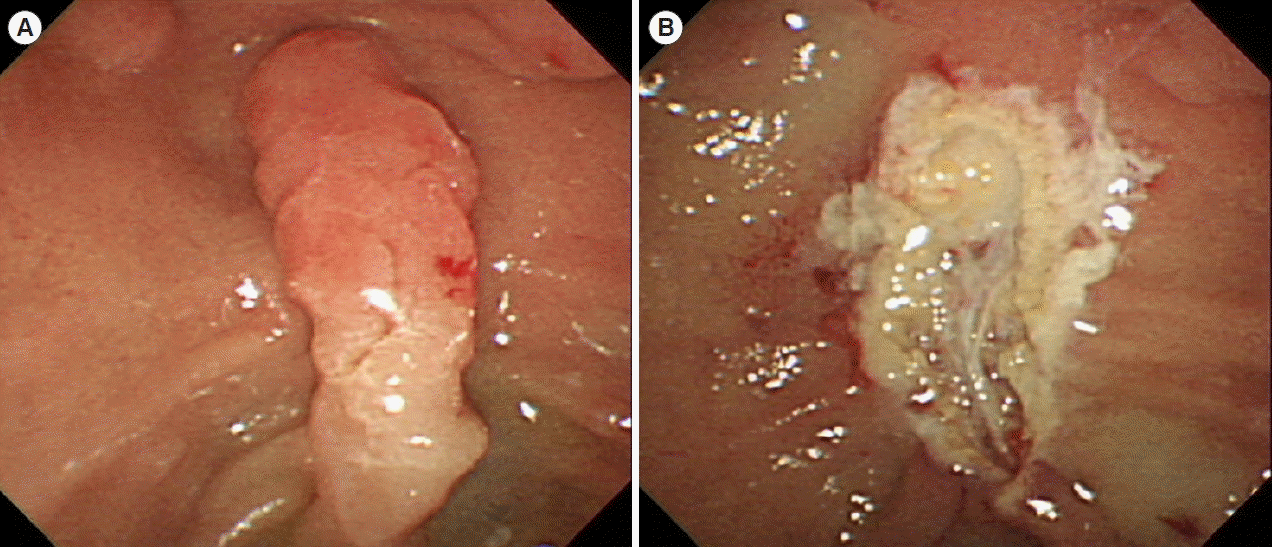
Table 1.
Table 2.
Table 3.
| Name | Clinical criteria | |
|---|---|---|
| Amsterdam criteria II [20] | Each of the following criteria must be fulfilled: | |
| 1. Three or more relatives with an HNPCC-associated cancer.a | ||
| 2. Two or more successive generations affected. | ||
| 3. One or more diagnosed before the age of 50 years. | ||
| 4. One should be a first-degree relative of the other two. | ||
| 5. Familial adenomatous polyposis should be excluded. | ||
| 6. Tumors should be verified by pathologic examination. | ||
| The revised Bethesda guidelines [19] | Tumors from individuals should be tested for microsatellite instability in the following situations. | |
| Requires at least one of the following: | ||
| 1. Colorectal cancer diagnosed in a patient who is less than 50 years of age. | ||
| 2. Presence of synchronous, metachronous colorectal, or other HNPCC-associated tumorsb, regardless of age. | ||
| 3. Colorectal cancer with the high level of microsatellite instability histologyc diagnosed in a patient who is less than 60 years of age. | ||
| 4. Colorectal cancer diagnosed in one or more first-degree relatives with an HNPCC-related tumor, with one of the cancers being diagnosed under age 50 years. | ||
| 5. Colorectal cancer diagnosed in 2 or more first- or second-degree relatives with HNPCC-related tumors, regardless of age. | ||
Table 4.
| Cancer | Modality | Recommendation | NCCN [10] | BSG/ACPGBI/UKCGG 2020 [6] | JSCCR 2020 [5] | ACG 2015 [26] |
|---|---|---|---|---|---|---|
| Colorectal cancer | Colonoscopy | Starting age | 20–25 years | 25 years for MLH1 and MSH2 mutation carriers | 20–25 years | 20–25 years |
| 2–5 years prior to the earliest colorectal cancer if diagnosed before age 25 years | 35 years for MSH6 and PMS2 mutation carriers | |||||
| Interval | 1–2 yearly | 2 yearly until age 75 years | 1–2 yearly | 2 yearly | ||
| 1 yearly in confirmed mutation carriers | ||||||
| Endometrial cancer | Endometrial biopsy | Starting age | 30–35 years | 30–35 years | 30–35 years | |
| Interval | 1–2 yearly | 1 yearly | 1 yearly | |||
| Total hysterectomy | A risk-reducing option | |||||
| Timing of total hysterectomy can be individualized | ||||||
| Ovarian cancer | Transvaginal ultrasound | Starting age | Data do not support routine ovarian cancer screening | 30–35 years | 30–35 years | |
| Interval | 1 yearly | 1 yearly | ||||
| BSO | A risk-reducing option | LS mutation carriers and who have finished child bearing, optimally at age 40–45 years | ||||
| Timing of BSO should be individualized | ||||||
| Gastric cancer | EGD | Starting age | 30–40 years | No convincing evidence to support the utility of gastric surveillance | 30–35 years | 30–35 years |
| Interval | 2–4 yearly | 1–3 yearly | 3–5 yearly | |||
| Helicobacter pylori | Screening and eradication | Screening and eradication | Screening and eradication | Treatment when found | ||
| Urothelial cancer | Urinalysis (or urine cytology) | Starting age | No clear evidence | 30–35 years | Not recommended unless there is a family history of the specific cancers | |
| 30–35 years | ||||||
| Interval | 1 yearly | 1 yearly | ||||
| Pancreatic cancer | MRI/MRCP/EUS | Starting age | 50 years with family history | Not recommended unless there is a family history of the specific cancers | ||
| Interval | Annual | |||||
| Prostate cancer | PSA | Starting age | 40 years | Not recommended unless there is a family history of the specific cancers | ||
| Digital rectal examination | ||||||
| Interval | Annual |
HNPCC, hereditary non-polyposis colorectal cancer; NCCN, National Comprehensive Cancer Network; BSG, British Society of Gastroenterology; ACPGBI, Association of Coloproctology of Great Britain and Ireland; UKCGG, United Kingdom Cancer Genetics Group; JSCCR, Japanese Society for the Cancer of the Colon and Rectum; ACG, American College of Gastroenterology; BSO, bilateral salpingo-oophorectomy; LS, Lynch syndrome; EGD, esophagogastroduodenoscopy; MRI, magnetic resonance imaging; MRCP, magnetic resonance cholangiopancreatography; EUS, endoscopic ultrasound; PSA, prostatespecific antigen.
Table 5.
| Modality | Recommendation | NCCN [10] | ASGE 2020 [26] | BSG/ACPGBI/UKCGG 2020 [6] | JSCCR 2020 [5] | ACG 2015 [33] |
|---|---|---|---|---|---|---|
| Colonoscopy | Starting age at screening | 10–15 years | 10–2 years | 12–14 years | 10 years | At puberty |
| Surveillance interval | 1 yearly | 1–2 yearly | 1–3 yearly | 1–2 yearly (AFAP: 2–3 yearly) | 1 yearly | |
| Sigmoidoscopy after colectomy with IRA or total proctocolectomy with IPAA | Starting age at screening | IRA: 6 months to 1 yearly | Subtotal colectomy: 6 months after surgery | IRA: 6 monthly | ||
| IPAA: 6 months to 1 yearly | Total colectomy: 1 year after surgery | IPAA: 1 yearly | ||||
| Surveillance interval | IRA: 6 months to 1 yearly | Subtotal colectomy: 6 months to 1 yearly | 1–3 yearly | |||
| IPAA: 6 months to 1 yearly | Total colectomy: 1–2 yearly | |||||
| Upper gastrointestinal endoscopy (duodenal or periampullary cancer) | Starting age at screening | 20–25 years | 20–25 years or before colectomy | 25 years | 20–25 years | Age 25–30 years |
| Surveillance interval | follow-up intervals based on the Spigelman stage | Every 0.5–4 years depending on Spigelman stage | ||||
| Upper gastrointestinal endoscopy (stomach cancer) | Starting age at screening | Need for specialized surveillance or surgery should be considered in presence of high-risk histologic features | 1 yearly | |||
| Surveillance interval | ||||||
| Jejunal ileal adenoma or cancer | Data to support any recommendation are lacking | |||||
| Thyroid ultrasound | Starting age at screening | Late teenage years | Late teenage years | |||
| Surveillance interval | 2–5 yearly | 1 yearly |
NCCN, National Comprehensive Cancer Network; ASGE, American Society for Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; ACPGBI, Association of Coloproctology of Great Britain and Ireland; UKCGG, United Kingdom Cancer Genetics Group; JSCCR, Japanese Society for the Cancer of the Colon and Rectum; ACG, American College of Gastroenterology; AFAP, atypical familial adenomatous polyposis; IRA, ileorectal anastomosis; IPAA, ileal pouch-anal anastomosis.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download